Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Effect of Vitamin D Status on Thyroid Dysfunction in Patients with COVID-19
*Corresponding author: Manana Machitidze, The University of Georgia, Tbilisi, Georgia.
Received: May 15, 2023; Published: May 24, 2023
DOI: 10.34297/AJBSR.2023.18.002534
Abstract
Aim/objective: Our study aimed to explore thyroid function in covid-19 patients and its correlation with vitamin D deficiency.
Background: Several studies have found that abnormal thyroid function was standard in patients with COVID-9 and that thyroid stimulating hormone (TSH) suppression was associated with higher levels of markers of inflammation. On the other hand, there is evidence that vitamin D may modulate the secretion of TSH.
Methods: Thyroid function was assessed in 195 unvaccinated COVID-19 patients (the target group) who presented with complaints suggesting disease of the thyroid gland by measuring serum free thyroxine (FT4), TSH, and anti-thyroid peroxidase antibody (TPOAb), and by cervical ultrasonography; the results were corresponding to subacute thyroiditis (SAT). Vitamin D status was determined at the initial presentation by measuring serum D (25-OH) levels. The target group was divided into three subgroups: I subgroup 58 patients with severe vitamin D deficiency (<12.5 ng/ml), II subgroup: 65 patients with moderate vitamin D deficiency (12.5-20 ng/ml), and III subgroup: 72 patients with vitamin D insufficiency (20-30 ng/ml). In addition, serum C-reactive protein (CRP) was measured in all the patients. The control group included 15 healthy individuals. After one month of treatment with corticosteroids, all the patients recovered from the symptoms of SAT.
Results and Conclusions: In all three subgroups, the mean plasma TSH concentrations and FT4 concentrations were lower in COVID-19 patients compared to controls. Significant positive correlations were recorded between serum 25(OH)D and TSH, and negative correlations - between serum 25(OH)D and TF4 in the I and II subgroups. TPO-Ab was increased significantly in all three subgroups compared to controls, with a significant negative correlation with serum 25(OH)D in the I and II subgroups. Our study confirmed that low vitamin D levels could be associated with an increased risk of developing SAT in COVId-19 patients. However, it did not find a relationship between vitamin D deficiency and the severity of Covid-19 related thyrotoxicosis, nor it revealed that the severity of vitamin D deficiency affects the outcome of SAT in COVId-19 patients.
Keywords: COVID-19, Vitamin D, Thyroid stimulating hormone (TSH), Free thyroxine (FT4), Anti-thyroid peroxidase antibody (TPO-Ab), Subacute thyroiditis (SAT).
Introduction
SARS-COV-2 is a novel coronavirus responsible for the COVID-19 global world pandemic that began in late 2019. COVID-19 infection can range from asymptomatic or mild presentation to critical illness and death. Severe COVID-19 disease can be manifested by acute respiratory distress syndrome (ARDS) and multiple organ failure [1- 5]. During the previous coronavirus outbreak with SARS-COV, some studies reported changes in thyroid function [6-8]. SARS-CoV-2 uses angiotensin-converting-enzyme 2 (ACE2) receptor to infect the host cells [9,10]. The mRNA encoding for the ACE-2 receptor is highly expressed in thyroid follicular cells, making them a potential target for SARS-COV-2 [11]. According to autopsy studies, the virus can enter any endocrine gland, including the pituitary gland, thyroid, parathyroids, adrenals, pancreas, and testis, and lead to transitory or definitive dysfunction [12,13].
Studies of thyroid dysfunction in patients with COVID-19 showed that the thyroid gland and the entire hypothalamic-pituitary-thyroid (HPT) axis could be damaged by SARS-CoV-2. COVID-19-related thyroid disorders could manifest as thyrotoxicosis, hypothyroidism, and non-thyroidal illness syndrome. Serum levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4) have been significantly lower in patients with SARS-CoV compared with those in the control group, irrespective of phases (acute and convalescent) [6,7]. A positive correlation was found between the severity of SARS and a decrease in T3 levels [6]. Some studies have reported cases of COVID-19-related primary hypothyroidism [14] and found that in-hospital mortality was higher in hypothyroid patients with COVID-19 compared to COVID-19 patients with euthyroidism.
The underlying mechanism of COVID19related endocrine dysfunctions may be inflammation, vessel damage, necrosis, and autoimmune processes [12,13,15]. Several studies found that abnormal thyroid function (particularly hyperthyroidism) is standard in patients with COVID-9 and that TSH suppression is associated with higher levels of markers of inflammation [14,16]. Data from minimally invasive autopsies from SARS-CoV-2 patients reported no abnormalities in the thyroid follicular morphology but displayed lymphocytic infiltration in the interstitium [17]. Though in some studies, follicular epithelial cell disruption was also noted. Wei, et al. reported that autopsies of SARS cases revealed follicular epithelial damage in the thyroid gland, with large numbers of cells exfoliated into the follicle and undergoing apoptosis, which indicates the destructive effect of the virus on the thyroid gland [18]. The studies suggest that these changes result from cytolytic recognition by T cells of viral and cellular antigens in thyrocytes [19]. Some data suggest a higher risk of autoimmune disorders (including autoimmune thyroiditis and Graves’ disease) after recovery of SARSCoV2 patients from the cytokine storm [12]. It was observed that patients with acute coronavirus infection presenting with thyrotoxicosis had statistically significantly higher levels of interleukin6 (IL6) [16]. Chen, et al. [20] described central hypothyroidism (low FT4 with inappropriately low/normal TSH) secondary to SARS-CoV-2 injury at the hypothalamus or pituitary level of the HPT axis [20].
COVID-19-related non-thyroidal illness (NTI) syndrome with a reduced level of T3 in serum with decreased or inappropriately normal TSH levels has been reported by several studies [14,16,21,22]. This condition may result from either a direct effect of the SARS virus on the cells of the pituitary gland or an indirect effect caused by the hyperactivation of circulating pro-inflammatory cytokines due to the SARS infection. The NTIS is thought to be an adaptive physiological mechanism instead of true thyroid dysfunction. In the prolonged phase of critical illness, the NTIS has been shown to be significantly correlated with mortality. [22].
Thyroid hormone levels were found to have prognostic value for predicting the severity of COVID-19 disease [23,24]. Chen et al. reported that low serum levels of FT3, FT4, and TSH in COVID-19 patients during admission were positively correlated with the severity of COVID-19 disease and in-hospital mortality among the patients [1,25]. Low TSH levels were an independent risk factor for mortality in COVID-19 presenting with NTI [26]. Gao, et al. [27] found that FT3 was remarkably lower in severely ill COVID-19 patients compared to non-severely ill cases, and it strongly correlated with mortality. However, other thyroid functional characteristics, including FT4, TSH, and FT3/FT4, were not significantly linked [27]. One meta-analysis studied COVID-19 in patients with pre-existing thyroid disease, concluding that the presence of thyroid disease was positively correlated with a more severe degree of COVID-19 infection [28].
Vitamin D deficiency is a global health problem prevalent in both developed and developing countries and is determined by low serum 25-hydroxy vitamin D (< 25 nmol/l) levels. Vitamin D has been proven to play an essential role in the modulation of inflammatory pathways and immune responses [29-31]. Low vitamin D levels are significantly correlated with the development of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, type 1 diabetes mellitus, multiple sclerosis, and autoimmune thyroid diseases [32,33]. Vitamin D displays its effect via intracellular vitamin D receptors (VDR), which are expressed in monocytes/macrophages, T cells, B cells, natural killer cells (NK), and dendritic cells (DCs) [34]. It modulates the phagocytic activity of macrophages and NK cells [30] and induces the microbicidal activity of phagocytes [35]. Low levels of vitamin D are associated with an increase in inflammatory cytokines, TNF-alpha, and IL6 [36,37]. Vitamin D suppresses the differentiation and maturation of antigen presenting DCs. The inhibitory effect of vitamin D on DC maturation and differentiation is very similar to that of glucocorticoids [38-40]. It downregulates T helper type 1 cell (Th1 cell) immune responses by inhibiting the production of pro-inflammatory cytokines such as IL-12, IFN-γ, IL-6, IL-8 TNF-α, and IL-9, and it upregulates the production of type 2 anti-inflammatory cytokines such as IL-4, IL-5, and IL-10 [41-43]. Vitamin D enhances the maturation of Th2 cells and the activation of T regulatory cells [44]. Vitamin D inhibits B lymphocyte proliferation and differentiation [45]. Taken together, the overall effect of vitamin D is anti-inflammatory and helps to prevent autoimmune development.
The ligand for the nuclear vitamin D receptor (VDR) is expressed in many tissues, including benign and malignant thyroid tissue. Low vitamin D receptor expression in papillary thyroid cancer positively correlates with low serum vitamin D levels and disease aggressiveness [46]. Vitamin D protects human thyrocytes from programmed cell death via increased B-cell lymphoma 2 (Bcl-2) expression [47]. Both Hashimoto thyroiditis and Grave’s disease have been found to be associated with lower vitamin D levels [47-49]. The findings of several studies suggest that vitamin D has a crucial role in regulating the thyroid-destroying autoimmune antibodies and the pituitary hormone TSH [50,51]. Though some studies revealed no significant association between vitamin D and autoimmune thyroiditis [52,53].
In a retrospective cohort study, low vitamin D status was associated with increased COVID-19 risk [54]. COVID-19 patients with acute respiratory failure showed a high prevalence of hypovita minosis D. Severe vitamin D deficiency could be a marker of poor prognosis in COVID-19 patients and is linked to significantly higher mortality risk [15]. Vitamin D deficiency has been associated with a significantly increased risk of pneumonia and an increase in thrombotic episodes, frequently observed in COVID-19 [55]. Some studies suggest that vitamin D supplementation could provide clinical benefits in disease progression in patients with COVID-19, irrespective of its serum levels at the time of the disease onset. However, more extensive clinical trials are needed to confirm this [56].
Methodology
In this study, we aimed to explore the characteristics of thyroid function in covid-19 patients and its possible correlation with vitamin D deficiency. The study included 195 unvaccinated COVID-19 patients (the target group) with complaints suggesting disease of the thyroid gland (the patients were referred to Contractor clinics of the University of Georgia hospital with fever (more than 38°C), fatigue, palpitations, and anterior neck pain). The patients were admitted to the hospital from February 2020 to February 2021. The patient’s physical examination revealed a painful, tender, slightly enlarged thyroid gland. Thyroid function was assessed at hospital admittance by measuring free thyroxine (FT4), thyroid stimulating hormone (TSH), and anti-thyroid peroxidase antibody (TPO-Ab) by chemiluminescent assay (Immulite 1000®, Diagnostic Product Corporation, LA, CA, USA). Cervical ultrasonography was performed for each patient. Ultrasonic examination of the thyroid gland showed enlarged thyroid, multiple diffuse hypoechoic areas, and decreased vascularity. These changes were suggestive of subacute thyroiditis (SAT). Vitamin D status was determined at the initial presentation by measuring serum D (25-OH) levels utilizing the spectrophotometric method. Serum C-reactive protein (CRP) was also measured in all the patients.
We categorized the patients into three clinically relevant subgroups based on the serum 25(OH)D levels: I subgroup, 58 patients with severe vitamin D deficiency (<12.5ng/ml) (29.7% of the target group), II subgroup included 65 patients with moderate vitamin D deficiency (12.5-20ng/ml) (33.3% of the target group), and III subgroup included 72 patients with vitamin D insufficiency (20- 30ng/ml) (36.9% of the target group). The control group included 15 healthy individuals. None of the participants had a history of thyroid disease or was on drugs affecting thyroid function at enrolment. None received glucocorticoids, dopamine/dobutamine, or iodinated contrasts before blood sampling for thyroid function tests. They were not on vitamin D supplements. The follow-up tests were conducted two weeks after the first presentation.
Data were analyzed using SPSS 25.0 for Windows. Parameters were tested for normality with the Shapiro-Wilk test. Pearson correlation tests estimated correlations. All tests were 2-tailed, and a p-value of less than 0.05 was considered significant.
Results
(Table 1) The mean plasma TSH and FT4 concentrations in all three subgroups were lower in COVID-19 patients than in controls. Significant positive correlations were recorded between serum 25(OH)D and TSH, and negative correlations - between serum 25(OH)D and TF4 in the I and II subgroups. TPO-Ab was increased significantly in all three subgroups compared to controls, with a significant negative correlation with serum 25(OH)D in the I and II subgroups (Figures 1,2).
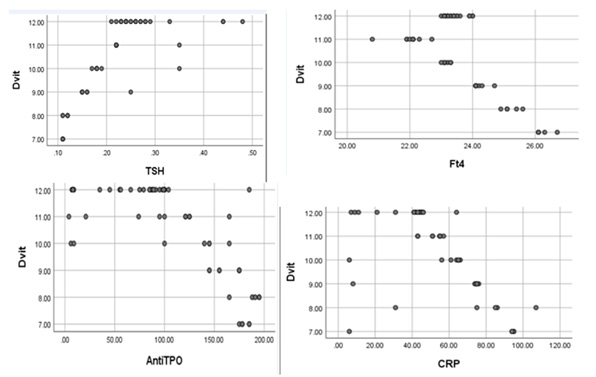
Figure 1: The first subgroup has correlations between the Vitamin D level and TSH, Ft4, TPO-Ab, and CRP.
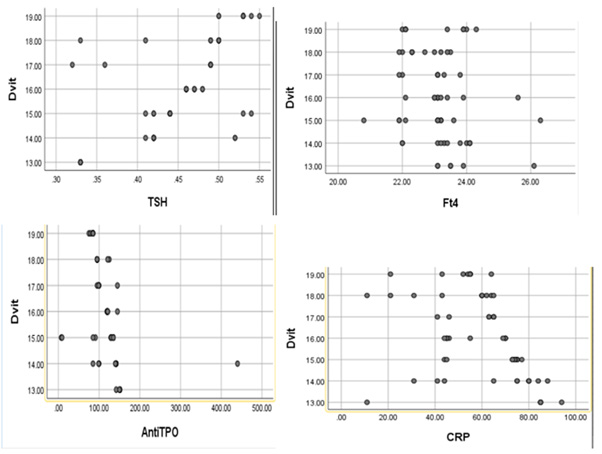
Figure 2: Correlations between the Vitamin D level and TSH, Ft4, TPO-Ab, and CRP in the second subgroup.
After the diagnosis of SAT, all the patients (except those with atypical thyroiditis) received corticosteroid treatment, while some were given ibuprofen for severe neck pain. After one month from the admittance, all the patients recovered from the symptoms.
Discussion
SARS-CoV-2 infection has been reported to have detrimental effects on multiple organ systems, including the hypothalamic-pituitary- thyroid (HPT) axis. Evidence suggests that thyroid function tests are altered during COVID-19, but the involved pathophysiological mechanisms still need to be clarified. Two mechanisms most likely might account for the changes in the thyroid gland observed in SARS-CoV-2 patients: An indirect effect via systemic inflammatory- immune responses to SARS-CoV-2 infection and a direct viral effect of SARS‐CoV‐2 virus.
SARS-CoV-2 could cause an increased immune response of T helper lymphocytes (Th1/Th17) that results in the activation of pro-inflammatory cytokines, including interleukins (IL1-IL6) and tumor necrosis factor α (TNFα). The release of large amounts of pro-inflammatory cytokines, described as cytokine storm syndrome, correlates with lung injury, multi-organ failure, and poor prognosis of severe COVID-19 cases. Increased immune responses of T helper lymphocytes and IL17-mediated cytokine signaling, associated with autoimmune thyroid disorders, have been detected in SARS-CoV-2 infections. [23]. Elevation of IL6 was detected in the acute phase, and it was significantly associated with thyrotoxicosis [48]. High IL6 is associated with low free triiodothyronine (FT3) [6].
Possible direct viral damage to the thyroid gland by the SARSCoV‐ 2 virus involves its interaction with ACE2 receptors. ACE2 is believed to play an essential role in the pathogenesis of coronavirus lung injury. SARS‐CoV and SARS‐CoV‐2 use ACE2 receptors in the thyroid gland as well as in the kidneys, adrenal glands, adipose tissue, endothelium, pancreas, testes, ovaries, and human pituitary gland to invade the host cells [9,10,57]. SARS‐CoV and SARS‐CoV‐2 use ACE2 receptors on thyroid cell membranes to enter the cell.
Chen et al. detected significantly lower TSH and TT3 in COVID-19 patients than in healthy control individuals and non- COVID-19 patients with pneumonia [58]. Moreover, the study found that the degree of the decrease in TSH and TT3 levels was positively correlated with the severity of the disease. TT4 level was not significantly different from the control group. After recovery without thyroid hormone replacement therapy, all the patients restored average values of thyroid hormones (TSH, FT3, FT4, TT3, and TT4).
Many studies reported subacute thyroiditis (SAT) associated with SARS-CoV-2 [59-62]. There have been reports of COVID-19-related SAT in patients who were not critically ill [63,64]. Also known as de Quervain thyroiditis, it is an inflammatory thyroid gland disease caused by a viral infection. It is typically characterized by three consecutive phases: 1. thyrotoxicosis during the first few months, 2. hypothyroidism for about three months, and 3. final restoration of euthyroidism [61]. SAT presents neck pain of varying degrees in the region of the thyroid gland, and a prodrome of generalized myalgias, low-grade fever, fatigue, and symptoms of upper respiratory inflammation characterizes it. In the initial phase, many patients present with clinical manifestations of mild to moderate thyrotoxicoses, such as tremors and palpitations [14]. The phase of thyrotoxicosis is usually followed by a phase of (transient) hypothyroidism with low free T4 and high thyroid stimulating hormone (TSH) levels. In most patients, euthyroid status is restored within 6-12 months. However, persistent hypothyroidism is observed in 10-15% of patients [65].
On SAT, thyrotoxicosis is caused by the release of preformed thyroid hormone into the circulation from the damaged thyroid follicular cells [66]. This causes transient thyrotoxicosis that most often self-resolves. Whether SAT represents a complication of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 is still unclear. Suppressed serum TSH, with elevated free thyroxine concentrations in our target group patients with SAT, indicated thyrotoxicosis. Our results are consistent with the study of Lania A, et al. [16] reporting that thyrotoxicosis is common in patients with COVID-19 [16]. Thyrotoxicosis could result from SARS-CoV-2 directly infecting the thyroid gland. TSH suppression appears to be associated with the excessive release of inflammatory cytokines, including IL-6, characterizing COVID-19, as cytokines decrease TRH and TSH secretion [16,26]. Another potential mechanism might be a direct suppressing effect of SARS-CoV-2 on the hypothalamus and/ or pituitary, similar to SARS-CoV-1 [8,18]. Thus, the results of the studies suggest that COVID-19, associated with systemic immune activation, may cause thyroid inflammation and result in hyperthyroidism. Though, we did not find lower TSH and higher FT4 plasma levels to be correlated with COVID-19 severity.
Most of the COVID-19 patients in our study had only mild-to-moderate fever and upper respiratory symptoms. However, nine patients (15,5%), eight patients (12%), and ten patients (13,9%) from the I, II, and III subgroups, respectively, were treated in intensive care units.
Neck pain was absent in 7 (12%), 5 (7.7%), and 9 (12.5%) patients in the I, II, and III subgroups, respectively. Therefore, their condition has been considered “atypical thyroiditis,” which is mainly characterized by “T4 thyrotoxicosis” and which differs from the classic form of SAT by the absence of neck pain [51]). Though unlike Muller, et al. [51], we did not find a correlation between atypical thyroiditis and the illness severity in our study.
The time from diagnosis of COVID-19 infection to typical symptoms of SAT was 5 to 22 days. All the patients of the target group (except those with atypical thyroiditis) received treatment with corticosteroids, while some of them were given ibuprofen for severe neck pain. Clinical symptoms were mainly relieved within a few days. The laboratory indexes of SAT returned to normal levels after one month in all the patients, which indicated a good prognosis. These results corresponded with the outcomes of several previous studies that showed complete remission of SAT in most cases; the thyrotoxicosis was transient, self-limited, and did not require specific anti-thyroid drugs [14,67]. Corticosteroid treatment lasted one month for all the patients.
Anti-thyroid peroxidase antibody (TPO-Ab), which enhances pro-inflammatory cytokines, is a cause of autoimmune thyroid disease [68]. TPO-Ab positivity could be associated with severe COVID-19 pneumonia and a potential adverse prognostic factor for progression to severe respiratory failure (SRF) [69]. SARS-CoV-2 might trigger thyroid autoimmunity in the context of a generalized immune response. Thus, TPO-Ab positivity could indicate exaggerated immune system activation in COVID-19 patients and an increased risk of severe/complicated illness. It could also indicate SARS-CoV-2-induced thyroiditis with a transient anti-thyroid antibody rise. Some studies reported that serum 25(OH)D levels in euthyroid patients with Hashimoto’s thyroiditis were inversely correlated with TPO-Ab levels. TPO-Ab levels were also significantly higher in vitamin D-deficient patients with Hashimoto’s thyroiditis [67,70]. A study found the prevalence of TPO-Ab positivity in women with vitamin D deficiency and insufficiency compared to vitamin D-sufficient individuals. [17]. Though, other studies revealed no association between vitamin D deficiency and TPO-Ab positivity and proposed that Vitamin D deficiency does not increase the risk of autoimmune thyroid diseases [70-72]. In our study, TPO-Ab was significantly increased compared to the control group. It negatively correlated with vitamin D deficiency in the I and II subgroups.
There is evidence that vitamin D binds to specific binding sites and is uptaken by the thyrotrophin of the anterior pituitary, which indicates that vitamin D may modulate the secretion of TSH [73-75]. Studies have shown that a reciprocal relationship exists between serum TSH and vitamin D levels in hypothyroid subjects [46,76]. It was demonstrated that vitamin D supplementation among hypothyroid patients for 12 weeks improved serum TSH and calcium concentrations, but it did not change serum T3 and T4 levels [51].
In subacute thyroiditis patients, vitamin D levels were significantly lower than in the healthy control group. [77]. The same study revealed no relationship between vitamin D level and disease prognosis. However, it showed that vitamin D deficiency may increase the rate of respiratory tract infections (especially influenza, measles, adenovirus, and retroviruses) and SAT development. Administered vitamin D weekly in autoimmune thyroid disorders (AITD) significantly reduced TPOAb titers [70]. The study revealed no correlation between the severity of COVID-19 symptoms and vitamin D status.
Serum C-reactive protein (CRP) is a sensitive non-specific biomarker for inflammation; as an acute phase reactant, it has been found to be elevated in inflammatory thyroid disorders [65,78]. Acute phase reactants are valuable alternatives to erythrocyte sedimentation rate (ESR) due to significantly higher false positivity and false negativity associated with the latter [79]. Serum C-reactive protein concentration is a general non-specific marker of inflammation, subacute thyroiditis, and COVID-19 disease severity. Studies found significantly higher serum CRP levels amongst SAT patients than Graves’ patients of similar age. CRP can be used as a diagnostic marker of SAT, especially when it may be confused with Graves’ disease, another common cause of thyrotoxicosis. (Ibid). Our study revealed an inverse correlation between serum 25(OH) D levels and C-reactive protein (CRP), which is in harmony with the findings of other studies [80].
Conclusion
SAT could be considered an adaptive body response during acute illness or damage to the hypothalamic-pituitary-thyroid function caused by SARS-CoV-2. However, it becomes pathological if prolonged. Hence, there is a need to pay attention to thyroid function during the infection and follow-up period. Muller et al. suggest routine assessment of thyroid function in COVID-19 patients requiring high-intensity care, as they frequently present with thyrotoxicosis as a form of SARS-CoV-2-related SAT [80]. We did not find the presence of thyrotoxicosis on SAT to be correlated with the severity of Covid-19. However, there should be awareness of the potential development of thyroid dysfunction at SARS‐CoV‐2, which may be masked by the manifestation of Covid-19 symptoms by the respiratory system and by dexamethasone administration in the treatment of COVID‐19 disease. Routine thyroid assays in hospitalized COVID‑19 patients could be encouraged to detect thyroid dysfunction. The latter may develop in both the acute phase, during the infection, and the convalescence phase, post-COVID condition; therefore, thyroid function should be assessed again after recovery [81-96].
Our study confirmed that low vitamin D levels could be associated with an increased risk of developing SAT; The present study did not find a relationship between vitamin D deficiency and the severity of Covid-19 related thyrotoxicosis; nor it revealed that the severity of vitamin D deficiency affects the disease outcome in COVId-19 patients. More studies are needed to delineate further the pathophysiologic mechanisms of thyroid involvement in SARSCoV- 2 infection and the related role of vitamin D status. In addition, long-term studies of thyroid dysfunction in covid-19 patients would be informative.
References
- Lang S, Liu Y, Qu X, Lu R, Fu W, et al. (2021) Association between thyroid function and prognosis of COVID-19: A retrospective observational study. Endocrine Research 46(4): 170-177.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) Covid-19: Consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229): 1033-1034.
- Li Y, He F, Zhou N, Wei J, Ding Z, et al. (2020) Organ function support in patients with coronavirus disease 2019: Tongji experience. Front Med 14(2): 232-248.
- Zaim S, Chong JH, Sankaranarayanan V, Harky A (2020) Covid-19 and Multiorgan response. Curr Probl Cardiol 45(8): 100618.
- Mehta OP, Bhandari P, Raut A, Kacimi SE, Huy NT (2021) Coronavirus disease (COVID-19): Comprehensive Review of Clinical Presentation. Frontiers in Public Health 8: 582932.
- Wang W, YE, YX, Yao H, (2003) Evaluation and observation of serum thd parathyroid hormone in patients with severe acute respiratory syndrome. Chinese journal of antituberculosis 25(4): 232.
- Wei L, Sun S, Xu C hong, Zhang J, Xu Y, et.al. (2007) Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol 38(1): 95-102.
- Leow MKS, Kwek DSK, Ng AWK, Ong KC, Kaw GJL, et al. (2005) Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clinical Endocrinology 63(2): 197-202.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426(6965): 450-454.
- Hoffmann M, Kleine Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-COV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2): 271-280.
- Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, et al. (2020) Detection of SARS-COV-2 receptor ACE-2 mrna in thyroid cells: A clue for covid-19-related subacute thyroiditis. Journal of Endocrinological Investigation 44(5): 1085-1090.
- Caron P (2020) Thyroid disorders and SARS-COV-2 infection: From pathophysiological mechanism to patient management. Ann Endocrinol 81(5): 507-510.
- Lazartigues E, Qadir MM, Mauvais Jarvis F (2020) Endocrine significance of SARS-COV-2’s reliance on ACE2. Endocrinology 161(9): 108.
- Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, et al. (2020) SARS-COV-2-related atypical thyroiditis. The Lancet Diabetes & Endocrinology 8(9): 739-741.
- Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, et al. (2020) Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest 44(4): 765-771.
- Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, et al. (2020) Thyrotoxicosis in patients with covid-19: The thyrcov study. European Journal of Endocrinology 183(4): 381-387.
- Yao XH, Li TY, He ZC, Ping YF, Liu HW, et al. (2020) A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 49(5): 411-417.
- Wei L, Sun S, Zhang J, Zhu H, Xu Y, et al. (2010) Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (sars)this paper is one of a selection of papers published in this special issue entitled “Second International Symposium on recent advances in basic, clinical, and social medicine” and has undergone the journal's usual peer review process. Biochemistry and Cell Biology 88(4): 723-730.
- Semikov VI, Aghayan DL, Shulutko AM, Khorobrykh TV, Aleksandrov YK, et al. (2021) Subacute thyroiditis after SARS‐COV‐2 infection. Clinical Case Reports 9(11).
- Chen L, Pei JH, Kuang J (2020) Moderators of the association between serum parathyroid hormone and metabolic syndrome in participants with elevated parathyroid hormone: NHANES 2003–2006. Horm Metab Res 52(07): 509-516.
- Fliers E, Bianco A C, Langouche L, Boelen A (2015) Thyroid function in critically ill patients. The Lancet Diabetes & Endocrinology 3(10): 816-825.
- Van den Berghe G (2014) Non-thyroidal illness in the ICU: A syndrome with different faces. Thyroid 24(10): 1456-1465.
- Beltrão FE, Beltrão DC, Carvalhal G, Beltrão FE, Brito A da, et al. (2021) Thyroid hormone levels during hospital admission inform disease severity and mortality in COVID-19 patients. Thyroid 31(11): 1639-1649.
- Moretti B, Papaleontiou M (2021) Prognostic value of thyroid hormone levels in hospitalized patients with moderate-to-severe COVID-19 infection. Clinical Thyroidology 33(12): 511-515.
- Chen Y, Li X, Dai Y, Zhang J (2022) The association between COVID-19 and thyroxine levels: A meta-analysis. Frontiers in Endocrinology 12: 779692.
- Gong J, Wang D kun, Dong H, Xia Q song, Huang Z yi, et al. (2021) Prognostic significance of low TSH concentration in patients with covid-19 presenting with non-thyroidal illness syndrome. BMC Endocr Disord 21(1): 111.
- Gao W, Guo W, Guo Y, Shi M, Dong G, et al. (2020). Thyroid hormone concentrations in severely or critically ill patients with covid-19. Journal of Endocrinological Investigation 44(5): 1031-1040.
- Hariyanto TI, Kurniawan A (2020) Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr 14(5): 1429-1430.
- Holick MF, & Chen TC (2008) Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr 87(4): 1080S-1086S.
- Müller K, Diamant M, Bendtzen K (1991) Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunology Letters 28(2): 115-120.
- Skrobot A, Demkow U, Wachowska M (2018) Immunomodulatory role of vitamin D: A Review. Adv Exp Med Biol 13-23.
- D Aurizio F, Villalta D, Metus P, Doretto P, Tozzoli R, et al. (2015) Is vitamin D A player or not in the pathophysiology of autoimmune thyroid diseases?. Autoimmunity Reviews 14(5): 363-369.
- Skaaby T, Husemoen LL, Thuesen BH, Linneberg A (2015) Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 50(1): 231-238.
- Sasaki H, Harada H, Handa Y, Morino H, Suzawa M, et al. (1995) Transcriptional activity of a fluorinated vitamin D analog on VDR-RXR-mediated gene expression. Biochemistry 34(1): 370-377.
- Wang TT, Nestel FP, Bourdeau Véronique, Nagai Y, Wang Q, et al. (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173(5): 2909-2912.
- Peterson C A, Heffernan ME (2008) Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. Journal of Inflammation 5(1): 5-10.
- Alhassan Mohammed H, Mirshafiey A, Vahedi H, Hemmasi G, Moussavi Nasl Khameneh A, et al. (2017) Immunoregulation of inflammatory and inhibitory cytokines by vitamin D3 in patients with inflammatory bowel diseases. Scand J Immunol 85(6): 386-394.
- Piemonti L, Monti P, Allavena P, Sironi M, Soldini L et al. (1999) Glucocorticoids affect human dendritic cell differentiation and maturation. The Journal of Immunology 162(11): 6473-6481.
- Piemonti L, Monti P, Sironi M, Fraticelli P, Leone B E, et al. (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. The Journal of Immunology 164(9): 4443-4451.
- Tang J, Zhou R, Luger D, Zhu W, Silver PB, et al. (2009) Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the th17 effector response. J Immunol 182(8): 4624-4632.
- Sassi F, Tamone C, D Amelio P (2018) Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 10(11): 1656.
- Colotta F, Jansson B, Bonelli F (2017) Modulation of inflammatory and immune responses by Vitamin D. Journal of Autoimmunity 85: 78-97.
- Gombart AF, Pierre A, Maggini S (2020) A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 12(1): 236.
- Aranow C (2011) Vitamin D and the immune system. J Investig Med 59(6): 881-886.
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, et al. (2007) Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179(3): 1634-1647.
- Kim MJ, Kim D, Koo JS, Lee JH, Nam KH (2022) Vitamin D receptor expression and its clinical significance in papillary thyroid cancer. Technol Cancer Res Treat 21.
- Wang SH, Koenig RJ, Giordano TJ, Myc A, Thompson NW, et.al. (1999) 1α,25-dihydroxyvitamin d3 up-regulates bcl-2 expression and protects normal human thyrocytes from programmed cell death. Endocrinology 140(4), 1649–1656.
- Li X, Wang G, Lu Z, Chen M, Tan J, et al. (2015) Serum 25-hydroxyvitamin D predict prognosis in radioiodine therapy of graves disease. Journal of Endocrinological Investigation 38(7): 753-759.
- Yasuda T, OkamotoY, Hamada N, Miyashita K, Takahara, M, et al. (2012) Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset graves’ disease. Endocrine 42(3): 739-741.
- Chahardoli R, Saboor Yaraghi AA, Amouzegar A, Khalili D, Vakili A, et al. (2019) Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO AB, Anti-Tg AB) and Thyroid Profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm Metab Res 51(05): 296-301.
- Talaei A, Ghorbani F, Asemi Z (2018) The effects of vitamin D supplementation on thyroid function in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Indian J Endocrinol Metab 22(5): 584-588.
- Musa IR, Gasim GI, Khan S, Ibrahim IA, Abo Alazm H, et al. (2017) No association between 25 (OH) vitamin D level and hypothyroidism among females. Open Access Macedonian Journal of Medical Sciences 5(2): 126-130.
- Kivity S, Agmon Levin N, Zisappl M, Shapira Y, Nagy E V, et al. (2011) Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol, 8(3): 243-247.
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, et al. (2020) Association of Vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 3(9): e2019722.
- Weir EK, Thenappan T, Bhargava M, Chen Y (2020) Does vitamin D deficiency increase the severity of COVID-19?. Clinical Medicine 20(4).
- Feiner Solís Á, Avedillo Salas A, Luesma Bartolomé MJ, Santander Ballestín S (2022) The effects of vitamin D supplementation in COVID-19 patients: A systematic review. International Journal of Molecular Sciences 23(20): 12424.
- Hamming I, Timens W, Bulthuis M, Lely AT, Navis GJ, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2): 631-637.
- Chen M, Zhou W, Xu W (2021) Thyroid function analysis in 50 patients with covid-19: A retrospective study. Thyroid 31(1): 8-11.
- Chong W H, Shkolnik B, Saha B, Beegle S (2021) Subacute thyroiditis in the setting of coronavirus disease 2019. The American Journal of the Medical Sciences 361(3): 400-402.
- Guven M (2020) Subacute thyroiditis in the course of Coronavirus Disease 2019: A case report. Journal of Endocrinology & Metabolism 10(3-4): 110-112.
- Sohrabpour S, Heidari F, Karimi E, Ansari R, Tajdini A, et al. (2021) Subacute thyroiditis in COVID-19 patients. Eur Thyroid J 9(6): 321-323.
- Feghali K, Atallah J, Norman C (2021) Manifestations of thyroid disease post covid-19 illness: Report of hashimoto thyroiditis, graves’ disease, and subacute thyroiditis. Journal of Clinical and Translational Endocrinology: Case Reports 22: 100094.
- Asfuroglu Kalkan E, Ates I (2020) A case of subacute thyroiditis associated with covid-19 infection. J Endocrinol Invest 43(8): 1173-1174.
- Ippolito S, Dentali F, Tanda ML (2020) SARS-COV-2: A potential trigger for subacute thyroiditis? insights from a case report. J Endocrinol Invest 43(8): 1171-1172.
- Pearce E N, Farwell A P, Braverman L E (2003) Thyroiditis. New England Journal of Medicine 348(26): 2646-2655.
- R Volpe, MW Johnston, N Huber (1958) Thyroid function in subacute thyroiditis. J Clin Endocrinol Metab 18(1): 65-78.
- Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, et al. (2015) Is vitamin D related to pathogenesis and treatment of Hashimoto s thyroiditis? Hellenic journal of nuclear medicine 18(3): 222-227.
- Khan F A, Al Jameil N, Khan M F, Al Rashid M, Tabassum H (2015) Thyroid dysfunction: an autoimmune aspect. Int J Clin Exp Med 8(5): 6677-6681.
- Assimakopoulos SF, Markantes GK, Papageorgiou D, Mamali I, Markou KB, et al. (2021) Low serum tsh in the acute phase of COVID-19 pneumonia: Thyrotoxicosis or a face of “non-thyroidal illness syndrome”? Clinical Chemistry and Laboratory Medicine (CCLM) 59(11).
- Chaudhary S, Dutta D, Kumar M, Saha S, Mondal SA, et al. (2016) Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian j endocrinol metab 20(3): 391-398.
- Sezgin G, Esref Ozer M, Uygur Bayramicli O, Melih Ozel A, Aksungar F (2011) Relationship of vitamin D deficiency and autoimmune thyroid diseases. European Journal of Internal Medicine 22.
- Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM (2012) Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. European Journal of Endocrinology 167(1): 43-48.
- Smith MA, McHenry C, Oslapas R, Hofmann C, Hessel P, et al. (1989) Altered TSH levels associated with increased serum 1,25-dihydroxyvitamin D3: a possible link between thyroid and parathyroid disease. Surgery 106(6): 987-991.
- Sar M, Stumpf W E, Deluca HF (1980) Thyrotropes in the pituitary are target cells for 1,25 dihydroxy vitamin D3. Cell And Tissue Research 209(1): 161-166.
- M C DEmden, J D Wark (1987) 1,25-dihydroxyvitamin D3 enhances thyrotropin releasing hormone induced thyrotropin secretion in normal pituitary cells. Endocrinology 121(3): 1192-1194.
- ElRawi H A, Ghanem N S, ElSayed N M, Ali H M, Rashed L A, et al. (2019) Study of vitamin D level and vitamin D receptor polymorphism in hypothyroid Egyptian patients. Journal of Thyroid Research: 3583250.
- Calapkulu M, Sencar ME, Sakiz D, Unsal IO, Ozbek M, et al. (2020) The importance of vitamin D level in subacute thyroiditis disease and the effect of vitamin D on disease prognosis. Endocr Pract 26(10): 1062-1069.
- Sari O, Tunc R, Kisakol G, Dostbil Z, Serdengect M (2007) Acute-phase response after radioiodine treatment in hyperthyroidism. The Endocrinologist 17(2): 89-91.
- Baruah MP, Bhattacharya B (2012) Significant role of serum CRP in differentiating inflammatory from non-inflammatory causes of thyrotoxicosis. Indian J Endocrinol Metab 16(6): 976.
- Kruit A, Zanen P (2016) The association between vitamin D and C-reactive protein levels in patients with inflammatory and non-inflammatory diseases. Clinical Biochem 49(7-8): 534-537.
- Chen W, Lei J, Li Z (2022) Thyroid function changes and COVID-19 severity: Egg or chicken? Endocrine 78(3): 436-440.
- Choi YM, Kim WG, Kim TY, Bae SJ, Kim HK, et al. (2014) Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid 24(4): 655-661.
- Croce L, Gangemi D, Ancona G, Liboà F, Bendotti G, et al. (2021) The cytokine storm and thyroid hormone changes in COVID-19. Journal of Endocrinological Investigation 44(5): 891-904.
- De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, et al. (2020) Marked T cell activation, senescence, exhaustion and skewing towards th17 in patients with covid-19 pneumonia. Nat Commun 11(1): 3434.
- Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis Stathopoulos I, Psaltopoulou T, et al. (2020). Organ-specific manifestations of COVID-19 infection. Clinical and Experimental Medicine 20(4): 493-506.
- Goswami R, Marwaha R K, Gupta N, Tandon N, Sreenivas V, et al. (2009) Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br J Nutr 102(3): 382-386.
- Hanley B, Naresh K N, Roufosse C, Nicholson A G, Weir J, et al. (2020) Histopathological findings and viral tropism in UK patients with severe fatal covid-19: A post-mortem study. The Lancet Microbe 1(6): E245-E253.
- He Z, Zeng G, Li X (2013) Chinese minimally invasive percutaneous nephrolithotomy (MPCNL): Overcoming the difficulties. Difficult Cases in Endourology 97-106.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Mattar SA, Koh SJ, Rama Chandran S, Cherng BP (2020) Subacute thyroiditis associated with covid-19. BMJ Case Reports 13(8): e237336.
- Mukhopadhyay S, Chaudhary S, Dutta D, Kumar M, Saha S, et al. (2016) Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J Endocrinol Metab 20(3): 391-398.
- Pearce E N, Bogazzi F, Martino E, Brogioni S, Pardini E, et al. (2003) The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid 13(7): 643-648.
- Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 46(5): 846-848.
- Taous A, Islam MS (1970) Thyroiditis: Differential diagnosis and management. Bangladesh Journal of Otorhinolaryngology 16(1): 48-53.
- Wang J, Lv S, Chen G, Gao C, He J (2015) Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 7(4): 2485-2498.
- Wu D, Yang XO (2020) Th17 responses in cytokine storm of covid-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3): 368-370.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.