Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Malaria Survey in Rural Villages of Anger Gute Area of Gida Ayana District, Western Ethiopia
*Corresponding author:Geletta Tadele, Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia.
Received:December 15, 2022; Published:February 15, 2023
DOI: 10.34297/AJBSR.2023.18.002424
Abstract
Background: Active malaria detection is required, for efficient identification of malaria cases or clusters of cases to eliminate malaria. Objective of this study was to explore prevalence of asymptomatic parasite carriage and active malaria cases among rural residents of Anger Gute area.
Methods: Community based cross-sectional survey was done among 413 individuals of two villages of Anger Gute area from 14 to 29, August 2020. Malaria infection was screened using RDT from finger-prick blood specimens. Data analysis was performed using SPSS version 20.0 and descriptive statistics was done using logistic regression. P values of less or equal to 0.05 were considered significant.
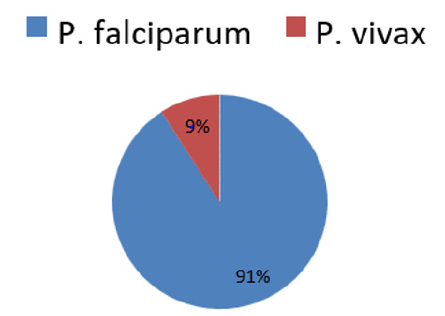
Result: prevalence of Plasmodium infection was 2.7 (11/413) as determined by RDT. Of these, 91% (10/11) were infected with Plasmodium falciparum and 9% (1/11) with Plasmodium vivax. 98.1% of study participants were asymptomatic upon clinical examination of malaria. Prevalence of malaria among asymptomatic individuals was 1.73% (7/405). 4 active malaria cases were detected from 8 febrile individuals (body temperature≥37.5°C). Malaria parasite infection was not associated with area of residence, age categories and ethnic group of study participants (P>0.05). But Plasmodium infection were more detected among males as compared with female (AOR=23.6, P=0.041). Out of total 413 respondents, 254(62.7%) had got malaria before this survey and majority of them did not know the type of malaria they diseased. 83.3% of respondents sleep under LLINs the night before the survey.
Conclusion and Recommendation: Overall prevalence of Plasmodium infection in this survey was 2.7%. Prevalence of malaria parasite among asymptomatic individuals was 1.73%. 4 active malaria cases were detected from 8 febrile individuals during community-based survey. Malaria parasites were more detected among males than females. 62.7% of respondents had got malaria before this survey. Molecular Techniques should be used to detect low density infections in this low transmission settings to accurately know prevalence of malaria parasite.
Keywords: Malaria survey, Asymptomatic malaria, Active case finding, Anger Gute Area
Introduction
Malaria is one of the leading health burdens in the developing world, especially in several sub Saharan Africa countries. Plasmodium falciparum is the most prevalent malaria parasite in African, accounting for 99.7% of estimated malaria cases in 2018 [1]. In Ethiopia, Malaria transmission is seasonal and predominantly unstable, peak malaria incidence occurs from September to December, following the main rainy seasons (June September) and from March to May, during and after the small rainy seasons (February-March) [2]. WHO has developed a plan to reduce the global malaria burden by 90% by 2030 and to move towards malaria elimination [3]. Malaria surveillance, monitoring & evaluation are critical to ensure efficient detection of malaria cases or clusters of cases should be investigated to identify risk factors, eliminate foci of transmission and maintain malaria-free status. Active case detection is important in elimination programmes by detecting symptomatic cases that are not detected by passive case detection and asymptomatic cases in the community [4].
Moving from malaria control to elimination requires national malaria control programs to implement strategies to detect both symptomatic and asymptomatic cases in the community [5]. Active case detection played a major role in interrupting malaria transmission in Sri Lanka [6]. Asymptomatic malaria is Plasmodium infections that lack typical clinical symptoms but are detectable by microscopy, rapid diagnostic test or molecular methods. Recent studies using molecular detection tools have shown that, in endemic populations, submicroscopic infections (parasitaemia that is below the limit of detection of microscopy or RDT, but detectable using molecular or other highly sensitive diagnostic methods) are common, whereas symptomatic and microscopically detectable infections comprise only a small fraction of all infections [7].
Statement of the Problem
Malaria remains an important public health concern as it causes an estimated 228 million cases and 405 000 deaths in 2018. Although incidence rate of malaria declined globally between 2010 and 2018, from 71 to 57 cases per 1000 population at risk, from 2014 to 2018, the rate of change slowed dramatically, reducing to 57 in 2014 and remaining at similar levels through to 2018 [1]. Malaria incidence in and around Anger Gute town from June to December 2018 was found to be 0.343. Trend analysis of malaria from 2014 to 2018 indicated a nearly unchanged number of malaria cases. This shows presence of low sustained malaria transmission In Anger Gute area [8]. Asymptomatic malaria remains a challenge for malaria elimination as it significantly influences transmission dynamics by a reservoir for malaria [9-11]. In low- malaria endemic setting asymptomatic infections were highly prevalent and responsible for most onward mosquito infections [12]. Asymptomatic malaria infections are prevalent in malaria endemic regions and most of these infections remain undiagnosed and untreated. Some community-based study in Ethiopia have shown that prevalence of asymptomatic malaria was 6.8% among school children in Sanja Town, Northwest Ethiopia [13] and it was 6.9% using RDT alone or prevalence was estimated to be 22.7% if RDT and PCR used in West Arsi Zone, Shalla district [14].
Significance of the Study
Household surveys in malaria endemic areas are one of the high priorities given from surveillance, monitoring and evaluation of malaria [2]. As progress is made towards malaria elimination, it is important to asses’ prevalence of asymptomatic and symptomatic malaria in community in different setting to support efforts in malaria elimination. Community based malaria survey was not done in the study area. This survey includes local community of all age groups to show prevalence of asymptomatic malaria. In addition, symptomatic malaria was detected by active case finding among study participants.
Objective General Objective
To assess occurrence of malaria among rural residences of Anger Gute area, Gida Ayana district, western Ethiopia.
Specific Objective
To assess prevalence of asymptomatic carriers and active malaria cases among rural residences of Anger Gute area. To assess the extent of malaria morbidity and malaria intervention/s used in Anger Gute area.
Methods
Study Area and Period
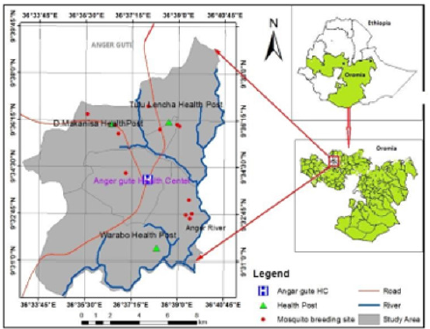
Figure 1:Study area Map, in and around Anger Gute town, East Wollega Zone, Oromia Regional State, Western Ethiopia.
Anger Gute area is found in Gida Ayana district in East Wollega Zone of Oromia, Regional State, and western Ethiopia (Figure 1). It is located about 360 km west of Addis Ababa along the main road connecting Nekemte to Bahir Dar and set in the Anger River Valley (upper Blue Nile Valley) in western Ethiopia. The altitude of the area is between 1200m to 1500m above sea level and on global positioning, the study area is located at latitude of N90 33’57” and longitude of E360 37’57. The area is affected by seasonal/unstable malaria transmission. This study was conducted from 14 to 29 August 2020.
Study Population
413 residents of Anger Gute area from two kebeles (Tull lencha and Warabo) were included in the study. Study participants were classified as asymptomatic carrier (harboring plasmodium parasitaemia without fever or clinical sign) or symptomatic malaria with a clinical malaria attack (with fever or clinical sign of malaria).
Inclusion Criteria
Anger Gute area residents, age greater than 6 months.
Exclusion Criteria
Symptomatic patients of malaria taking antimalarial drugs during study period were excluded.
Study Design
A community based cross-sectional study was conducted.
Sample Size Determination/
129 and 130 Sample size was determined using a single population formula, by assuming an expected 131 proportion of malaria 22.7% in asymptomatic individuals detected by RDT and PCR in Ethiopia 132 [14], 4% margin of error, and 95% confidence of certainty.

413 individuals were included in the survey.
Sampling Techniques
Random sampling (lottery method) was used to select two kebeles (Tull Lencha and Warabo) from eight malaria endemic kebeles of Anger Gute area. One village was selected from each 141 kebeles for door-to-door sampling. The number of individuals participated in this study was 142 proportional to total population size in selected villages 143.
Data Collection Techniques
Data were collected by laboratory diagnosis of Plasmodium using Malaria RDT kit (CareStar TM, Pf/Pv (HRP2/ PLDH)). In addition, socio-demographic characteristics, history of malaria 146 infection and prevention of the disease were assessed by interviewing study participants. The 147 questionnaire is initially prepared in English and then translated in to local languages for data 148 collection.
Sample Collection
After consenting the head of household, household members were requested to give finger-prick blood samples. An experienced clinician, member of data collector, examined presence or absence of sign and symptoms of malaria. Digital thermometers were used to measure body temperature. Individuals with body temperature≥37.5°C were considered febrile. A finger-prick blood sample was collected by a laboratory technician for RDT tests. This survey targeted all selected volunteers and household members to detect any asymptomatic infection or clinical malaria found in the community for active malaria case detection.
Statistical Analysis/
SPSS 20.0 statistical software package (SPSS, Inc, Chicago, USA) was used for statistical analysis. Data analysis of factors associated with Plasmodium infections was done using binary logistic regression. P- value of less or equal to 0.05 was considered significant.
Ethical Consideration
This research proposal was approved by the Research Ethical Review Committee of Akillu Lema Institute, AAU. Permission to undertake the research was obtained from regional, zonal and worda health offices. Verbal consent was obtained from household heads before administering the questionnaire. A parent or guardian of any child participant provided informed consent on their behalf. Assent was also taken from children. Study participants tested malaria positive by RDT were treated based on Ethiopia’s malaria treatment guideline: Artemether-lumefantrine for P. falciparum and chloroquine for P. vivax.
Variables
Dependent variables: Prevalence of malaria parasite.
Independent variables: Place of residence, age, sex, malaria morbidity, LLINs utilization.
Results
Socio Demographic Characteristics
A total of 413 individuals were included in the survey. 58.8% and 41.2% of study participants were recruited from Tull Lencha and Warabo kebeles respectively. Nearly half percent of study participants were under 15 years of age. Of the total 413 study participants, 56.7% were females and majority (87.2%) of them were Amhara in ethnic (Table 1).
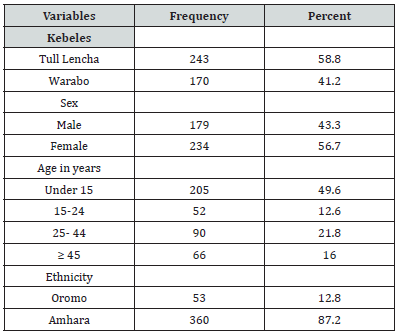
Table 1:Socio-demographic characteristics of study participants, Anger Gute area, 14 to 29 August 2020(n= 413).
History of Malaria Before this Survey
Out of total 413 respondents, 254(62.7%) had got malaria before this study. The majority of these did not know the type of malaria they diseased. In addition, 72.1% of these got malaria in the past five years. About 83.3% of respondents sleep under LLINs the night before the survey (Table 2).
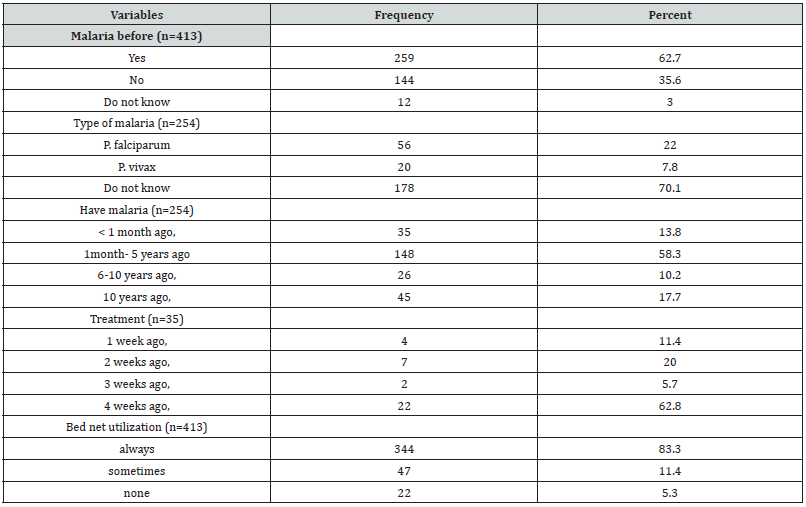
Table 2:Study participants’ malaria history and bed net utilization in Anger Gute area, 14 to 29 August 2020.
Prevalence of Malaria
Among 413 study participants screened, Plasmodium infection was detected in 2.7% (11/413). Out of these, ten individuals were diagnosed with Plasmodium falciparum and one individual was detected with Plasmodium vivax. A mixed infection of P. vivax and Plasmodium falciparum were not detected (Figure 2).
The vast majority of study participants (98.1%) were asymptomatic upon clinical examination of malaria. Of these, malaria parasites were detected in 7 individuals. Hence, prevalence of malaria among asymptomatic individuals was 1.73% (7/405). 4 active malaria cases (3 from Warabo and 1 from Tull lencha kebele) were detected from 8 febrile individuals identified during community-based survey. The occurrence of malaria parasite was 36.5 times more in symptomatic patients as compared to asymptomatic individuals (Table 3).
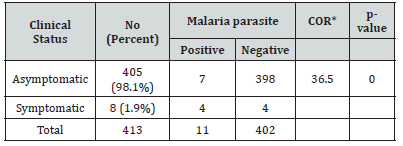
Table 3:Occurrence of malaria parasite among asymptomatic and symptomatic individuals in Anger Gute area, 14 to 29 August 2020(n= 413).
No significance difference in malaria parasite infection in areas of residence, age categories and 213 Ethnic group of study participants (P>0.05). In Anger Gute area, Plasmodium infection were 214 more detected among Males as compared with female (AOR= 23.6, P=0.041) (Table 4).
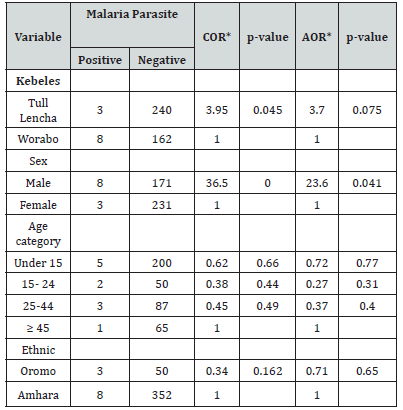
Table 4:Socio-demographic characterizes of study participants in relation to malaria parasite 216 infection in Anger Gute area, 14 to 29 August 2020(n=413).
Note*: COR*: Cumulative Odds Ratio; AOR*: Adjust Odds Ratio.
Discussion
Monitoring malaria using health facility-based surveys has challenges and limitations [15]. So, community-based malaria survey is needed to assess prevalence of asymptomatic carriers and active cases to support striving to ward malaria elimination. Overall prevalence of Plasmodium infection in this communitybased survey was 2.7%. This prevalence was lower than study reported from Maseno, Kenya [16] using microscopy and Nigeria that use RDT for detection [17]. This difference might be related to the level of malaria transmission in the study sites.
Prevalence of malaria parasite in this study was comparable to some community-based study done in Ethiopia, among all age group in malarious areas of Ethiopia using RDT [18], among school children in Sanja Town, Northwest Ethiopia using microscopy [13] and in West Arsi Zone using RDT [14]. 91% of malaria parasites detected in this survey were P. falciparum. The Predominance of P. falciparum in this finding was in line with Ethiopia MIS-2015, among malaria positive cases P. falciparum and P. vivax infection were 82% and 8% respectively by RDT [18]. In this study, Malaria parasites were more detected among males than females. This finding was similar to study done in Gambia [15], western India [19], and Ethiopia [20]. However, it contrasts study done in Kenya [16]. Risk factor of this socio- demographic characteristic to malaria parasite infection was related to division of labour as a result of gender roles may play a significant part in determining exposure to female Anopheles mosquitoes [21].
In this survey, occurrence of malaria parasite was 36.5 times more in symptomatic patients as compared to asymptomatic individuals. This might be related to the limit of RDT to detect parasites in asymptomatic individuals and/ or symptomatic individuals are more likely have malaria parasites than asymptomatic carriers. 62.4% of study participants had got malaria before this survey. This finding was higher than study reported from Ethiopia [22]. This increase in mortality of malaria in Anger Gute might be related to the level of malaria transmission in the areas. Although knowledge of study participants on malaria transmission, symptoms and preventive measures was good [22,23], this study showed that most of study participants did not differentiate the type of malaria they diseased. Malaria patients should be informed of the type of malaria they are ill with as severity, recurrence and treatment type differed by type of malaria parasite that cause the disease.
About 83.3% of respondents slept under LLINs the night before the survey. This utilization of bed nets was higher than study done in this study area 7 years ago [24] and Ethiopia MIS report of 2015 [18].
Limitation of the Study
Individuals previously treated for malaria may test positive for RDT within 14days after treatment, as antigens often persist after treatment. In addition, RDT has low sensitivity for low parasite densities.
Conclusion
Overall prevalence of Plasmodium infection in this survey was 2.7%. Prevalence of malaria parasite among asymptomatic individuals was 1.73%. 4 active malaria cases were detected in 8 febrile individuals. Malaria parasites were more detected among males than females. 62.7% of respondents had got malaria before this survey. About 83.3% of respondents slept under LLINs the night before the survey.
Recommendation
Molecular Techniques should be used to detect low density infections in this low transmission settings to accurately know prevalence of malaria parasite.
Conflict of Interest
No conflict of interest..
Acknowledgement
None.
References
- (2019) WHO. Global malaria report.
- (2019) President’s malaria initiative. Ethiopia malaria operational plan for year.
- (2015) WHO. Global technical strategy for malaria 2016-2030.
- Malaria surveillance, monitoring & evaluation: a reference manual.
- Cara SG, Kelly CS, Gawrie NL, Christina R, Tashi T, et al. (2013) Active case detection for malaria elimination: a survey among Asia Pacific countries. Malaria Journal 12: 358.
- Renu Wickremasinghe, Sumadhya Deepika Fernando, Janani Thillekaratne, Panduka Mahendra Wijeyaratne, Ananda Rajitha Wickremasinghe (2014) Importance of active case detection in a malaria elimination programme. Malar J 13: 186.
- Teun Bousema, Lucy Okell, Ingrid Felger, Chris Drakeley (2014) Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12(12): 833-840.
- Mebrate D, Oljira K, Geleta T (2018) Malaria Incidence and Associated Risk Factors in and Around Anger Gute Town, Western Ethiopia. Research Square.
- Dolie D Laishram, Patrick L Sutton, Nutan Nanda, Vijay L Sharma, Ranbir C Sobti, et al. (2012) The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 11(1): 29.
- Carlos A Guerra, Daniel T Citron, Guillermo A García, David L Smith (2019) Characterising malaria connectivity using malaria indicator survey data. Malar J 18(1): 440.
- José Rodrigues Coura, Martha Suárez Mutis, Simone Ladeia Andrade (2006) A new challenge for malaria control in Brazil: Asymptomatic Plasmodium infection - A Review. Mem Inst Oswaldo Cruz 101(3): 229-237.
- Fitsum G Tadesse, Hannah C Slater, Wakweya Chali, Karina Teelen, Kjerstin Lanke, et al. (2018) The Relative Contribution of Symptomatic and Asymptomatic Plasmodium vivax and Plasmodium falciparum Infections to the Infectious Reservoir in a Low-Endemic Setting in Ethiopia. Clin Infect Dis 66(12): 1883-1891.
- Ligabaw Worku, Demekech Damtie, Mengistu Endris, Sisay Getie, Mulugeta Aemero (2014) Asymptomatic Malaria and Associated Risk Factors among School Children in Sanja Town, Northwest Ethiopia. Int Sch Res Notices.
- Lemu Golassa, Nizar Enweji, Berhanu Erko, Abraham Aseffa, Göte Swedberg (2013) Detection of a substantial number of submicroscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J 12: 352.
- Abraham Rexford Oduro, Ernest Tei Maya, James Akazili, Frank Baiden, Kwadwo Koram, et al. (2016) Monitoring malaria using health facility-based surveys: challenges and limitations. BMC Public Health 16: 354.
- Rachel Jenkins, Raymond Omollo, Michael Ongecha, Peter Sifuna, Caleb Othieno, et al. (2015) Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J 14: 263.
- Titilope M Dokunmu, Cynthia U Adjekukor, Omolara F Yakubu, Adetutu O Bello, Jarat O Adekoya, et al. (2019) Asymptomatic malaria infections and Pfmdr1 mutations in an endemic area of Nigeria. Malar J 18(1): 218.
- (2015) Ethiopian Public Health Institute. Ethiopia Nationa l Malaria Indicator Survey.
- Sulabha Pathak, Mayuri Rege, Nithya J Gogtay, Umesh Aigal, Surya Kant Sharma, et al. (2012) Age-Dependent Sex Bias in Clinical Malarial Disease in Hypoendemic Regions. PLoS One 7(4): e35592.
- Ayenew Addisu, Yalewayker Tegegne, Yenesew Mihiret, Abebaw Setegn, Ayalew Jejaw Zeleke (2020) A 7-Year Trend of Malaria at Primary Health Facilities in Northwest Ethiopia. Journal of Parasitology Research.
- (2007) WHO. Gender, Health and Malaria.
- M Legesse, W Deressa (2009) Community awareness about malaria, its treatment and mosquito vector in rural highlands of central Ethiopia. Ethiop J Health Dev 23(1).
- Parsa Sanjana, Mazie J Barcus, Michael J Bangs, Sahat Ompusunggu, Iqbal Elyazar, et al. (2006) Survey of community knowledge, attitudes, and practices during a malaria epidemic in central java, indonesia. Am J Trop Med Hyg 75(5): 783-789.
- G Tadele, A Samuel, E Adeba (2014) Replacement of Long Lasting Insecticide Treated Nets in Malarious Kebeles of Gida Ayana District, East Wollega Zone, Ethiopia. Sci Technol Arts Res J 3(2): 162-166.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.