Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
An Initial Evaluation of the Effectiveness of a Dietary Supplement Regimen on the Effects of Post-COVID Infection
*Corresponding author: Leonard Sonnenschein, The Sonnenschein Institute, 6617 NW 24th Ave., Boca Raton, FL 33496, USA.
Received: May 19, 2023; Published: May 22, 2023
DOI: 10.34297/AJBSR.2023.18.002528
Abstract
The prevalence of COVID-19 (Coronavirus) infection has been categorized as pandemic since November 2019. Currently the pandemic is slowdown, but now the prolonged COVID amid other derived medical conditions are emerging. The identification of Coronavirus infection by various assessments is well-known including initial diagnosis by symptoms. Some of the symptoms have been seen to persist beyond the normal incubation time and some additional symptoms have arisen to be termed as post-COVID-19 or Long COVID which include loss of energy, memory issues, persistence of pain and sleeplessness. In this study, 15 randomized adult post-COVID infection participants were evaluated with a 3-day control followed by a 5-day Electrocide® treatment protocol. All the participants noted a significant increase in energy. 88% of the participants noted a significant decrease in pain. 75% of the participants noted a significant improvement in sleep. As in our previous studies [1], the elimination of active COVID-19 infection (diagnosed by fever, coughing, runny nose, brain fog, headache, and pain) can be achieved in an average of 6 days with the use of Electrocide®. The current study furthermore demonstrates the effectiveness of the Electrocide® as a 7-day treatment for post-COVID infection. A specially formulated enzyme formula added to the Electrocide® regime was also evaluated against a placebo control and it was found that there was rapid recovery from a number of symptoms associated with long haul, chronic COVID-19 as well as vaccine effects including peripheral neuropathy, eye blurriness, and heart issues. The current study furthermore demonstrates the effectiveness of the Electrocide® and enzyme formula as a 7-day treatment for many of the symptoms of COVID-19, COVID-19 vaccine, and COVID-19 long haul.
Keywords: COVID-19, Long COVID, COVID-19 vaccine, Electrocide®, Dietary supplement, Wellbeing, Inflammation, Antipathogen, Recovery
Abbreviations: BRVO: Branch Retinal Vein Occlusion; CDC: Center for Disease Control and Prevention; CHD: Congenital Heart Disease; DIC: Disseminated Intravascular Coagulation; DVT: Deep Vein Thrombosis; FDPs: Fibrin Degradation Products; GBS: Guillain-Barre Syndrome; MERS: Middle East Respiratory Syndrome; MI: Myocardial Infarction; PASC: Post-Acute Sequelae of COVID-19; PE: Pulmonary Embolism; RVO: Retinal Vein Occlusion; SARS: Severe Acute Respiratory Syndrome; SARS-Cov-2: Severe Acute Respiratory Syndrome Coronavirus 2; T2DM: Type 2 Diabetes Mellitus; TLC: The Lovelady Center; VST: Venous Sinus Thrombosis
Literature Review
COVID-19 Symptoms and Management
COVID-19 symptoms commonly include [2] fever, shortness of breath, cough, and viral pleurisy. Viral nucleic acid amplification tests play an important role [3] in identifying SARS-CoV-2 infection and helped in managing populations from exposure.
Previous Studies
The Effect of an Aqueous Electricidal Solution on General Well Being: Solution4USA’s dietary supplement, referred to here as “Electrocide®” is a new type of aqueous nutraceutical which is antipathogenic in nature due to pathogen specific electrical charge properties. Thousands of people have been treated with this product when delivered through an existing network of medical professionals validating its efficacy and safety. Using FDA guidelines, “Electrocide®” was evaluated [1] for wellness benefits as a potential investigational drug under medical supervision using 52 volunteers in an institutional setting (The Lovelady Center (TLC) in Birmingham, Alabama) using quantitative measurements of vital signs along with two qualitative self-reported questionnaires. The results indicate a highly significant improvement in vital signs and a significant reduction in various physiological and emotional conditions. At the start of the study, the average temperature and oxygen values were subnormal, indicating poor health status, whereas, by the end of the study, vital signs were all normal. During the study, a cohort study (proof of concept) was conducted with 14 additional individuals, in the TLC general population, who were identified and diagnosed as COVID-19 positive. After 6 days (on average) of “Electrocide ®” administration, a shift of their COVID-19 status switched to negative, and a complete reversal of symptoms were observed several times faster than predicted by the CDC. Of particular interest were oxygen levels because a previous in-vitro pilot study indicated that dissolved oxygen levels doubled with “ELECTROCIDE®” -It is postulated that this is an important mechanism promoting an immediate sense of wellness.
Historical and Current Conditions Including COVID-19 Requiring Anti-Pathogenic Surveillance and Proper Immunogenic Support: Everyone on the planet will eventually be exposed to COVID-19 [4]. The question of who will get ill, who will die and who will survive will depend more on appropriate preparations than on a vaccine. In addition, this current pandemic threat may pose a harbinger of what is yet to come. The key lesson learned is to strengthen our immunological support and surveillance. In this way, we will have greater capacity to address future variant viral strains. This brief research-based communication explores possible treatments and some of the historical, societal, and economic possible sequelae resulting from pandemics.
COVID-19 Effects on Organs
The COVID-19 pandemic has infected millions worldwide, leaving a global burden [5] for long-term care of COVID-19 survivors. Post-COVID (i.e., short-term) and long-COVID (i.e., long-term) effects, can lead to local and systemic pathophysiological outcomes of other coronavirus-related diseases such as Middle East Respiratory Syndrome (MERS) and severe acute respiratory syndrome (SARS). Other COVID-19 pathology is characterized by cytokine storm that results to endothelial inflammation, microvascular thrombosis, and multiple organ failures in affected additional human systems, including: (i) immune system (e.g., Guillain-Barré syndrome, rheumatoid arthritis, pediatric inflammatory multisystem syndromes such as Kawasaki disease), (ii) hematological system (vascular hemostasis, blood coagulation), (iii) pulmonary system (respiratory failure, pulmonary thromboembolism, pulmonary embolism, pneumonia, pulmonary vascular damage, pulmonary fibrosis), (iv) cardiovascular system (myocardial hypertrophy, coronary artery atherosclerosis, focal myocardial fibrosis, acute myocardial infarction, cardiac hypertrophy), (v) gastrointestinal, hepatic, and renal systems (diarrhea, nausea/vomiting, abdominal pain, anorexia, acid reflux, gastrointestinal hemorrhage, lack of appetite/constipation), (vi) skeletomuscular system (immune-mediated skin diseases, psoriasis, lupus), (vii) nervous system (loss of taste/smell/hearing, headaches, spasms, convulsions, confusion, visual impairment, nerve pain, dizziness, impaired consciousness, nausea/vomiting, hemiplegia, ataxia, stroke, cerebral hemorrhage), (viii) mental health (stress, depression and anxiety) have been discovered.
The COVID-19 pandemic that first became apparent in Wuhan, China, is now infecting millions all over the world. COVID-19 has extensive effects on virtually all the organs [6] It causes inflammation, endotheliosis, vasoconstriction, hypercoagulability, and edema. Lymphocytopenia, elevated D-dimer, elevated Fibrin Degradation Products (FDPs), and Disseminated Intravascular Coagulation (DIC) have been observed. Deep Vein Thrombosis (DVT), venous thromboembolism, Pulmonary Embolism (PE), systemic and pulmonary arterial thrombosis and embolism, ischemic stroke, and Myocardial Infarction (MI). In the heart it can cause acute coronary syndrome, congestive heart failure, myocarditis, and arrhythmias. Kidney injury is usually secondary to systemic abnormalities. Stroke has been reported in young patients with delirium and seizures being common. Anosmia (loss of smell) and impaired sense of taste are largely reported. Lactate dehydrogenase elevation has been recorded and various skin manifestations including patchy erythematous rashes.
Other Infectious Processes and COVID-19
The pandemic outbreak of COVID-19 has resulted in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [7], evidenced by a massive inflammatory cytokine storm leading to multi-organ damage including that of the brain and testes leading to infertility. While the lungs, heart, and brain are identified as the main sites of action of SARS-CoV-2-mediated pathogenesis, reports on its testicular infections have been a subject of debate. The brain and testes are physiologically synchronized by the action of gonadotropins and sex steroid hormones. Though the evidence for the presence of the viral particles in the testicular biopsies and semen samples from COVID-19 patients are highly limited, the occurrence of testicular pathology due to abrupt inflammatory responses and hyperthermia is increasingly reported.
Heart Effects and COVID-19
Arrhythmias in COVID-19 patients [8] are commonly associated with hypoxia, myocardial ischemia, cytokines, inflammation, electrolyte abnormalities, pro-arrhythmic or QT-prolonging medications, and underlying heart conditions such as severe congestive heart failure, inherited arrhythmia syndromes, or congenital heart conditions. In the pediatric population, multisystem inflammatory syndrome can lead to cardiac injury and arrhythmias. Arrhythmias and cardiac arrests are most prevalent in the critically ill COVID-19 intensive care unit patient population.
COVID-19 Effects on Children
COVID-19 is an infectious disease caused by the severe acute respiratory syndrome corona virus 2 with significant cardiovascular implications [9]. Given the severe COVID-19 conditions observed in adults with underlying cardiac involvement, there is concern that patients with pediatric and congenital heart disease (CHD) may likewise be at increased risk for severe infection. COVID-19 cardiac manifestations include myocarditis, arrhythmia, and myocardial infarction.
Vision Effects
COVID-19 infection has been implicated in Retinal Vein Occlusion (RVO). Medical personnel should be aware of RVO in patients with COVID-19 infection who are presenting with blurred vision and severe pneumonia [10]. Additionally, Branch Retinal Vein Occlusion (BRVO) could be a COVID-19 vaccine-related thrombotic adverse event [11].
Peripheral Neuropathy and Neurological Effects
The COVID-19 pandemic is an issue of global significance that has taken the lives of many across the world. The pulmonary manifestations (Severe Acute Respiratory Syndrome-SARS) of COVID-19 have been well described in the literature. The neurological manifestations of SARS-CoV-2 infection are growing rapidly, as evidenced by several reports including Guillain-Barré syndrome (GBS) and its variants, dysfunction of taste and smell, and muscle injury are numerous examples of COVID-19 PNS (Peripheral Nervous System) disease, hemorrhagic and ischemic stroke, encephalitis, meningitis, encephalopathy acute disseminated encephalomyelitis, endotheliosis, and venous sinus thrombosis.9 Due to multifactorial and complicated pathogenic mechanisms, COVID-19 poses a largescale threat to the whole nervous system.
Loss of Smell
The loss or change in sense of smell (anosmia), is one of the most common symptoms of COVID-19 experienced by more than 50% of those affected [12]. Possible reasons for anosmia, may be local inflammation in the nasal epithelium (olfactory cleft syndrome), early apoptosis of olfactory cells, changes in olfactory cilia and odor transmission, damage to microglial cells, effect on olfactory bulbs, epithelial olfactory injury, and impairment of olfactory neurons and stem cells. The angiotensin-converting enzyme 2 receptor is a significant player in the mechanism of anosmia in COVID-19 patients. Options for patients who are suffering from the effects of COVID-19 including anosmia may be evidenced by changes in eating habits and other factors affecting mental health [13].
COVID-19 Co-Morbidity and Risk Factors
Fibrin(ogen) amyloid microclots and platelet hyperactivation have been reported in South African patients with COVID-19 and Long COVID/Post-Acute Sequelae of COVID-19 (PASC) [14]. In 845 patients’ hypertension, high cholesterol levels (dyslipidemia), cardiovascular disease and Type 2 Diabetes Mellitus (T2DM) were found to be the most important comorbidities. Additionally, fatigue, brain fog, loss of concentration and forgetfulness, shortness of breath, as well as joint and muscle pain were significant. Microclot and platelet pathologies were associated with Long COVID/PASC symptoms that persisted after the recovery from acute COVID-19. Recently a case study has clearly demonstrated the use of dietary supplements can reverse PASC [15].
Post-COVID-19 Effects Including Long COVID
Long COVID, the prolonged illness and fatigue suffered by those infected with SARS-CoV-2, is placing an increasing burden on individuals and society [14]. Long COVID links with other post-viral illnesses such as myalgic encephalomyelitis/chronic fatigue syndrome which may result by the stimulation of the vagus nerve. These mechanisms may underlie long COVID symptoms, with a focus on impaired oxygen delivery due to micro-clotting and disruption of cellular energy metabolism.
Many patients with mild or severe COVID-19 do not make a full recovery and have a wide range of chronic symptoms includes post-viral chronic fatigue syndrome, sequelae in multiple organs and the effects of severe hospitalization/post-intensive care for weeks or months after infection, often of a neurological, cognitive, or psychiatric nature [16]. Most related symptoms are fatigue, dyspnea, anxiety, depression, and impaired attention, concentration, memory and sleep. Abnormal autoimmune and inflammatory response may play an important role.
This post-COVID-19 study [17] of several hundred patients showed only 10.8% of all subjects having no manifestation after recovery from the disease while a large percentage of subjects suffered from several symptoms and diseases including fatigue (72.8%), other manifestations like stroke, renal failure, myocarditis, and pulmonary fibrosis were reported. There was a relationship between the presence of other comorbidities and severity of the disease. It is recommended that those affected by COVID-19 should undergo long-term monitoring for evaluation and treatment of symptoms and conditions that might be precipitated with new coronavirus infections.
Vaccine Effects
Those infected with SARS-CoV-2 (also identified here as COVID-19) have been shown to have adverse reactions to SARSCoV- 2 vaccinations and show a tropism for neuronal structures and tissues [18] Neurological side effects of SARS-CoV-2 vaccines can include headache, Guillain-Barre syndrome (GBS), Venous Sinus Thrombosis (VST), and transverse myelitis. Guillain-Barré syndrome (GBS) is an immune-mediated inflammatory polyradiculoneuropathy, which commonly leads to a very high level of neurological disability. GBS can be linked to SARS-CoV-2 infection and COVID-19 vaccination that can induce GBS and its variants [19]. There is a causal relationship between SARS-CoV-2 vaccination and new-onset nephropathies, based on the appearance of Urinary Abnormalities and/or renal insufficiency shortly after vaccination; it is hypothesized that the immune response to the COVID-19 vaccine may be a trigger of nephropathies [20].
COVID-19 Treatments
Among outpatients studied with mild to moderate COVID-19, treatment with ivermectin, compared with placebo, did not significantly improve time to recovery. These findings do not support the use of Ivermectin in patients with mild to moderate COVID-19 [21]. Four stages of COVID-19 infection have been identified: 1. upper respiratory tract infection; 2. the onset of dyspnea and pneumonia; 3. a worsening clinical scenario dominated by a cytokine storm and the consequent hyperinflammatory state; and 4. death or recovery.
Currently, no treatment can act specifically against the SARSCoV- 2 infection [22]. Based on the pathological features and different clinical phases of COVID-19, particularly in patients with moderate to severe COVID-19, the classes of drugs reported to be used are antiviral agents, inflammation inhibitors/antirheumatic drugs, low molecular weight heparins, plasma, and hyperimmune immunoglobulins. Nirmatrelvir/ritonavir (“Paxlovid”) is a combination protease inhibitor that blocks replication of SARS-CoV-2 (the virus that causes COVID-19) and has been shown to reduce the risk for hospitalization and death among patients with mild to moderate COVID-19 who are at risk for progression to severe disease. There are complications experienced from this product including hospitalizations, drug interactions and mixed effectiveness [23].
Food Supplements for COVID-19 Recovery
Many COVID-19 patients experience fatigue, extreme tiredness and symptoms that persist beyond the active phase of the disease. Nutritional protocols and supplements that can mitigate, alleviate, or relieve the associated chronic fatigue, gastrointestinal disorders and continuing inflammatory reactions. Naturally occurring food supplements, such as acetyl L-carnitine, hydroxytyrosol and vitamins B, C and D have shown significant promise in the management of post-COVID syndrome. Food supplementation can aid the recovery of patients with long COVID [24].
Health and Wellness Measurements
A proof-of-concept study with data has shown the feasibility for a wearable, wireless pressure sensor to provide real time monitoring in the intensive care unit setting for COVID-19 patients [25].
Introduction to this Study
Previously we have studied the etiology of COVID-19 and various natural products on peoples’ wellbeing and coping with infection [4] In July, 2021 we published a pre-clinical trial which demonstrated an electricidal drink could help people cope with a number of issues including rapid recovery from COVID-19 symptoms (fever, cough, congestion, breathing/lung issues, runny nose, brain fog, fatigue and loss of smell in 6 days associated with COVID-19 including SARS-CoV-2 infection as well as depression, anxiety, pain, arthritis, asthma, heart irregularities , sleep, memory loss, and stomach issues in the general population [1].
Recently we demonstrated phytotherapy management in a vaccinated patient with peripheral vascular disease and its effect on arterial blood flow obstruction which had positive results in 7- days in decreasing the effects of blood clots presumably formed due to previous COVID-19 vaccination [15]. The objective of this study was to evaluate the effectiveness of Electrocide in liquid and capsule form along with seeing if there is subsequent benefit(s) to using a combined enzyme and liver, gallbladder, kidney and lymphatic system detoxification process to improve the outcomes from Long-Haul COVID-19, post-Vaccine symptoms and after-effects of COVID-19 infection.
Supplement Information
The Electrocide
ELECTROCIDE® liquid Ingredients: Alumina, Boron, Calcium, Chromium, Copper, Iron, Magnesium, Manganese, Potassium, Phosphorous, Treated Energy Water, Silicon, Sodium, Zinc, and other trace elements as found in natural seawater.
ELECTROCIDE® CAPSULE Ingredients: Calcium, Iodine, Iron, Manganese, Magnesium, Molybdenum, Selenium, Zinc, additional trace ingredients including chromium, brown rice, vanadium, watercress, and other herbs Electrocide has been shown to be a broad-spectrum anti-pathogen product in the form of a heterogenous powder or liquid form based upon laboratory studies including examples of viral, bacterial, and fungus samples that are 99.9999% annihilated through this process [1]. The Electrocide is the ceramic-based particle that’s inside the liquid slurry product and the dry form as a powder in a capsule. The Electrocide particle in an aqueous environment creates a very strong positive charge (40%+). It has been demonstrated that negatively charged particles to it. When the negative particle and positive particle come together, there’s a presumed reaction similar to an electrical discharge that creates a cellular disruption in the negative charged particle [1]. Healthy cells have somewhat neutral and/or positive charge. Primarily all pathogens, whether they are bacterial or viruses or protozoa, or fungus have a net negative charge, and when the positive charged Electrocide product comes in contact with the negative charge, it has been shown to destroy the pathogen [1] Based on in vitro studies the pathogen is literally ripped apart and those small remnant pieces are hypothesized to stimulate the Immune System to develop immunity against the destroyed pathogen creating an automatic vaccine process. Healthy cells, it is presumed are not affected by Electrocide.
When taking the product, whether it’s in the liquid form or in the capsule form, there is an increase in the dissolved oxygen. In the lab, almost a doubling of the dissolved oxygen upon adding Electrocide [1]. Increased oxygen can improve the oxidative process throughout the body. It allows oxygen to the blood, so that when people with COVID-19 were experiencing cytokine storms as soon as they would take Electrocide, their blood oxygen would go up and they would go out of the cytokine storm into a healing situation.2 There have been numerous reports of a number of people who had pneumonia from cytokine storm, the pneumonia was gone in a matter of the days after using the Electrocide dietary supplement, sometimes in as short duration of a day and a half, symptoms would be all gone [2]. But also, the Electrocide is theorized to create a process in the blood that basically improves the blood’s ability to kill the pathogens as well, begin a healthy transformation called the hypochlorous acid process (a chlorine dioxide product) [1]. The chlorine dioxide simulates a natural antipathogen process in the blood. So, when the Electrocide has an interaction with the pathogen, it is thought to be stimulating the hypochlorous acid process that allows for the whole body to go into the killing mode of the pathogen. The whole body in only a few minutes is now transformed because of the presence of hypochlorous acid. This process engages phagocytosis but also transforms if people have been in a long-term chronic inflammation cycle into the healing acute inflammation. This immediately starts decoupling that infectious process by stimulating healing. Acute inflammation is also known as healing, also has been shown to begin almost immediately once the person takes the Electrocide as proven with microscopic studies [1]. Electrocide has been demonstrated anecdotally to increase healing in people who cut their hand in only days rather than weeks. Electrocide, also as a topical antipathogen has been seen to reduce fungal and bacterial infections where the direct action of reversing the inflammation occurs by the aspect of the Electrocide. The Electrocide is postulated to form changes in the solution ions, those ions are further activated in terms of their electrical potential in terms of healing [2]. The Electrocide most likely engages the healing process, transforming a chronic inflammation into acute inflammation by dealing directly with killing the pathogens and increasing the dissolved oxygen that improves the healing process.
Protein Enzyme
Protein Enzyme Capsules Ingredients: Enzymes: serrapeptase, nattokinase, lumbrokinase, tryptophan, protease, amylase, glucoamylase are mixed with willow bark, dandelion, milk thistle, fulvic acid, sheep sorrel, burdock root, slippery elm, tudca, brown rice and other proprietary trace ingredients. Protein Enzyme product contains nattokinase, serrapeptase and lumbrokinase in a proprietary mixture which have been shown to have benefits for heart, gut, brain, blood vessel, nerve and lung health. Nattokinase has potent fibrinolytic activity, antihypertensive, anti-atherosclerotic, and lipid-lowering, antiplatelet, and neuroprotective effects [26]. The role of mucolytics, for example proteolytic drug of natural origin, serratiopeptidase, has been suggested to protect the body from respiratory pathogens ascribed to their expectorant action, and are considered important as an adjuvant in the management of COVID-19 [27]. Earthworm protease (Lumbrokinase) functions in anti-thrombosis by its fibrinolytic activity and inhibiting platelets aggregation, and anti-fibrosis by its decreasing fibronectin, collagen and laminin, showing a broad substrate specificity [28].
There are detox programs [29] for health concerns like arthritis, allergies/asthma, candida, GERD, infertility, and weight loss. The Protein Enzyme product used here also has detoxes for liver, kidneys, gallbladder and lymphatic system and amino acids and other dietary components that improve the blood flow. What has been reported is that clogged capillaries become unclogged and brain and heart function is totally changed. Silymarin is a bioflavonoid complex extract derived from dry seeds of Milk thistle (Silybum marianum) has low toxicity, favorable pharmacokinetics, powerful antioxidant, detoxifying, preventive, protective and regenerative effects and side effects similar to placebo make silymarin extremely attractive and safe for therapeutic use [30]. The medicinal properties of silymarin have been studied in the treatment of Alzheimer’s disease, Parkinson’s disease, sepsis, burns, osteoporosis, diabetes, cholestasis, and hypercholesterolemia. Silymarin is a neuro-, nephro- and cardio-protective in the damage of different etiologies due to its strong antioxidant potentials. Protein Enzyme herbal products including dandelion have also been reported to improve the cognitive capacity as postulated by improving brain function, neural function, blood flow and blood oxygenation [31]. In vitro and in vivo scientific investigations have confirmed dandelion’s pharmacological potential by showing antioxidant, antibacterial, anti-inflammatory, antiviral, cytotoxic, diuretic and hepatoprotective properties. Responsible for these activities are bioactive metabolites belonging to different classes, including sesquiterpenoids, caffeoylquinic acids and flavonoids. Several people who have previously presented with dementia, families report no longer have dementia after use.
Protocol (Table 1).
Directions for Taking Electrocide® Drink: Take on an empty stomach: No food, supplements or medications at least 30 minutes to one hour before and after taking the Electrocide® Drink. Best not to take on an Overnight empty stomach. Better to take 1 hour after breakfast or another meal or snack, to avoid any queasiness that can be associated with taking vitamins or minerals in a completely empty stomach. Bedtime is a good time to take, as the body has time to rest and heal, but products can be taken any time of the day or night. Shake bottle well and drink entire contents: Add more water, shake again and drink to make sure that all solution is taken from the bottle. Plain water can be consumed after taking the ELECTROCIDE ® Drink. Do not take dairy or citrus. Hold the ELECTROCIDE® drink in your mouth and swish around for 20 seconds or so before swallowing. It is a good idea to ADD SUGAR: a small amount (equivalent of approximately 1/2 a sugar packet) of raw sugar, coconut sugar, maple syrup, date syrup or agave but NOT stevia, should be added to the bottle to counteract any queasiness from taking minerals on an empty stomach and enhance absorption. If you take the drink and feel queasy, take some extra sugar, maple syrup etc. from a spoon and it should quickly help. Be sure to open the bottle carefully and not spill any of the ELECTROCIDE® Drink when adding in sweetener. If you need more room in the bottle, take a sip to make more room than add sweetener, shake and take immediately.
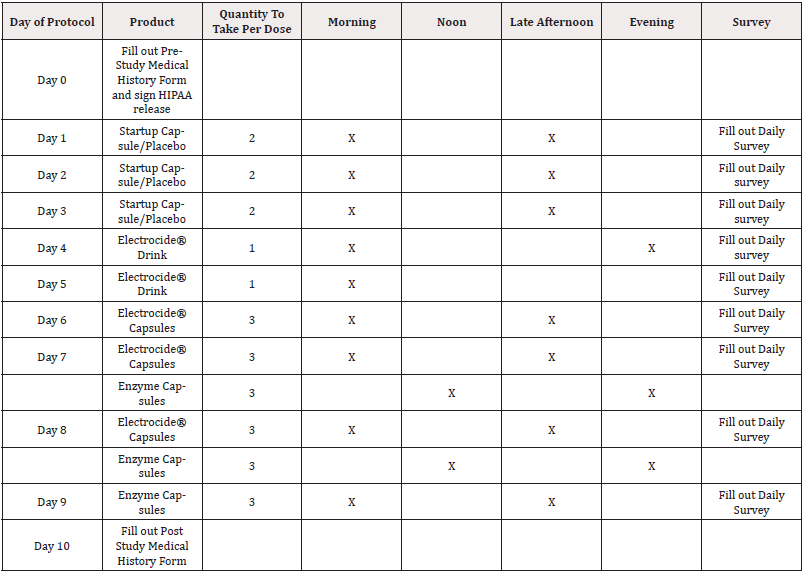
Table 1: Study Protocol as presented to the participants without control/placebo info.
Note*: Day1-4= Control Days| Day 5= Electrocide® Drink Day| Day 6-8= Electrocide® and Enzyme Capsules| Day9- Enzyme Capsule.
***If you weigh 180 pounds or more, you will be given (1) Electrocide® 4X Drink to equal the (1) dose mentioned above.
***Stop taking any magnesium and zinc supplements while taking this protocol.
Directions for Taking Startup, Electrocide® Capsules and Enzyme Capsules: Take at least 30 minutes away from other, food, supplements, and medications.
***IMPORTANT: Drink A FULL 8 ounces of water with the capsules to ACTIVATE them.
Study Parameters
a) 15 adult participants were randomly chosen to participate out of a larger pool of volunteers. Pre- and Post- Study interviews were performed independently with each participant and Daily VAS scale surveys were filled out evaluating wellness conditions. Each participant filled out a HIPAA release and received detailed protocol and product information.
b) Control measurements with placebo products were taken during the first 3 days of trial,
c) Protocol design was approved and supervised by a medical doctor.
d) Data were tabulated and student t-tests were run to evaluate statistical significance of data with Exploratory Statistical Significance noted at 80% confidence level (p=0.2).
Pre-Study Findings
(Figures 1,2)
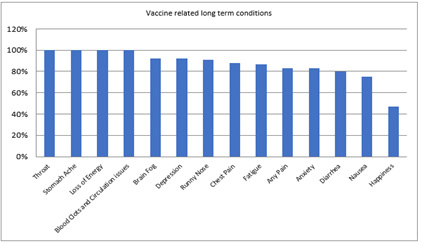
Post Vaccine Related Issues of Those Participants in this Research Study: 86% were Pfizer Vaccinated while 14% were Johnson & Johnson Vaccinated (Figure 3).
Note*: 43% had immediate post-vaccine reaction including swollen lymph nodes, pain and fever which generally subsided within a few weeks after injection(s).
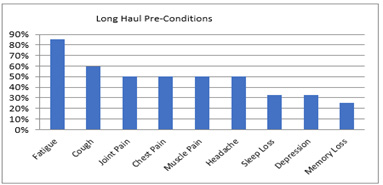
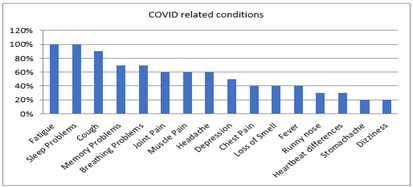
Figure 1: Long Haul Pre-Conditions Summary of pre-survey findings.
Note*: Long Haul Pre-Conditions rank order; n=8.
Results
Recovery Data and Statistics
Post Vaccine Results: From the results analysis, the trends with confidence level of 80%, we can see that the protocol had effects on patients with other Illnesses, Shortness of Breath, High Blood Pressure, Arm Pain, Depression/Anxiety and Diabetes (Table 2).
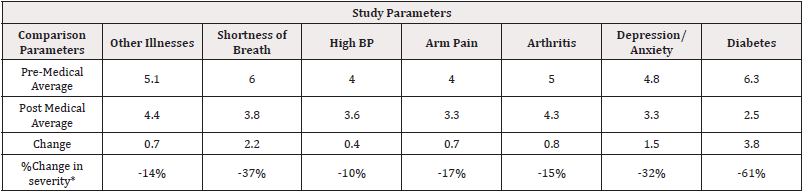
Table 2: Medical Exploratory Statistical Significance Pre-Post Data.
Note*: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
Long Haul Recovery Data: Paired t-test analysis comparing Pre- and Post- Long haul COVID symptoms among patients showed significant difference (p=0.02) while trends with 80% confidence indicate that the protocol had beneficial effects on patients with symptoms for Fatigue, Cough, Memory/Concentration Problems, Sleep Problems, Muscle Pain, Headache, Depression/Anxiety symptoms as shown in (Table 3).
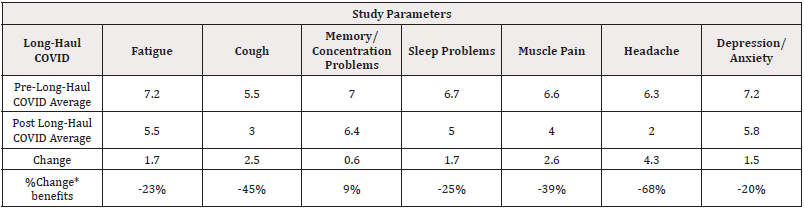
Table 3: Pre-Long Haul COVID versus Post Long Haul COVID Exploratory Statistical Significance effects after 7-Day Program.
Note*: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
10-Day Protocol Exploratory Statistical Significance Comparisons
Effects of Liquid Electrocide Compared to Control: On the 10-day protocol, the first three days were control while patients were served with Electrocide drink on day 4 and 5, Electrocide and Enzyme capsule were served on day 6- 8 while Enzyme capsule were given on day 9 as shown in (Table 1). With exploratory significance (80% level of confidence), patients were less sleepy, tired, or fatigued after using the Electrocide drink as compared to the control days. The same trend was observed for patients who reported lesser throat issues, less chest pain and less brain fog or inability to think clearly in the symptoms compared to the control day as shown in (Table 4).
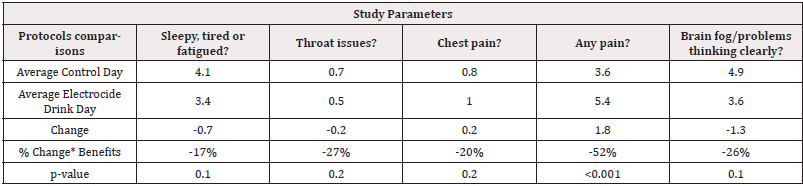
Table 4: Control days versus Electrocide Drink Exploratory Statistical Significance comparisons.
Note*: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
Effects of Capsule: With 80% level of confidence using exploratory statistical significance comparisons, the results show that patients were less nauseous, had less pain, diarrhea, runny nose and sleepy, tired or fatigued after using the Electrocide capsule compared to using the Electrocide drink as shown in (Table 5).
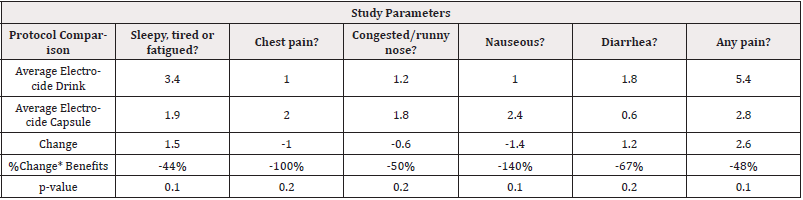
Table 5: Electrocide drink versus Electrocide Capsules Exploratory Statistical Significance comparisons.
*Note: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
Electrocide Liquid Versus Electrocide & Enzyme Capsule Effectiveness: With 80% level of confidence using exploratory statistical significance comparisons 15-19% improvements, the trends show that patients were less fatigued, better able to breathe and less nauseous when they used the Electrocide capsule, and Protein Enzyme as compared to using the Electrocide drink as noted in Table 6. Additionally, patients reporting 37-200% improvement in Pain reduction, control of Diarrhea, reduction of Nausea and improved circulation with changes shown in (Table 6).
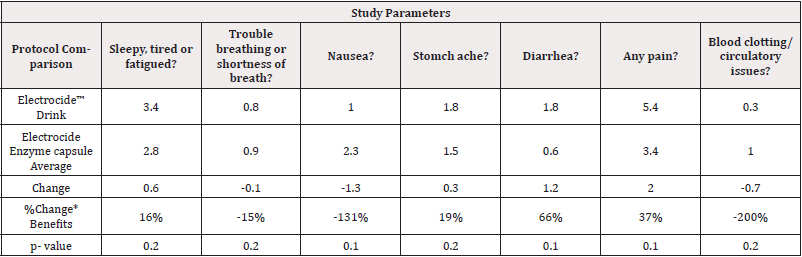
Table 6: Electrocide drink versus Electrocide Enzyme Capsules Exploratory Statistical Significance comparisons.
Note*: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
Comparison of Electrocide Liquid and Capsules + Enzymes to Control Days: There was a statistically significant change in any pain observed by patients after using the Electrocide and Enzyme capsule as compared to the control days as shown in (Table 7). The patients also were less sleepy, tired or fatigued as compared to control and the observation was statistically significant. The patients further reported to be less nauseous after use of the Electrocide and Enzyme capsule with statistically significant change noted as shown in (Table 7). With 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients had fewer headaches, observed reduction in the shortness of breath and observed improvement in blood circulation issues and less blood clotting as shown in (Table 7). Statistically significant changes were noted p<0.05 or less for Pain, Fatigue and Nausea.
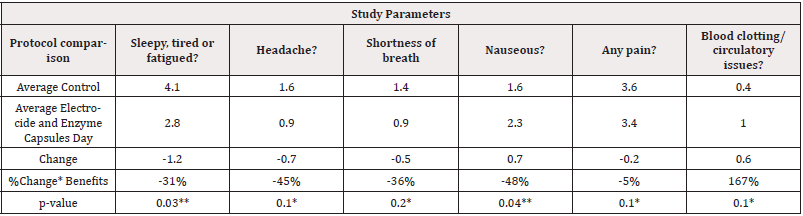
Table 7: Control Versus Electrocide and Enzyme Capsules Exploratory Statistical Significance comparisons.
Note*: *80% exploratory statistical significance level, ** p<0.05 significant.
Comparison of Control (Day1-4) to Final Day 9 Total Program: With 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients had less throat issues, reduction in the congestion or running nose, less any other pain and also less brain fog and were able to think more clearly when they used the Enzyme capsules in the final day of the study as compared to the control days as shown in (Table 8).
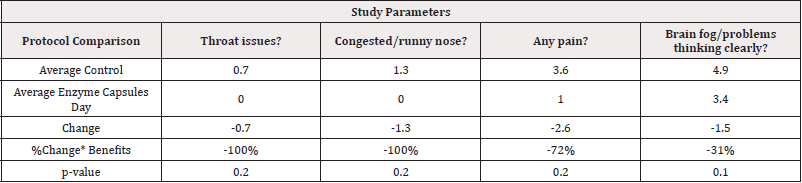
Table 8: Control Versus Enzyme Capsules (summary effect) Exploratory Statistical Significance comparisons.
Note*: *80% exploratory statistical significance level Legend: (-) =decreased symptoms. (+) =improved conditions.
Effects of Protein Enzyme
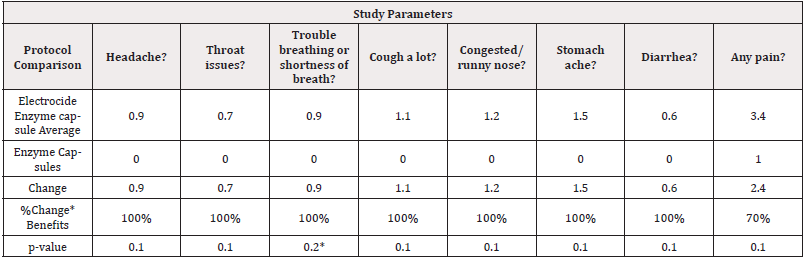
Day 6-8 Electrocide Treatment Compared to Day 9 Enzymes Only: When comparing observation between Electrocide & Enzyme capsules versus the Enzyme capsules alone, with an 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients reported 100% stomachache, headache disappear, disappearance breathing or shortness of breath, no longer coughing, did not have congestion or running nose, and diarrhea also with a 70% reduction in pain overall as shown in (Table 9).
Medical Conditions
Pre- and Post-Treatment Interview results (n=15):
Pre-Treatment
a) 100% Shortness of Breath
b) 100% Pain
c) 50% Weakness/Fatigue
Post-Treatment
a) Increased Energy
b) Increased Oxygen
c) Better Breathing
d) Better Sleep
e) Significant Pain Improvement
f) Increased Sense of Wellbeing
Discussion
Medical Significance Pre-Post Data
100% of the participants noted a significant increase in energy. 88% of the participants noted a significant decrease in pain. 75% of the participants noted a significant improvement in sleep. As in a previous study [1], the elimination of active COVID-19 infection (diagnosed by fever, coughing, runny nose, brain fog, headache, and pain) can be achieved in an average of 6 days with the use of Electrocide ®. The current study furthermore demonstrates the effectiveness of the Electrocide® as a 7-day treatment for post-COVID infection. A specially formulated enzyme combination with the regimen of Electrocide® (in capsule and liquid form) was evaluated with a placebo control and it was found that there was rapid recovery from loss of energy, pain, brain fog, with improved sleep other symptoms associated with Long Haul, Chronic COVID-19 and Vaccine Effects including peripheral neuropathy, eye blurriness, and heart issues were improved with the addition of Protein Enzyme formulation. Use of the Electrocide liquid showed rapid recovery response in the first 2 days with sequential benefit from the capsule version of Electrocide. From the medical results analysis, the trends with exploratory confidence level of 80%, the protocol had effects on patients with other Illnesses, Shortness of Breath, High Blood Pressure, Arm Pain, Depression/Anxiety and Diabetes as shown in (Table 2).
Long Haul Recovery Data
Comparing Pre- and Post- Long Haul COVID symptoms among patients showed exploratory significant difference (p=0.02) with 80% confidence indicating that the protocol had beneficial effects on patients with Fatigue, Cough, Memory/Concentration Problems, Sleep Problems, Muscle Pain, Headache, Depression/Anxiety symptoms as shown in (Table 3).
Discussion of Treatment Effectiveness
On the 10-day protocol, the first three days were controlled while patients were served with Electrocide drink on day 4 and 5, Electrocide and Enzyme capsule were served on day 6- 8 while Enzyme capsule were given on day 9 as shown in (Table 1).
Effects of Liquid Electrocide Compared to Control: With exploratory significance (80% level of confidence), patients were less sleepy, tired, or fatigued after using the Electrocide drink as compared to the control days. The same trend was observed for patients who reported lesser throat issues, less chest pain and less brain fog or inability to think clearly in the symptoms compared to the control day as shown in (Table 4).
Effects of Electrocide Capsule compared to Electrocide Drink: With 80% level of confidence using exploratory statistical significance comparisons, the results show that patients were less nauseous, had reduced pain, diarrhea control, runny nose relief and less sleepy, tired or fatigued after using the Electrocide capsule compared to using the Electrocide drink as shown in (Table 5).
Electrocide Liquid versus Electrocide & Enzyme Capsule combination Effectiveness: With 80% level of confidence using exploratory statistical significance comparisons trends show that patients were less fatigued, better able to breathe and less nauseous when they used the Electrocide capsule, and Protein Enzyme as compared to using the Electrocide drink as noted in Table 6. Additionally, patients reporting greater improvement in Pain reduction, control of Diarrhea, reduction of Nausea and improved circulation compared to Liquid Electrocide alone as shown in (Table 6).
Comparison of Electrocide Liquid & Capsules + Enzymes to Control Days: There were statistically significant changes (p<0.05) in Pain, Fatigue and Nausea observed by patients after using the Electrocide and Enzyme capsules as compared to the control days as shown in (Table 7). With 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients had fewer headaches, observed reduction in the shortness of breath and observed improvement in blood circulation issues/less blood clotting as shown in (Table 7).
Comparison of Control (Day1-4) to Final Day 9 total program: With 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients had less throat issues, reduction in the congestion or running nose, less any other pain and also less brain fog and were able to think more clearly when they used the Enzyme capsules as compared to the control days as shown in (Table 8).
Effects of Electrocide + Protein Enzyme compared to Day 9 Enzymes Only: When comparing observation between Electrocide & Enzyme capsules versus the Enzyme capsules alone, with an 80% level of confidence using exploratory statistical significance comparisons, the trend shows that patients reported 100% stomachache relief, headache disappearance, restoral of normal breathing, no longer coughing, did not have congestion or running nose, no diarrhea also with a 70% reduction in Pain overall as shown in (Table 9). Overall implications of this study are that this combined Electrocide and Protein Enzyme protocol is a safe (no side-effects were reported) and effective method for improving the wellbeing of COVID-19 sufferers both in the infections stage [1], post-COVID-19 and COVID-19 vaccine recovery phases from the various effects. Additionally, many people have poor gut conditions, and those poor gut conditions lead to problems not only in the gut, but problems throughout the rest of the body. It is hypothesized that annihilation of pathogens in the gut and providing better nutrition health and wellbeing can be restored for many [32].
Conclusions
The current study furthermore demonstrates the effectiveness of the Electrocide® as a 7-day treatment for Post-COVID infection [2] A specially formulated enzyme combination with the regimen of Electrocide® (in capsule and liquid form) was evaluated with a placebo control and it was found that there was rapid recovery from a number of symptoms associated with Long Haul, Chronic COVID-19 and Vaccine Effects including peripheral neuropathy, eye blurriness, and heart issues. In this study, by combining the uses of Electrocide and Protein Enzyme as a program with the many of the symptoms of COVID-19, COVID-19 vaccine and COVID-19 Long Haul were ameliorated which suggest that these findings may be used as a basis for other wellness protocols [33].
Conflict of Interest
There are no conflicts of interest for any of the parties involved.
Acknowledgements
Many thanks to George Madiou, Dr. Glen Rein, Lawrence Salvo, Karen Stein, Joel Westermarck and the voluntary participants for their invaluable help to make this research project a reality!.
References
- Leonard Sonnenschein, Tiberious Etyang, Rutu Shah, Ruth Frischer, Glen Rein, et al. (2021) The Effect of An Aqueous Electricidal Solution on General Well Being. Am J Biomed Sci & Res: 13(4).
- Khan M, Khan H, Khan S, Nawaz M (2020) Epidemiological and clinical characteristics of coronavirus disease (COVID-19) cases at a screening clinic during the early outbreak period: a single-centre study. J Med Microbiol 69(8): 1114-1123.
- Oleynick C (2020) Symptoms of Pleurisy as the Initial Presentation of COVID-19. Am J Case Rep 24(21): e925775.
- Sonnenschein L, Weinberg R, Cotter HT, Salvo LA, Etyang T, et al. (2020) Historical and Current Conditions Including COVID-19 Requiring Anti-Pathogenic Surveillance and Proper Immunogenic Support: 8(4).
- Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos N O, et al. (2021) Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 13(4): 700.
- Jain U (2020) Effect of COVID-19 on the Organs. Cureus 12(8): e9540.
- Selvaraj K, Ravichandran S, Krishnan S, Radhakrishnan RK, Manickam N, et al. (2021) Testicular Atrophy and Hypothalamic Pathology in COVID-19: Possibility of the Incidence of Male Infertility and HPG Axis Abnormalities. Reprod Sci 28(10): 2735-2742.
- Varney JA, Dong VS, Tsao T, Sabir MS, Rivera AT, et al. (2022) COVID-19 and arrhythmia: An overview. J Cardiol 79(4):468-475.
- Alsaied T, Aboulhosn JA, Cotts TB, Daniels CJ, Etheridge SP, et al. (2020) Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J Am Heart Assoc 9(12): e017224.
- Gaba WH, Ahmed D, Al Nuaimi RK, Dhanhani AA, Eatamadi H, et al. (2020) Bilateral Central Retinal Vein Occlusion in a 40-Year-Old Man with Severe Coronavirus Disease 2019 (COVID-19) Pneumonia. Am J Case Rep 29(21): e927691.
- Daiana Roxana Pur, Lulu Liane Catherine Danielle Bursztyn, Yiannis Iordanous (2022) Branch retinal vein occlusion in a healthy young man following mRNA COVID-19 vaccination. American Journal of Ophthalmology Case Reports 26: 101445.
- Najafloo R, Majidi J, Asghari A, Aleemardani M, Kamrava SK, et al. (2021) Mechanism of Anosmia Caused by Symptoms of COVID-19 and Emerging Treatments. ACS Chem Neurosci 12(20): 3795-3805.
- Andrea XP, Joceline LM, Jose OF, Jose PO (2023) Human Nasal Epithelium Damage as the Probable Mechanism Involved in the Development of Post-COVID-19 Parosmia. Indian J Otolaryngol Head Neck Surg 14: 1-7.
- Astin R, Banerjee A, Baker MR, Dani M, Ford E, et al. (2023) Long COVID: mechanisms, risk factors and recovery. Exp Physiol. 108(1): 12-27.
- Selene Manga, Scott Weiss, Shirley Zelikovsky, Leonard Sonnenschein (2023) Phytotherapy Management in a Vaccinated Diabetic Patient with Foot Ulceration and Peripheral Vascular Disease: A Case Study of a Dietary Supplement and Its Effect on Arterial Blood Flow.
- Carod Artal FJ (2021) Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol 72(11): 384-396.
- Kamal M, Abo Omirah M, Hussein A, Saeed H (2021) Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 75(3): e13746.
- Finsterer J (2022) Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand 145(1): 5-9.
- Zheng X, Fang Y, Song Y, Liu S, Liu K, et al. (2023) Is there a causal nexus between COVID-19 infection, COVID-19 vaccination, and Guillain-Barré syndrome?. Eur J Med Res 28(1): 98.
- Fenoglio R, Lalloni S, Marchisio M, Oddone V, De Simone E, et al. (2022) New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am J Nephrol 53(4): 325-330.
- Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Gentile N, et al. (2022) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 328(16): 1595-1603.
- Stasi C, Fallani S, Voller F, Silvestri C (2020) Treatment for COVID-19: An overview. Eur J Pharmacol 889: 173644.
- Malden DE, Hong V, Lewin BJ, Ackerson BK, Lipsitch M, et al. (2022) Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment - California, December 2021-May 2022. MMWR Morb Mortal Wkly Rep 71(25): 830-833.
- Naureen Z, Dautaj A, Nodari S, Fioretti F, Dhuli K, et al. (2021) Proposal of a food supplement for the management of post-COVID syndrome. Eur Rev Med Pharmacol Sci 25(1 Suppl): 67-73.
- Franz CK, Murthy NK, Malik GR, Kwak JW, D Andrea D, et al. (2022) The distribution of acquired peripheral nerve injuries associated with severe COVID-19 implicate a mechanism of entrapment neuropathy: a multicenter case series and clinical feasibility study of a wearable, wireless pressure sensor. J Neuroeng Rehabil 19(1): 108.
- Hongjie Chen, Eileen M McGowan, Nina Ren, Sara Lal, Najah Nassif, et al. (2018) Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomarker insights 13: 1177271918785130.
- Charu Sharma, Niraj Kumar Jha, MF Nagoor Meeran, Chandragouda R Patil, Sameer N Goyal, et al. (2021) Serratiopeptidase, a serine protease anti-inflammatory, fibrinolytic, and mucolytic drug, can be a useful adjuvant for management in COVID-19. Frontiers in Pharmacology 12: 603997.
- Xiu Mei Wang b, Shi Chao Fan b, Yao Chen a b, Xiao Feng Ma c, et al. (2019) Earthworm protease in anti-thrombosis and anti-fibrosis. Biochimica et Biophysica Acta (BBA)-General Subjects 1863(2): 379-383.
- Linda Page (2008) Healthy Healing's Detoxification: Programs to Cleanse, Purify & Renew published by Healthy Healing, Inc.
- Nataša Milić, Nataša Milošević, Ljiljana Suvajdžić, Marija Žarkov, Ludovico Abenavoli, et al. (2013) New Therapeutic Potentials of Milk Thistle (Silybum marianum). Natural product communications 8(12): 1801-1810.
- Laura Grauso, Stefano Emrick, Bruna de Falco, Virginia Lanzotti, Giuliano Bonanomi, et al. (2019) Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochemistry Reviews 18: 1115-1132.
- Tao Zuo, Xiaojian Wu, Weiping Wen, Ping Lan (2021) Gut microbiome alterations in COVID-19. Genomics, proteomics & bioinformatics 19 (5): 679-688.
- Jha NK, Ojha S, Jha SK, Dureja H, Singh SK, et al. (2021) Evidence of Coronavirus (CoV) Pathogenesis and Emerging Pathogen SARS-CoV-2 in the Nervous System: A Review on Neurological Impairments and Manifestations. J Mol Neurosci 71(11): 2192-2209.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.