Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Metabolic Reprogramming and Cancer: 2022
*Corresponding author:Susinjan Bhattacharya, School of Agriculture and Allied Sciences, The Neotia University, India.
Received:February 16, 2021; Published:March 30, 2023
DOI: 10.34297/AJBSR.2023.18.002465
Abstract
Cancer is a disease that is considered a silent killer. Early therapeutic interventions can lead to a cure for the disease. The causal reasons for cancer are many. At the molecular level, metabolic reprogramming is a process for self-renewal and survival by cancer cells. Dietary constituents are an important factor that leads to metabolic transitions and induces cellular reprogramming. The process is initiated by inducing changes in metabolism leading to the supply of nutrients and energy to the tumour cells, and this is linked to the induction of epithelial mesenchymal transition (EMT) and vascular mimicry (VM). Thermodynamic changes that get distinguished between proliferating and non-proliferating cells might also be a possible reason for cellular reprogramming. The entire process of metabolic reprogramming is linked to diet-driven changes and bioenergetics. The work here elucidates in brief the dependency of metabolic reprogramming on different factors, or stages and highlights possibilities for therapeutic interventions as part of cancer therapeutics.
Keywords: Metabolic Reprogramming; Cell Fate Decisions; EMT; Vascular Mimicry; Thermodynamic Constraints; Dietary Components
Abbreviations: TME: Tumour Micro Environment; Myc/c-Myc: Myelocytomatosis/c- Myelocytomatosis; Ras: Rat sarcoma virus; HIF-1: Hypoxia Inducible Factor-1; p53: Protein 53; POU1F1: Pituitary-Specific POU-homeo Domain Transcription Factor; HNF: Hepatocyte Nuclear Factor; MODY: Maturity-Onset Diabetes of the Young; TNFα: Tumour Necrosis Factor-α; JAK-STAT: Janus Kinase/Signal Transducers and Activators of Transcription; EMT: Epithelial Mesenchymal Transition; MET: Mesenchymal Epithelial Transition; CTC: Circulating Tumour Cells; CSC: Cancer Stem Cells; TGF β: Transforming Growth Factor β; BMP: Bone Morphogenetic Proteins; ZEB: Zinc Finger E-box-binding homeobox 1; TCA cycle: Tricarboxalic Acid Cycle/ Kreb’s Cycle ; miRNA/miR: microRNA; OVOL: Ovo Like Protein; FOXA1: Forkhead box protein A1; EC: Endothelial Cells; VSMC: Vascular Smooth Muscle Cell; VEC: Vascular Endothelial Cell Function; LPS: Lipopolysaccharide; IL: Interleukin FAS: Fatty Acid Synthase; Hh: Hedgehog; Smad: Suppressor of Mothers Against Decapentaplegic; EGF: Epidermal Growth Factor; FGF: Fibroblast Growth Factor; HGF: Hepatocyte Growth Factor; SHH: Sonic Hedgehog; IGF-1R: Insulin-like Growth Factor 1 Receptor; Pin-1: Peptidylprolyl Cis/Trans Isomerase, NIMA-Interacting 1; SHIP 2: SH2-domain-containing Inositol Phosphatase 2; PFK-1: Phosphofructokinase-1; GAPDH: Glyceraldehyde-3-Phospahte Dehydrogenase; ADP: Adenosine Diphosphate; ATP: Adenosine Triphosphate; VM: Vascular Mimicry; Nrf2: Nuclear Factor Erythroid 2–Related Factor 2; NF-κB: Nuclear Factor kappa
Introduction
The silent killer which goes by name of cancer, if detected at an early stage, can lead to a cure by therapeutic intervention with maximum chances. Though model-based year-wise predictions of cancer trends cannot be a possible eye opener as the incidences vary from year to year for manifold reasons, there might be 1.9 million new cancer cases as diagnosed along with 609, 360 deaths in 2022 at USA [1]. Globally in 2020, there was 10 million deaths due to cancer [2]. The year 2020 reported 18.1 million cancer cases worldwide with 9.3 million in men and 8.1 million in women [3]. The incidence of cancer cases worldwide rose to 21 % with 16 % deaths from 2010-19 and cancer incidence in India has increased at an annual average annual rate of 1.1-2 % during 2010-19 [4].
India occupies the third position worldwide in cancer incidences [5], and there are reports of the rise in breast cancer in men in India [6,7]. India has reported an estimate of around 40 lakh cancer cases and 22.54 lakh deaths from 2018-2020 [8]. The increase in the burden of cancer has been linked to socio-demographic index and food intake apart from other factors [9].
Cancer is a disease that can affect any body part, and a recognized phenomenon of cancer is the very fast creation of abnormal cells growing beyond their usual boundaries, which can lead to metastasis. Early detection and screening can help to reduce mortality due to cancer [10]. Diet can be an important component to reducing cancer aggressiveness and progression [6,11], but it is of high importance that diet as a therapeutic intervention must progress along with the mode of primary treatment. Diet makes cells undergo metabolic programming in normal cells as well as reprogramming in cancer cells, wherein the latter is a hallmark of malignancy [12]. Dietary restriction also leads to metabolic reprogramming and can be thought of as a therapeutic approach as the process extends the lifespan of an organism and the process is linked to energy intake restriction without essential nutrient deficiency [13]. Furthermore, mitochondrial involvement in metabolic reprogramming is an additional prime factor [14,15].
The tumour microenvironment (TME) involves the interaction of metabolic reprogramming with tumour cells and non-tumour cells, suggesting therapeutic strategies to target metabolic interventions [12]. With the growing incidences of cancer, it is necessary to investigate the metabolic reprogramming occurring in cancer cells and the possible dietary interventions along with the first-line therapy as medical care for cancer patients [16]. Additionally, targeted therapy adapted in cancer cure can either target tumour cells to be killed or can help tumour cells to grow in TME. Thus, targeted therapeutics can either act as cytostatic and or as precision medicine, as they act on specific molecular targets [17]. Such therapeutics might be influenced by food intake and diet, followed by metabolic reprogramming that also leads to cellular reprogramming. This review summarizes the influence of diet on metabolic reprogramming and possible targets as a therapeutic intervention in cancer cure. However, discussion on a single tumour as a type and mitochondrial lead metabolic reprogramming has not been done in the present paper. Metabolic reprogramming acts as a switch in cellular proliferation and cell reprogramming [18]. The dependence of cellular reprogramming on metabolic reprogramming and linkage to epithelial mesenchymal transition (EMT) has also been reviewed here in brief. Cell growth and proliferation is dependent on metabolism [19,20]. The entire process of metabolic reprogramming is dependent upon many factors and processes, or pathways, referred to as ‘stages’ in the present manuscript, affected by dietary components. Dietary food intake components can be from plant, animal, and microbial sources, and is referred to as ‘dietary factors’ as food component representative of any food source in this review. Detailed discussion on the subtopics is beyond the scope of this review, and the current review in a concise way is highlighting the stages, and usage of dietary components that can influence metabolic reprogramming and cell fate decisions.
Metabolic Reprogramming
Metabolic reprogramming leads to the development and progression of cancer [21]. The TME rich with a heterogenous environment associated with the ‘Warburg effect’ shows a fast response of tumour cells to hypoxia and hypo-nutrient conditions. The TME is characterized by reduced pH, lessened oxygen apart from various metabolic changes, which combinatory leads to changes in immune cells in the microenvironment, increase in various tumourrelated immune cells, decrease in inhibitory cells, and release of various toxic metabolites [22]. Intermediates from biochemical pathways also lead to metabolic reprogramming (Figure 1). This leads to changes in tumour cell bioenergetics, and the process named ‘metabolic reprogramming’ is a necessity for malignancy and tumour progression. Additional metabolic reprogramming of cancer stem cells (CSC) makes CSC show metastatic potential leading to resistance against cancer therapeutics [23,24]. Cancer stemness results from vascular mimicry (VM) [25]. However, blood supply in tumour cells is provided by vasculogenic mimicry [26].
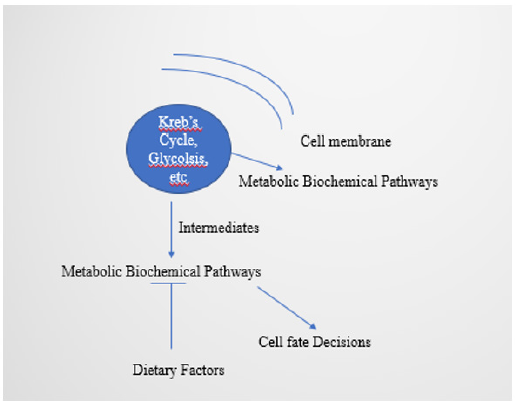
Figure 1:Intermediates from the biochemical pathways and metabolic reprogramming, regulated by dietary factors
It is not only metabolic changes, but also epigenetic changes that help in the metabolic adaptation of the cancer cells in TME. Additionally, non-tumour cells in TME also undergo metabolic reprogramming [12]. The altered metabolic pathway sustains a pool of nutrients and energy for the cancer cells to grow. Furthermore, metabolic pathway activity in a such cancerous cellular environment is influenced by transcriptional programs involving oncogenes and tumour suppressor genes [27].
Stages
Metabolic reprogramming induces cellular reprogramming and initiation of cancer in pathological conditions. The process involves phenomenal changes referred to herein as ‘stages’, or ‘steps’. A detailed discussion of the steps is beyond the scope of this review, and a concise view of the stages is discussed below.
Factors involved in metabolic reprogramming: Advances in biological research have proved that many of the signaling pathways changed by gene mutations regulate cancer cell metabolism, and can lead to conditions, like aerobic glycolysis or the ‘Warburg effect’. Reports evidence aberrations in the proto-oncogenes, Myc or Ras leading to glycolytic phenotype by HIF 1α – mediated metabolic reprogramming [28]. Key regulators of the processes are three transcription factors, namely HIF-1, c-Myc, and p53. As an example, the risk of malignant tumour occurrence increases due to changes in enzyme activity of α-ketoglutarate-dependent dioxygenase resulting from the increased levels of 2-hydroxyglutarate due to mutations in gene encoding isocitrate dehydrogenase [29]. Additionally, metabolic reprogramming of breast cancer cells and fibroblast activation occurs due to the transcription factor POU1F1 by regulating gene encoding lactate dehydrogenase A [30].
An example of complexity arising due to transcription factor defect is MODY resulting due to alterations in HNF1α. MODY1 results due to alterations in HNF4α, whereas MODY4 is due to mutations in PDX1 and insulin synthesis defect [30,31]. Apart from the internal factors, there are also external factors, like cytokine IL-4/IL-4R signaling leads to elevated uptake of glucose and glutamine via their transporters to stimulate breast cancer cell growth. Furthermore, alteration of functions of metabolic nodes due to IL-6, TNF α, IL-17, and IL-1β are seen in patients tumourigenic for breast, pancreatic, and colon [32]. The IL-6 in TME also activates the JAK-STAT3 pathway for immune, epithelial, and endothelial cells [33-35]. Besides this, cytokines and chemokines can also mediate metabolic interactions between host and tumour cells in TME. There can also be hormone receptors which act as transcription factors, like androgen and estrogen receptors in breast cancer. Metabolite cross-feeding also leads to tumourigenesis [36,37].
The T-cell activation induces transcription factors, HIF 1α and Myc, and the absence of Myc stops activated glycolysis and glutaminolysis in T cells. HIF 1 α plays a role in the regulation of immune cell effector functions, and also plays an important role in the maturation of dendritic cells and T cell activation [28]. However, Myc-based metabolism was seen to be linked to the polyamine biosynthesis via glutaminolysis, suggesting a myc-dependent metabolic transcriptome drives metabolic reprogramming in activated, primary T lymphocytes [38]. HIF induction leads to the expression of Carbonic anhydrase IX (CA IX), monocarboxylate transporter 4, and programmed death ligand 1, wherein CA IX is needed for tumour progression under conditions of hypoxia [28]. Metabolic reprogramming in cancer is helped by another transcription factor, Nrf 2 (Nuclear factor erythroid-2–related factor-2) [39].
Thus, disturbances in the metabolic activities due to environment, mutation, and metabolic insults affect transcription at the level of epigenetic and transcriptional activities leading to a significant effect on oncogenesis [40]. The long non-coding RNAs also modulate metabolic reprogramming and cancer progression [41]. Researchers also have observed the role of ubiquitination and deubiquitination in tumour cell metabolic reprogramming, especially dysregulation of these processes leads to cancer [42]. Hindrances to mitochondrial apoptosis are additional players in the cancer initiation and progression [43]. Metabolites by themselves can prove to be oncogenic by interfering with cell signaling as well as inhibiting cell differentiation [44].
Metabolic reprogramming and cytoskeletal changes: Metabolic reprogramming is a part of physiological cell proliferation and tumourigenesis [45]. Cellular growth and proliferation are also linked to changes in cytoskeletal dynamics of a cell. The cytoskeleton also plays an important role in tumour cell aggressiveness and EMT [46]. Mitochondrial Hsp 90 is one of the important mediators of tumour cell motility when nutrients are limited in human glioblastoma, prostate, lung, breast, melanoma, and fibroblast cell lines, which in turn also acts as the upstream regulator tumour cell bioenergetics. In a nutshell, cytoskeletal dynamics, including the release of cell motility factor, FAK is controlled by metabolic forces [47]
Cell Fate Decisions
Cell fate decisions are inter-twinned with metabolic shifts and are essential for the development of pluripotency [48]. Metabolic reprogramming also plays an important role in cell fate transitions and is essential for cell differentiation at the embryonic stage, as well as in tumour development and progression. It is not only the metabolic networks, but also mitochondrial distribution that acts to regulate the divisional balance between stem cells in asymmetric and symmetric divisions, finally affecting tissue homeostasis [49].
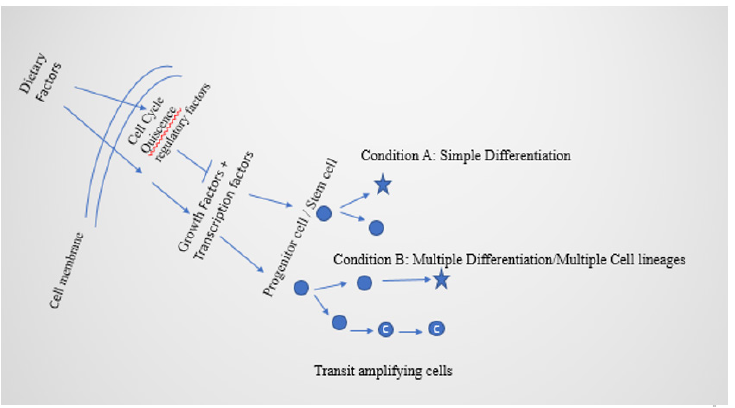
Alterations in metabolic activities can affect post-translational modifications, by affecting gene expression for cell differentiation. This helps in regulating not only the cell fate decisions, but also epigenetic modifications [50]. Dietary factors regulate cellular proliferation and cell fate decisions by metabolic shifts, by affecting functions of cell cycle quiescence factors (Figures 2 and 3). These processes lead toward EMT and EMT-MET. Networking between cell organelles, like lysosomes, also plays a critical role in metabolic transitions and fate decisions of stem cells for defining cell identity [51]. In brief, controlling factors for cell fate decisions are metabolic activities, reactive oxygen species, intracellular pH, and cell morphology [52].
Metabolic reprogramming and Epithelial-Mesenchymal Transition
The phenomenon of epithelial-mesenchymal transition refers to loss of epithelial cellular characteristics and gain of mesenchymal traits in epithelial cells [18]. On the other side, metabolic reprogramming is also linked to the acquirement of EMT traits. The regulatory process of EMT involves specific transcription factors, microRNAs, epigenetic modifications as well as long non-coding RNAs (lncRNAs) and metabolic reprogramming coordinates the transitory process [53]. The process is linked to the generation and expression of cancer stem cell features, and changes in metabolic ways enable the survival of tumour cells in changed environmental conditions [54]. The EMT is also understood to be a process involved with most of the metastatic state of cancer [55,56].
Glycolytic enzymes in metabolic reprogramming plays a role in EMT induction making use of glycolytic flux. The process of EMT advancement is linked to abnormal lipid metabolism and amino acid metabolism in cancerous cells [57-60]. Furthermore, EMT is also regulated by produces from the metabolic pathways by transcription factors in the EMT process as well as epigenetic regulators [18], like some products from the glycolytic pathway can induce EMT, as well as some products from the same glycolytic pathway, can inhibit EMT [53]. The process of EMT in metastasis can also involve entry of CTCs in peripheral blood, wherein CTCs can show the presence of hybrid epithelial-mesenchymal markers. Additionally, EMT can also lead to the formation of CSCs [61], which can switch between glycolysis and oxidative phosphorylation [62]. Furthermore, CSCs proliferate and grow towards the formation of multiple cell lineages leading to tumour heterogeneity (Figure 2) to express their differentiation potential [63]. On the other side, EMTgenerated CSCs can switch to MET, and the process is useful in the initiation of pluripotency [64,65]. Reprogramming is affected by EMT and metabolic regulatory processes, through different factors like histone modification, and DNA methylation. The importance of metabolism in deciding cell fate is evidenced by the studies of substrate utilization [66,67]. Many signaling networks, like Notch, TGF-β, and BMP plays role in the regulatory part of the process [18,68]. The transcription factor TWIST, part of basic helix-loophelix (bHLH) transcription factors involved in EMT apart from playing role in the formation of cancer stem cells, functions in lipid metabolism in adipose tissue, and also plays role in inflammation and insulin resistance [69]. Metabolic reprogramming has been reported to be involved with type 2 diabetes and breast cancer [70]. Another transcription factor involved in EMT, ZEB1 is important in adipogenesis [71]. However, ZEB 1/2, though influenced by TCA cycle end products, also influences glycolysis and can also divert glycosphingolipid metabolism [72,73]. On the other side, miR-200 can inhibit EMT by targeting ZEB 1/ 2 [74]. Additionally, TP53 can downregulate ZEB 1/ 2 expression by targeting miR 192 and 200 [75]. The miR200 member(s) can also inhibit signaling networks, like Wnt and Notch pathways [76,77]. Apart from this, lncRNAs also play role in the activation, or inhibition, of EMT, and research works are expanding knowledge on their role in metabolism and cancer cell metabolism associated with EMT [78,79]. However, EMT can be suppressed also by the OVOL 1 / 2 transcription factors, and OVOL2 and ZEB 1 can mutually repress each other [80,81]. Nevertheless, though little is known about the role of transcription factors to suppress MET, FOXA1 can reduce lipid accumulation in human hepatocytes, and interactions between FOXA1 activity and ZEB 1 and SNAIL (Snail belongs to zinc finger protein, SNAI1) can be another way to study cancer cell metabolism [53,82].
Vascular Mimicry
Vascular mimicry (VM) involves the formation of blood vessellike structures by aggressive tumours and is connected to the process of EMT [83-85]. The process of VM is linked to cancer stemness and autophagy [25]. One of the reasons for perturbed vascular functions is due to the disturbed arterial blood flow, induction of metabolic reprogramming through HIF-1α resulting in activation of endothelial cells, vascular inflammation, and atherosclerosis. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium [86].
An interaction between EC, VSMC, and immune cells regulates the response between pathological and physiological states [87]. Furthermore, nitric oxide is a critical modulator of VEC, and metabolic reprogramming leads toward the migration of VEC in an anoxic environment [84]. Factors like LPS, IL-1, and TNF-α activate VEC leading to changed VEC metabolic activities with enhanced glycolysis, upregulated FAS [88]. The cumulative effect of activities leads to increased proliferation, migration and VEC dysfunction, and vascular diseases [84,89].
Dietary Components in Metabolic Reprogramming
The tumour cell in cancer changes their metabolic pathway as they enter metabolic reprogramming which is one of the characteristic features of cancer [90]. The purpose is to provide tumour cells with essential energy, signaling intermediate and precursors to support biosynthesis, growth, proliferation, and metastasis [91].
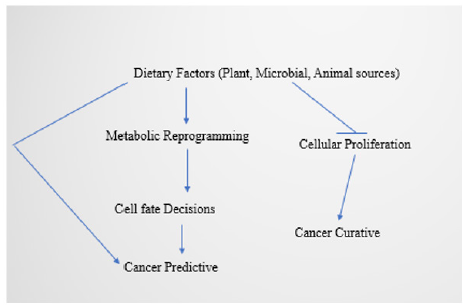
Figure 4:Schematic illustration of therapeutic importance of dietary factors in metabolic reprogramming.
Diet and food intake influence microbial composition and the healthy metabolic activity of the consumer. Plant-based food is understood to help in maintaining a healthy gut microbiota. Food intake is also understood in terms of food ingredients and food supplementation that includes microbial sources also [92]. Food intake not only includes components from plant-based sources, but also microbial and animal sources (Figure 4). Gut microbiota shows ecosystem shifts, and is related to metabolic transitions [93].
Diet and nutrition are also two of the major essential requirements to control cancer cell metabolism [94]. Gut microbiome undergoes shifts in changes with regard to diet consumption and lifestyle, and such shifts can result in a change in gut microflora composition. The gut microbiome due to diet changes can affect tumour development, progression, and therapy [95,96]. Gut microbiota also affects systemic metabolic reprogramming and local metabolic reprogramming [97]. Cell motility in malignant cancers is affected by EMT signalling. Dietary components, like luteolin and quercetin, can reduce EMT signalling and inhibit metastasis in cervical cancer [98]. Gut microbiota is influenced by dietary components. A high fiber diet can lead to the generation of short-chain fatty acids that offers manifold health benefits [99]. However, fatty acids, like palmitic acid and a high-fat diet also led to cancer and EMT by activating TGF- β and β-catenin [100,101]. Reports evidence the ability of dietary energy balance to modulate EMT and cancer progression [102].
Resveratrol found in grapes, peanuts, cranberries, etc. was seen to inhibit EMT factors and TNF-β-induced factors for tumour progression [103-105]. Similarly, silibinin, a flavonolignan found in milk thistle (Silybum marianum) was reported to modulate levels of EMT markers and stop EMT and remove colorectal CSCs by blocking the Wnt/ β-catenin signaling pathway [106,107]. The nucleus sourced β-catenin is a transcriptional activator of EMT target genes and stem cell markers [108]. Zerumbone, from the Zingiberaceae family, was reported to upregulate miR-200c and inhibit cancer progression, EMT, and CSC functions [108-110].
Fucoxanthin, belonging to marine carotenoids and abundant in macro- and microalgae was also reported to induce apoptosis and inhibit EMT and CSC invasion [111,112]. The EMT-related markers are also regulated by scutellarein, derived from apigenin, and found in Scoparia dulcis, Artemisia douglasiana, as well as by tetramethyl ether found in Acacia carneorum, Acacia fasciculifera, and Pongamia pinnata [113,114], and by cyclopamine, a steroidal alkaloid isolated from the corn lily (Veratrum californicum) [115-117].
The TGF β signaling is also linked to cancer metabolism and EMT [118]. Nanoparticle-coated α-mangostin (α-Mangostin, a natural xanthonoid found in bark and dried sap of Garcinia mangostana L.) could inhibit colorectal cancer growth and EMT by downregulating GSK3β/β-catenin /CDK6 signaling pathway [119- 121]. Curcumin obtained from turmeric and analogues of curcumin modulated signaling network, miRNAs and EMT and anticancerous effect on colorectal cancer stem cells [122]. Additionally, triptolide suppressed EMT by downregulating EMT transcription factors [123]. Low folate metabolic stress in the colon by reprogramming Hh pathway transdifferentiated human colon adenocarcinoma cells to EMT with deep tissue invasion [124]. Baicalin, a natural flavonoid observed in Scuttelaria spp. could inhibit EMT by stopping the TGFβ/Smad pathway [124-126]. Interaction of Nrf2 and NF-κB with glucosinolates can leads to the inhibition of cancer cell growth [127]. Thus, natural plant-derived chemicals can not only modulate different stages of cancer progression but also can inhibit EMT.
Phenomenal Relatedness of The Processes in Metabolic Reprogramming
Though not common in all cancer types, in the majority of tumorous conditions EMT drives the development of cancer [128,129]. Epithelial-mesenchymal transition is also a part of the developmental process and irrespective of development or disease, EMT involves complex networking of pathways and different factors [130-132]. The process of EMT initiation and advancement is dependent upon many signaling molecules, like EGF, FGF, HGF, TGF β, BMP, SHH, Notch, and Wnt signaling pathway, etc., as well as β -catenin–dependent canonical and β -catenin–independent noncanonical WNT signaling pathways [133-135]. A few of these signaling networks are in turn modulated by dietary, or food components. The transcription factor family, SNAIL can not only change epithelial cell polarity, but also inhibit apoptosis and cell cycle, as well as induce the formation of CSCs. However, TGF β in turn induces SNAIL expression not only in cellular development, but also in organ development. The TGF β again can be inhibited by Baicalin [24]. The transcription factor, Twist also drives the development of CSC phenotypes, and due to the levels of expression of Twist in specific precursor cell types, is useful as a cancer biomarker [53]. However, triptolide can down-regulate SNAIL, Twist, and Slug (Slug: Zinc finger transcription factor) [123].
Targeted Therapeutic Interventions
Therapeutic intervention is a necessity for curing cancer. Understanding of therapeutic intervention needs study in the appropriate cell lines followed by studies in model systems with final clinical trial studies. Of all the types of cancer, there are numerous reports about studies on breast cancer. This is not only regarding the growing importance of breast cancer of all types of cancer, but also there are advantages to the availability of negative and positive breast cell lines for experimentation. Different therapeutic approaches can be adapted for cancer cure, wherein targeted therapy can be practiced along with the standard therapeutic approaches [17] Targeted therapy can act on specific molecular targets, and exemplarily targets can be the cell cycle molecules, like cyclins [136,137]. The other targets that can be used are: IGF-1R, Pin-1, Nicastrin, SHIP 2, Syndecan 1, and proinflammatory cytokines [138]. Metabolic pathway products can be also used for targeted therapy [139]. Chemical cell death kinase inhibitors and miRNA can be also used in targeted therapy [140- 142]. Recent reports evidence the usage of miRNA for metabolic reprogramming of chimeric antigen receptor T-cells [143].
Mitochondria plays an important role in tumour metabolism. Another important target for therapeutic purposes can be Parkin, an E3 ubiquitin ligase, a regulator of mitochondrial integrity, which not only plays role in the early onset of Parkinson’s disease but also in cancer [144]. Metabolic remodeling is a necessity for the cells to support energy for cytoskeletal remodeling needed for cellular responses, cell migration, EMT, and changes in cell morphology. The process is linked to the rearrangement of actin bundles and the binding of glycolytic enzymes to actin fibers [145]. Actin interacts with three of the glycolytic enzymes, PFK-1, aldolase, and GAPDH, wherein binding of PFK-1 to actin is by electrostatic forces and binding is dependent upon ADP concentrations over ATP concentration but independent of its substrate, F-6-P (fructose- 6-phosphate) [146,147]. In turn, aldolase binds preferentially to F-actin, whereas GAPDH binds directly to F-actin [145]. Cell migration and proliferation need energy, nutrients, and metabolic activities, and metabolic activities in proliferating cells differ from that in non-proliferating activities [45,145]. Intracellular transport in cancer metabolism is also linked to cytoskeletal dynamics and functioning [148]. Furthermore, the use of VM inhibitors along the standard anti-angiogenesis treatment and drugs targeting hypoxia signaling might be of help in angiogenesis treatment [85].
Thermodynamic Constraints
Thermodynamic constraints and their consequences have been well studied in microorganisms. Understandings from those studies can help understand more about the metabolic constraints in mammalian cells. Life depends on the laws of thermodynamics. Thermodynamics explains the mechanism of chemical transport of materials into- and out- of the cells [149,150]. Any type of cellular work is understood from the viewpoint of enthalpy, entropy and Gibb’s free energy. Ion channels are essential for cellular signaling, and their functioning depends on thermodynamic shifts. These channels work by responding to changes in the membrane potentials [151]. Metabolic reprogramming may lead to the development of thermodynamic constraints [152], which might be beneficial for the proliferating cells and can be hypothesized to distinguish between proliferating and non-proliferating cells and this might lead them to be far away from equilibrium [153]. This needs to be investigated in mammalian cell line experimentations. Thermodynamic theories highlight the principal limitations in microbial growth [154]. There are enumerable studies in this regard to understand the physiology of microorganisms and their survival in a niche. Studies in metabolic networks can be related to the maximum entropy production [155]. This understanding might be possible for reinforcement of the knowledge from microbial syntrophy studies, wherein this refers to a process of metabolic interaction between microbial partners in an environmental condition [156]. Metabolic interactions can also modulate metabolic rates [157].
Conclusion
Somatic cells can be reprogrammed to iPSCs by use of defined transcription factors, and the process is called somatic cell reprogramming [158]. The process can be hypothesized to be regulated by dietary factors, or diet inducible factors. Despite the fact that the MET is an essential requirement for reprogramming, the sequential process of EMT-MET at the initiation stage of reprogramming can increase reprogramming efficiency [18,65]. Reports from Liu et al. [65] revealed that temporary EMT can generate iPSCs with an efficiency of 600% at the basal level. The regulatory process of EMT also involves the functioning of noncoding RNAs like miRNA [83]. However, it is not known whether the functioning of those miRNAs is in turn being influenced by diet/dietary factors. Reprogramming can shed new light on the therapeutical approaches to cancer [65]. It is the metabolic shift that regulates EMT in metastasis apart from the pathway metabolites that control epigenetically [66,159]. The phenomenon of drug treatment resistance has been also linked to EMT [160,161]. Thus, EMT by itself can be an addressing factor to study and cure drug resistance [53]. Vascular mimicry has been also linked to EMT [83]. This leads to the question if vascular mimicry can also be regulated by metabolic reprogramming, which needs to be studied. There are numerous reports about the usage of plant and microbial products with antimicrobial properties that can be possibly used for therapeutic purposes, and it might be possible to use many of them for metabolic reprogramming [162,163]. Metabolites can transcriptionally regulate genes, and metabolic reprogramming can be predictive for cancer detection, and might be therapeutic as part of cancer precision medicine [37,164]. Cytokines and TNF-α can play a role in cancer pathogenesis as well as cancer prediction (Figure 4) [165-168]. Dietary phytochemicals are evidenced to regulate EMT [169]. Additionally, derived cytokines can play a role in abnormal glucose and lipid metabolism [170,171]. Furthermore, Kreb’s cycle intermediate, citrate plays role in both immunity and inflammation [172]. In recent reports, the therapeutical side of metabolic reprogramming also evidences the use of fibroblasts in the microenvironment of pacemaker cardiomyocytes at the sinoatrial node to drive metabolic reprogramming [173]. Cells in higher eukaryotes can either proliferate or show senescence. Both of these states are regulated by extrinsic and intrinsic stimuli and environmental factors [174,175]. Dietary factors provide growth factors and other necessities for influencing the states and can be hypothesized to regulate cellular proliferation or senescence mechanisms (Figures 2 and 3). Proliferation, including in nerve cells leads to the generation of transit-amplifying cells (TAC) [176- 178]. Senescence can be triggered by stress, and stress induced by serum deprivation can lead to a quiescent stage (G0 phase of the cell cycle) [179-181]. Both senescence and proliferation are important in tissue regeneration which can be modulated by dietary factors [182,183].
Lastly to state, it is the diet that makes cells undergo metabolic reprogramming [184]. This is also because dietary phytochemicals targets signaling pathways of cancer stem cells, which prospects the development of phytomedicines and pharmaceutical development for cancer therapeutics [104,185,186].
Future Directions
There are different types of cellular proliferation, like differentiation, de-differentiation, and trans-differentiation.
Understandings can be directed to look at the cross connections
between these types of cell proliferation and metabolic switches.
The dosage requirement of nutritional components has not been
discussed in the present work. However, elaborated discussion on
this for colorectal cancer has been done by [187]. Furthermore,
research works can be directed to understand the induction of
metabolic reprogramming under appropriate dosage requirement
from a specific food component. It will also be essential under
such circumstances to know the total signaling network within a
normal and malignant cell that can be induced. Though there are
significant advances achieved in the studies of EMT and metabolic
reprogramming, the unanswered questions need to be looked in:
1) Can diet/dietary factors be the driving force to initiate
EMT and metabolic reprogramming?
2) Will dosage or quantum of diet in terms of energy
and diet/dietary component initiate EMT and metabolic
reprogramming?
3) Can the transcription factors and other factors including
noncoding RNAs involved in EMT, EMT-MET, metabolic
reprogramming, and cell fate decisions be decided by specific
dietary components qualitatively and quantitatively?
4) Can the gut microflora also influence cell fate decisions?
5) In the situation where somatic reprogramming is linked
to EMT, can conditional reprogramming be linked to influences
from dietary factors as well as EMT and EMT-MET?
A defined answer to the questions can help in therapeutic interventions in cancer and can be of help in situations where resistance to therapies arises. Understanding metabolic decisions will be a major hallmark to understand development and disease biology.
Conflict of Interest
The author declares no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- (2022) Cancer Facts and Figures 2022. American Cancer Society
- (2021) Global Cancer Observatory. Globocan.
- (2023) Worldwide cancer data. World cancer research fund.
- (2022) India’s cancer burden: Cases and deaths increased last decade, COVID-19 widens screening gap. Down to Earth.
- (2022) World cancer day 2022: Most common cancers in India and why they are rising. World Cancer Day.
- Bhattacharya S (2021a) A view of breast cancer and diet therapy. Oral presentation at the Sciinova Group’s Virtual Congress on Breast Cancer research.
- Yadav SS, Bhattacharya, S (2021) Breast cancer scenario: a review. Cancer Pages 2(2): 15.
- (2022) 40 lakh cancer cases, 22.54 lakh deaths reported in India in 3 years: Govt. India Today.
- (2022) India’s cancer burden to rise to 29.8 million in 2025: ICMR report. Livemint.
- (2022) Cancer-WHO. World Health Organization.
- Mitra S, Dash R (2018) Natural products for the management and prevention of breast cancer. Evid Based Complement Alternat Med. 2018: 8324696.
- Xie SZ, Pan JJ, Xu JF, Zhu WW, Qin LX (2022) The critical function of metabolic reprogramming in cancer metastasis. Aging and Cancer 3(1): 20-43.
- Anderson RM, Weindruch R (2007) Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol 35: 18-38.
- Seyfried TN, Chinopoulos C (2021) Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites 11(9): 572.
- Duraj T, Carrión Navarro J, Seyfried TN, García Romero N, Ayuso Sacido A (2021) Metabolic therapy and bioenergetic analysis: The missing piece of the puzzle. Mol Metab 54: 101389.
- Bhattacharya S (2021b) Diet and cancer metabolic reprogramming. Cancer Rep Rev 5: 1-4.
- Bhattacharya S (2021c) Breast cancer and targeted therapy. Cancer Rep Rev 5: 1-6.
- Lai X, Li Q, Wu F, Lin J, Chen J, et al. (2020) Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons From Somatic Cell Reprogramming. Front Cell Dev Biol 8: 760.
- Zhu J, Thompson CB (2019) Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol 20(7): 436-450.
- Ishida T, Nakao S, Ueyama T, Harada Y, Kawamura T (2020) Metabolic remodeling during somatic cell reprogramming to induced pluripotent stem cells: involvement of hypoxia-inducible factor 1. Inflamm Regen 40: 8.
- Faubert B, Solmonson A, Deberardinis RJ (2020) Metabolic reprogramming and cancer progression. Science 368(6487): eaaw5473.
- Zhao L, Liu Y, Zhang S, Wei L, Cheng H, et al. (2022) Impacts and mechanisms of metabolic reprogramming of tumor microenvironment for immunotherapy in gastric cancer. Cell Death Dis 13(4): 378.
- Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exptl Clinical Cancer Res 34: 111.
- Yang E, Wang X, Gong Z, Yu M, Wu H, et al. (2020) Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduction and Targeted Therapy 5: 242.
- Welchman SL, Emdad L, Sarkar D, Das SK, Fisher PB (2020) Vascular mimicry: Triggers, molecular interactions, and in vivo models. Adv Cancer Res 148: 27-67.
- Luo Q, Wang J, Zhao W, Peng Z, Liu X, et al. (2020) Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol 13(1): 19.
- Meiliana A, Dewi NM, Wijaya A (2021) Metabolic reprogramming and molecular rewiring in cancer. Indones Biomed J 13(2): 114-139.
- Corcoran SE, O’Neill LAJ (2016) HIF1α and metabolic reprogramming in inflammation. J Clin Invest 126(10): 3699-3707.
- Soga T (2013) Cancer metabolism: key players in metabolic reprogramming. Cancer Sci 104(3): 275-281.
- Martínez Ordoñez A, Seoane S, Avila L, Eiro N, Macía M, et al. (2021) POU1F1 transcription factor induces metabolic reprogramming and breast cancer progression via LDHA regulation. Oncogene 40(15): 2725-2740.
- Antal Z (2021) Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines 2(4): 485-501.
- Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, et al. (2014) IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA 111(35): E3641–E3649.
- Lan T, Chen L, Wei X (2021) Inflammatory cytokines in cancer: comprehensive understanding and clinical progress in gene therapy. Cells 10(1): 100.
- Jin W (2020) Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells 9(1): 217.
- Galoczova M, Coates P, Vojtesek B (2018) STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett 23: 12.
- Dey P, Kimmelman AC, Depinho RA (2021) Metabolic co-dependencies in the tumor microenvironment. Cancer Discov 11(5): 1067-1081.
- Knaap JAVD, Verrijzer CP (2016) Undercover: gene control by metabolites and metabolic enzymes. Genes Dev 30(21): 2345-2369.
- Wang R, Dillon CP, Shi ZL, Milasta S, Carter R, et al. (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35(6): 871-882.
- Zhao J, Lin X, Meng D, Zeng L, Zhuang R, et al. (2020) Nrf2 mediates metabolic reprogramming in non-small cell lung cancer. Front Oncol 10: 578315.
- Martín Martín N, Carracedo A, Torrano V (2018) Metabolism and Transcription in Cancer: Merging Two Classic Tales. Front Cell Dev Biol 5: 119.
- Tan YT, Lin JF, Li T, Li JJ, Xu RH, et al. (2021) LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond) 41(2): 109-120.
- Sun T, Liu Z, Yang Q (2020) The role of ubiquitination and deubiquitination in cancer metabolism. Molecular Cancer 19: 146.
- Cazzaniga M, Bonanni B (2015) Relationship between metabolic reprogramming and mitochondrial activity in cancer cells. understanding the anticancer effect of metformin and its clinical implications. Anticancer Res 35(11): 5789-5796.
- Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21(3): 297-308.
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1): 11-20.
- Bhattacharya S (2021d) Cytoskeleton and epithelial mesenchymal transition. Cancer Pages 2(2): 16.
- Caino CM, Chae YC, Vaira V, Ferrero S, Nosotti M, et al. (2013) Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest 123(7): 2907-2920.
- Cliff TS, Dalton S (2017) Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Curr Opin Genet Dev 46: 44-49.
- Ito K, Ito K (2016) Metabolism and the control of cell fate decisions and stem cell renewal. Annu Rev Cell Dev Biol 32: 399-409.
- Tarazona OA, Pourquie´ O (2020) Exploring the influence of cell metabolism on cell fate through protein post-translational modifications. Dev Cell 54(2): 282-292.
- Julian LM, Stanford WL (2020) Organelle cooperation in stem cell fate: lysosomes as emerging regulators of cell identity. Front Cell Dev Biol 8: 591.
- Tatapudy S, Aloisio F, Barber D, Nystul T (2017) Cell fate decisions: emerging roles for metabolic signals and cell morphology. EMBO Rep 18(12): 2105-2118.
- Georgakopoulos Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A (2020) EMT factors and metabolic pathways in cancer. Front Oncol 10: 499.
- Morandi A, Taddei ML, Chiarugi P, Giannoni E (2017) Targeting the metabolic reprogramming that controls epithelial-to-mesenchymal transition in aggressive tumors. Front Oncol 7: 40.
- Ribatti D, Tamma R, Annese T (2020) Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl Oncol 13(6): 100773.
- Nan Zhang MM, Seng AN, Cai S, Li Q, Yang L, et al. (2021) Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol 22(8): E358-E368.
- Luo W, Hu H, Chang R, Zhong J, Knabel M, et al. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145(5): 732-744.
- Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, et al. (2013) Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24(2): 213-228.
- Kim NH, Cha YH, Lee J, Lee SH, Yang JH, et al. (2017) Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat Commun 8: 14374.
- Swinnen JV, Brusselmans K, Verhoeven G (2006) Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 9(4): 358-365.
- Dongre A, Weinberg R (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20(2): 69-84.
- Yu L, Lu M, Jia D, Ma J, Ben Jacob E, et al. (2017) Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res 77(7): 1564-1574.
- Clarke M, Dick J, Dirks P, Eaves C, Jamieson C, Jones D, et al. (2006) Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66(19): 9339-9344.
- Nieto MA (2013) Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342(6159): 1234850.
- Liu X, Sun H, Qi J, Wang L, He S, et al. (2013) Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT-MET mechanism for optimal reprogramming. Nat Cell Biol 15(7): 829-838.
- Ryall JG, Cliff T, Dalton S, Sartorelli V (2015) Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17(6): 651-662.
- Wu J, Ocampo A, Belmonte J (2016) Cellular metabolism and induced pluripotency. Cell 166(6): 1371-1385.
- Zhou Z, Yang X, He J, Liu J, Wu F, et al. (2017) Kdm2b regulates somatic reprogramming through variant PRC1 complex dependent function. Cell Rep 21(8): 2160-2170.
- Dobrian AD (2012) A tale with a Twist: a developmental gene with potential relevance for metabolic dysfunction and inflammation in adipose tissue. Front Endocrinol 3: 108.
- Martin SD, Mcgee SL (2018) Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J Endocrinol 237(2): R35-R46.
- Gubelmann C, Schwalie PC, Raghav SK, Roder E, Delessa T, et al. (2014) Identification of the transcription factor ZEB1 as a central component of the adipogenic gene regulatory network. Elife 3: e03346.
- Grassian AR, Lin F, Barrett R, Liu Y, Jiang W, et al. (2012) Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/MicroRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT). J Biol Chem 287(50): 42180-42194.
- Mathow D, Chessa F, Rabionet M, Kaden S, Jennemann R, et al. (2015) Zeb1 affects epithelial cell adhesion by diverting glycosphingolipid metabolism. EMBO Rep 16(3): 321-331.
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, et al. (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9(6): 582-589.
- Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, et al. (2011) p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208(5): 875-883.
- Saydam O, Shen Y, Würdinger T, Senol O, Boke E, et al. (2009) Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol 29(21): 5923-5940.
- Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, et al. (2011) The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J 30(4): 770-782.
- Zhao XY, Lin JD (2015) Long noncoding RNAs: a new regulatory code in metabolic control. Trends Biochem Sci 40(10): 586-596.
- Gugnoni M, Ciarrocchi A (2019) Long noncoding RNA and epithelial mesenchymal transition in cancer. Int J Mol Sci 20(8): 1924.
- Hong T, Watanabe K, Ta C, Villarreal Ponce A, Nie Q, et al. (2015) An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLoS Comput Biol 11(11): e1004569.
- Roca H, Hernandez J, Weidner S, Mceachin RC, Fuller D, Sud S, et al (2013) Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS ONE 8(10): e76773.
- Moya M, Benet M, Guzman C, Tolosa L, Garciam Monzon C, et al. (2012) Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS ONE 7(1): e30014.
- Kotiyal S, Bhattacharya S (2015) Epithelial mesenchymal transition and vascular mimicry in breast cancer stem cells. Crit Rev Eukaryot Gene Expr 25(3): 269-280.
- Peng H, Wang X, Du J, Cui Q, Huang Y, et al. (2021) Metabolic reprogramming of vascular endothelial cells: basic research and clinical applications. Front Cell Dev Biol 9: 626047.
- Wei X, Chen Y, Jiang X, Peng M, Liu Y, et al. (2021) Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer 20(1): 7.
- Wu D, Huang RT, Hamanaka RB, Krause M, Oh M-J, et al. (2017) Hif-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. ELife 6: e25217.
- Bonacina F, Dalt LD, Catapano AL, Norata GD (2021) Metabolic adaptations of cells at the vascular-immune interface during atherosclerosis. Mol Aspects Med 77: 100918.
- Magnuson DK, Maier RV, Pohlman TH (1989) Protein kinase C: apotential pathway of endothelial cell activation by endotoxin, tumor necrosis factor, and interleukin-1. Surgery 106(2): 216-222.
- Pan S, World CJ, Kovacs CJ, Berk BC (2009) Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol 29(6): 895-901.
- Thankamony AP, Saxena K, Murali R, Jolly MK, Nair R (2020) Cancer stem cell plasticity - a deadly deal. Front Mol Biosci 7: 79.
- Phan LM, Yeung SCJ, Lee MH (2014)Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med 11(1): 1-19.
- O’Keefe SJD (2019) Plant-based foods and the microbiome in the preservation of health and prevention of disease. Am J Clin Nutr 110(2): 265-266.
- Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, et al. (2019) Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients 11(10): 2393.
- Bose S, Allen AE, Locasale JW (2020) The molecular link from diet to cancer cell metabolism. Mol Cell 78(6): 1034-1044.
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, et al. (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342(6161): 967-970.
- Nakatsu G, Li X, Zhou H, Sheng sJ, Wong SH, et al. (2015) Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun 6: 8727.
- Savkovic SD (2020) Gut microbes effects on host metabolic alterations in health and disease. Gut Microbes 11(3): 249-252.
- Lin TH, Hsu WH, Tsai PH, Huang YT, Lin CW, et al. (2017) Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial–mesenchymal transition signaling. Food Funct 8: 1558-1568.
- Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, et al. (2019) The effects of vegetarian and vegan diets on gut microbiota. Front Nutr 6: 47.
- Huang F, Sun B, Wang X, Jian X, Du Q, et al. (2021) Dietary palmitic acid promotes tumor growth and epithelial mesenchymal transformation in prostrate cancer. Ressearch Square.
- Kwapisz O, Górka J, Korlatowicz A, Kotlinowski J, Waligórska A, et al. (2021) Fatty Acids and a High-Fat Diet Induce Epithelial–Mesenchymal Transition by Activating TGF-β and β-Catenin in Liver Cells. Int J Mol Sci 22(3): 1272.
- Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, Hursting SD (2012) Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res 5(7).
- https://doi.org/10.1158/1940-6207.CAPR-12-0034
- Buhrmann C, Yazdi M, Popper B, Shayan P, Goel A, Aggarwal BB, Shakibaei M (2018) Resveratrol chemosensitizes tnf-β-induced survival of 5-fu-treated colorectal cancer cells. Nutrients 10(7): 888. https://doi.org/10.3390/nu10070888
- Hashem S, Ali TA, Akhtar S, Nisar S, Sageena G, Ali S, Al-Mannai S, Therachiyil L, Mir R, Elfaki I, Mir MM, Jamal F, Masoodi T, Uddin S, Singh M, Haris M, Macha M, Bhat AA (2022) Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomedicine & Pharmacotherapy 150: 113054. https://doi.org/10.1016/j.biopha.2022.113054
- Resveratrol, Linus Pauling Institute, Oregon State University (2022) https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/resveratrol. Accessed 12 January 2023
- Sameri S, Saidijam M, Bahreini F, Najafi R (2021) Cancer chemopreventive activities of silibinin on colorectal cancer through regulation of e-cadherin/β-catenin pathway. Nutr Cancer 73: 1389-1399. https://doi.org/10.1080/01635581.2020.1800764
- Pubchem (2022a) Silibinin. C25H22O10-Pubchem, https://pubchem.ncbi.nlm.nih.gov/compound/Silibinin.Accessed 27 October 2022
- Dermani FK, Amini R, Saidijam M, Pourjafar M, Saki S, Najafi R (2018a) Zerumbone inhibits epithelial-mesenchymal transition and cancer stem cells properties by inhibiting the β-catenin pathway through miR-200c. J Cell Physiol 233(120): 9538-9547. https://doi.org/10.1002/jcp.26874
- Dermani FK, Amini R, Saidijam M, Najafi R (2018b) miR-200c, a tumor suppressor that modulate the expression of cancer stem cells markers and epithelial-mesenchymal transition in colorectal cancer. J Cell Biochem 119(7): 6288-6295. https://doi.org/10.1002/jcb.26880
- Kalantari K, Moniri M, Moghaddam AB, Rahim RA, Ariff AB, Izadiyan Z, Mohamad R (2017) A review of the biomedical applications of zerumbone and the techniques for its extraction from ginger rhizomes. Molecules 22(10):1645. https://doi.org/10.3390/molecules22101645
- Kim SM, Jung Y-J, Kwon O-N, Cha KH, Um B-H, Chung D, Pan C-H (2012) A potential commercial source of fucoxanthin extracted from the microalgae Phaeodactylum tricornutum. Appl Biochem Biotechnol 166(7): 1843-55. https://doi.org/10.1007/s12010-012-9602-2
- Terasaki M, Mima M, Kudoh S, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M (2018) Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol Rep 40(1): 414-424.
- https://doi.org/10.3892/or.2018.6398
- Pubchem (2022b) Scutellarin. C15H10O6-Pubchem. https://pubchem.ncbi.nlm.nih.gov/compound/Scutellarein. Accessed 27 October 2022
- Pubchem (2022c) Fisetin tetramethyl ether. C19H18O6-Pubchem. https://pubchem.ncbi.nlm.nih.gov/compound/Fisetin-tetramethyl-ether. Accessed 27 October 2022
- Pubchem (2022d) Cyclopamine-A sonic hedgehog gene pathway antagonist. https://aphios.com/products/research-chemicals-apis/cyclopamine/ Accessed 27 October 2022
- Batsaikhan BE, Yoshikawa K, Kurita N, Iwata T, Takasu C, Kashihara H, Shimada M (2014) Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer Res 34(11): 6339-6344.
- Pereira CV, Duarte M, Silva P, Bento DSA, Duarte CMM, Cifuentes A, García-Cañas V, Bronze MR, Albuquerque C, Serra AT (2019) Polymethoxylated flavones target cancer stemness and improve the antiproliferative effect of 5-fluorouracil in a 3d cell model of colorectal cancer. Nutrients 11(2): 326. https://doi.org/10.3390/nu11020326
- Hua W, Dijke PT, Kostidis S, Giera M, Hornsveld M (2020) TGFβ induced metabolic reprogramming during epithelial to mesenchymal transition in cancer. Cellular and Molecular Life Sciences 77(11): 2103–2123. https://doi.org/10.1007/s00018-019-03398-6
- Dey A, Nanda B, Nandy S, Mukherjee A, Pandey DK (2020) Implications of phytochemicals as disease-modifying agents against Huntington’s disease (HD): Bioactivity, animal models, and transgenics, synergism and structure-activity studies. Studies in Natural Products Chemistry 67: 27-79. https://doi.org/10.1016/B978-0-12-819483-6.00002-3
- Chandra-Boinpelly V, Verma RK, Srivastav S, Srivastava RK, Shankar S (2020) α-Mangostin encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J Cell Mol Med 24: 11343-11354. https://doi.org/10.1111/jcmm.15731
- Wu AT, Yeh YC, Huang YJ, Mokgautsi N, Lawal B, Huang TH (2022) Gamma-mangostin isolated from garcinia mangostana suppresses colon carcinogenesis and stemness by downregulating the GSK3β/β-catenin/CDK6 cancer stem pathway. Phytomedicine 95: 153797. https://doi.org/10.1016/j.phymed.2021.153797
- Hewlings SJ, Kalman DS (2017a) Curcumin: A review of its’ effect on human health. Foods 6(10): 92. https://doi.org/10.3390/foods6100092
- Hewlings SJ, Kalman DS (2017b) Curcumin: a review of its’ effects on human health. Foods 6: 92. https://doi.org/10.3390/foods6100092
- Acikgoz E, Tatar C, Oktem G (2020) Triptolide inhibits CD133(+) /CD44(+) colon cancer stem cell growth and migration through triggering apoptosis and represses epithelial-mesenchymal transition via downregulating expressions of Snail, Slug, and Twist. J Cell Biochem 121(5-6): 3313-3324. https://doi.org/10.1002/jcb.29602
- Feng HC, Lin JY, Hsu SH, Lan WY, Kuo CS, Tian YF, Sun DP, Huang RS (2017) Low folate metabolic stress reprograms DNA methylation-activated sonic hedgehog signaling to mediate cancer stem cell-like signatures and invasive tumour stage-specific malignancy of human colorectal cancers. Int J Cancer 141(12): 2537-2550. https://doi.org/10.1002/ijc.31008
- Pubchem (2022e) Baicalin. C21H18O11-Pubchem.
- https://pubchem.ncbi.nlm.nih.gov/compound/Baicalin. Accessed 27 October 2022
- Donald G, Hertzer K, Eibl G (2012) Baicalein – an intriguing therapeutic phytochemical in pancreatic cancer. Curr Drug Targets 13(14): 1772–1776. https://doi.org/10.2174/138945012804545470
- Soundararajan P, Kim JS (2018) Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 23(11): 2983. https://doi.org/10.3390/molecules23112983
- Yang B, Bai H, Sa Y, Zhu P, Liu P (2020) Inhibiting EMT, stemness and cell cycle involved in baicalin induced growth inhibition and apoptosis in colorectal cancer cells. J Cancer 11(8): 2303-2317.
- https://doi.org/10.7150/jca.37242
- Tarin D (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 6: 5996–6000. https://doi.org/10.1158/0008-5472.CAN-05-0699
- Kotiyal S, Bhattacharya S (2016) Events of molecular changes in epithelial-mesenchymal transition. Critical Reviews in Eukaryotic Gene Expression 26(2): 163–171.
- https://doi.org/10.1615/CritRevEukaryotGeneExpr.2016016307
- Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142. https://doi.org/10.1038/nrm1835
- Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial mesenchymal transitions in development and disease. Cell 139: 871–890. https://doi.org/10.1016/j.cell.2009.11.007
- Nieto MA (2011) The ins and outs of the epithelial to mesenchymal transition in health and disease. Ann Rev Cell Dev Biol 27: 347–76. https://doi.org/10.1146/annurev-cellbio-092910-154036
- Nieto MA, Huang RY-J, Rebecca JAA, Jean TP (2016) EMT: 2016. Cell 166: 21–45. https://doi.org/10.1016/j.cell.2016.06.028
- Zaravinos A (2015) The regulatory role of microRNAs in EMT and cancer. J Oncol Volume 2015, Article ID 865816. https://doi.org/10.1155/2015/865816
- Masoud V, Pagès G (2017) Targeted therapies in breast cancer: New challenges to fight against resistance. World J Clin Oncol 8: 120-134. https://doi.org/10.5306/wjco.v8.i2.120
- Wang J, Xu B (2019) Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduction and Targeted Therapy. 4: 34.
- https://doi.org/10.1038/s41392-019-0069-2
- Kotiyal S, Bhattacharya S (2014) Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Comm 453: 112–116. http://dx.doi.org/10.1016/j.bbrc.2014.09.069
- Schiliro C, Firestein BL (2021) Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 10: 1056. https://doi.org/10.3390/cells10051056
- Schrump DS, Chen A, Consoli U (1996) Inhibition of lung cancer proliferation by antisense cyclin D. Cancer Gene Ther 3: 131-135.
- Otto T, Sicincki P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17: 93-115. https://doi.org/10.1038/nrc.2016.138
- Bhattacharya S (2021e) miRNA and cancer. In: Murdaca G (ed) New Frontiers In Medicine And Medical Research, Book Publisher International, vol. 15, pp. 173-184.
- https://doi.org/10.9734/bpi/nfmmr/v15/12373D
- Rad SMAH, Halpin JC, Tawinwung S, Suppipat K, Hirankarn N, Mclellan AD (2022) MicroRNA-mediated metabolic reprogramming of chimeric antigen receptor T cells. Immunology & Cell Biol 100(6): 424-439. https://doi.org/10.1111/imcb.12551
- Agarwal E, Goldman AR, Tang H-Y, Kossenkov AV, Ghosh JC, Languino LR, Vaira V, Speicher DW, Altieri DC (2021) A cancer ubiquitome landscape identifies metabolic reprogramming as target of Parkin tumour suppression. Sci Adv 7: eabg7287. https://doi.org/10.1126/ciadv.abg7287
- Dewane G, Salvi AM, Demali KA (2021) Fueling the cytoskeleton – links between cell metabolism and actin remodeling. J Cell Sci 134(3): jcs248385. https://doi.org/10.1242/jcs.248385
- Roberts SJ, Somero GN (1987) Binding of phosphofructokinase to filamentous actin. Biochemistry 26: 3447-3442. https://doi.org/10.1021/bi00386a028.
- Roberts SJ, Somero GN (1989) Properties of the interaction between phosphofructokinase and actin. Arch Biochem Biophys 269: 284-294. https://doi.org/10.1016/0003-9861(89)90110-0
- Sneeggen M, Guadagno N, Progida C (2020) Intracellular transport in cancer metabolic reprogramming. Front Cell Dev Biol 8: 597608. https://doi.org/10.3389/fcell.2020.597608
- Fulweilerlab (2022) Thermodynamics and microbial metabolism.
- https://www.fulweilerlab.com/uploads/3/7/6/7/3767379/1-s2.0-s0065288105480037-main.pdf.
- Accessed 12 January 2023
- Surfguppy (2020) Chemistry online resources for students and teachers.
- https://surfguppy.com/thermodynamics/difference-between-enthalpy-entropy-gibbs-free-energy-themodynamics/Accessed 12 January 2023
- Zhang XC, Yang H, Liu Z, Sun F (2018) Thermodynamics of voltage-gated ion channels. Biophys Rep 4(6): 300-319. https://doi.org/10.1007/s41048-018-0074-y
- Großkopf T, Soyer OS (2016) Microbial diversity arising from thermodynamic constraints. The ISME Journal. 10: 2725–2733. https://doi.org/10.1038/ismej.2016.49
- Cook J, Pawar S, Endres RG (2021) Thermodynamic constraints on the assembly and diversity of microbial ecosystems are different near to and far from equilibrium. PLoS Comput Biol 17(12): e1009643. https://doi.org/10.1371/journal.pcbi.1009643
- Saadat NP, Nies T, Rousset Y, Ebenhoh E (2020) Thermodynamic limits and optimality of microbial growth. Entropy 22(3): https://doi.org/10.3390/e22030277
- Vallino JJ, Huber JA (2018) Using maximum entropy production to describe microbial biogeochemistry over time and space in a meromictic pond. Front Environ Sci 6: 100.
- https://doi.org/10.3389/fenvs.2018.00100
- Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37: 384–406.
- https://doi.org/10.1111/1574-6976.12019
- Braissant O, Astasov-Frauenhoffer M, Waltimo T, Bonkat G (2020) A review of methods to determine viability, vitality, and metabolic rates in microbiology. Front Microbiol 11: 547458. https://doi.org/10.3389/fmicb.2020.547458
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676.
- https://doi.org/ 10.1016/j.cell.2006.07.024
- Kang H, Kim H, Lee S, Youn H, Youn B (2019) Role of metabolic reprogramming in epithelial-mesenchymal transition (EMT). Int J Mol Sci 20: 2042.
- https://doi.org/10.3390/ijms20082042
- Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, et al (2011) Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg 92: 1794–804. https://doi.org/10.1016/j.athoracsur.2011.07.032
- Smith B, Bhowmick N (2016) Role of EMT in metastasis and therapy resistance. J Clin Med 5: 17. https://doi.org/10.3390/jcm5020017
- Dixit A, Gulati B, Sharma G, Bhatia G, Priya R, Bhattacharya S (2021) Evaluation of phytochemical and antimicrobial activity of Ocimum spp. Integr Food Nutr Metab 8: 1-4.
- https://doi.org/10.15761/IFNM.1000299
- Sharma A, Gupta P, Bhattacharya S (2015) Evaluation of antibacterial activity of Lactobacillus spp. on selected food spoilage bacteria. Recent Patents on Food, Nutrition & Agriculture 7(1): 9-13. https://doi.org/10.2174/2212798407666150309125110
- Zhang H, Tang S (2021) Metabolic reprogramming and cancer precision medicine: a narrative review. Precis Cancer Med 4: 35. https://dx.doi.org/10.21037/pcm-21-27
- Sethi JK, Hotamisligil GS (2021) Metabolic Messengers: tumour necrosis factor. Nature Metabolism 3: 1302–1312. https://doi.org/10.1038/s42255-021-00470-z
- Silva LB, Neto APDS, Maia SMAS, Guimaraes CDS, Quidute IL, Carvalho AdAT, Junior SA, Leao JC (2019) The Role of TNF-α as a Proinflammatory Cytokine in Pathological Processes. The Open Dentistry Journal 13: 332-338. https://doi.org/10.2174/1874210601913010332
- Mohammadi M, Gozashti MH, Aghadavood M, Mehdizadeh MR, Hayatbakhsh MM (2017) Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Reports of Biochemistry & Molecular Biology Vol.6, No.1.
- Popa C, Netea MG, van Riel PLCM, van der Meer JWM, Stalenhorf AFH (2007) The role of TNF-a in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48: 751-762. https://doi.org/10.1194/jlr.R600021-JLR200
- Das B, Sarkar N, Bishayee A, Sinha D (2019) Dietary phytochemicals in the regulation of epithelial to mesenchymal transition and associated enzymes: A promising anticancer therapeutic approach. Seminars in Cancer Biol 56: 196-218. https://doi.org/10.1016/j.semcancer.2018.11.007
- Shi J, Fan J, Su Q, Yang Z (2019) Cytokines and Abnormal Glucose and Lipid Metabolism. Front Endocrinol 10: 703. https://doi.org/10.3389/fendo.2019.00703
- Kern HA, Ranganathan S, Li C, Wood L, Ranganathan G (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745–E751. https://doi.org/10.1152/ajpendo.2001.280.5.e745
- Williams NC, O’Neill LAJ (2018) A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front Immunol 9: 141.
- https://doi.org/ 10.3389/fimmu.2018.00141
- Chou PC, Liu C-M, Weng C-H, Yang K-C, Cheng M-L, Lin Y-C, Yang R-B, Shyu B-C, Shyue S-K, Liu J-D, Chen S-P, Hsiao M, Hu Y-F (2022) Fibroblasts drive metabolic reprogramming in pacemaker cardiomyocytes. Circulation Res 131(1): 6-20. https://doi.org/10.1161/CIRCRESAHA.121.320301
- Oshima J, Campisi J (1991) Fundamentals of cell proliferation: control of the cell cycle. J Dairy Sci 74: 2778-2787. https://doi.org/10.3168/jds.S0022-0302(91)78458-0
- Kumari R, Jat P (2021) Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 9: 645593.
- https://doi.org/10.3389/fcell.2021.645593
- Homem CCF, Repic M, Knoblich JA (2015) Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci 16(11): 647–659. https://doi.org/10.1038/nrn4021.
- Bueno C, Martinez-Morga M, Martinez S (2019) Non-proliferative neurogenesis in human periodontal ligament stem cells. Scientific Rep 9: 18038. https://doi.org/10.1038/s41598-019-54745-3
- Rangel-Huerta E, Maldonado E (2017) Transit amplifying cells in the fast lane from stem cells towards differentiation. Stem Cell Int Volume 2017, Article ID 7602951: 10 pages, https://doi.org/10.1155/2017/7602951
- Liu H, Adler AS, Segal E, Chang HY (2007) A transcriptional program mediating entry into cellular quiescence. PLoS Genet 3(6): e91. https://doi.org/10.1371/journal.pgen.0030091
- Marescal O, Cheeseman IM (2020) Cellular mechanisms and regulation of quiescence. Dev Cell 55(3): 259-271. https://doi.org/10.1016/j.devcel.2020.09.029.
- Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14(6): 329-340. https://doi.org/10.1038/mrm3591
- Hsu Y-C, Li L, Fuchs E (2014) Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157(4): 935-949. https://doi.org/10.1016/j.cell.2014.02.057.
- Rumman M, Dhawan J, Kassem M (2015) Concise Review: Quiescence in adult stem cells: Biological significance and relevance to tissue regeneration. Stem Cells 33(10): 2903-2912. http://dx.doi.org/10.1002/stem.2056
- Brasiel PGDA (2020) Luquetti SCPD. Metabolic programming and nutrition. In : https://doi.org/10.5772/intechopen.92201.
- Liskova A, Kubatka P, Samec M, Zubor P, Mlyncek M, Bielik T, Sameul SM, Zulli A, Kwon TK (2019) Büsselberg D. Dietary Phytochemicals targeting cancer stem cells. Molecules 24: 899. https://doi.org/10.3390/molecules24050899
- Gupta PK, Saraff M, Gahtori R, Negi N, Tripathi SK, Kumar J, Kumar S, Aldhayan SH, Dhanasekaran S, Abomughaid MM, et al (2021) Phytomedicines Targeting Cancer Stem Cells: Therapeutic Opportunities and Prospects for Pharmaceutical Development. Pharmaceuticals 14: 676. https://doi.org/10.3390/ph14070676
- Liao W, Zhang L, Chen X, Xiang J, Zheng Q, Chen N, Zhao M, Zhang G, Xiao X, Zhou G, Zeng J, Tang J (2023) Targeting cancer stem cells and signalling pathways through phytochemicals: A promising approach against colorectal cancer. Phytomedicine 108:154524. doi: 10.1016/j.phymed.2022.154524. Epub 2022 Oct 31. PMID: 36375238.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.