Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Para-Chlorophenyl Alanine Induces Aggressive Behavior by Serotonin Depletion in Male Rats and Increases Tryptophan Hydroxylase two and GABAA α1 mRNA Expression in the Olfactory Bulb
*Corresponding author: Ricardo Cabrera, Biomedical Research Institute (INBIOMED-IMBECU-CONICET), Faculty of Medical Sciences, University of Mendoza, Argentina.
Received: May 13, 2023; Published: May 17, 2023
DOI: 10.34297/AJBSR.2023.18.002524
Abstract
Decreased serotonin (5-HT) has long been linked to increased aggressive behavior. Tryptophan Hydroxylase (TPH) is an enzyme involved in the 5-HT synthesis, and para- chlorophenyl alanine (pCPA) inhibits its activity. TPH2 mRNA expression presence has been mainly described in the raphe complex rodent’s brain. 5-HT-producing neurons in the raphe project their axons to the olfactory bulb, considered a relevant structure in rodents for establishing social interactions, including aggressive behavior. However, the relationship between the olfactory bulb and aggression in a pCPA 5-HT depletion model has not been studied.
Moreover, receptor subunit GABAα1 has been found in the olfactory bulb, and 5-HT depletion could affect GABAA receptor expression in different brain areas. Thus, we aimed to evaluate aggressive behavior, serotonergic activity, and the TPH2 and GABAAα1 mRNA expression, in the olfactory bulb, after a single pCPA (300mg/kg) or vehicle i.p. administration in male rats. Aggression was tested using a resident intruder test. The olfactory bulb was obtained, and neurochemical and molecular techniques were used to measure 5-HT, 5-HIAA, TPH2, and GABAAα1 mRNA expression, respectively. pCPA administration increased aggressive behavior parameters without affecting locomotion, non-social or social interaction. 5-HT levels were decreased after pCPA administration and its turnover rate, although there were no significant changes in 5-HIAA. TPH2 mRNA expression was increased. GABAAα1 mRNA expression was increased in the olfactory bulb. Our results apport evidence to the serotonergic deficiency hypothesis of aggression and highlight the olfactory bulb’s Role as an essential structure for understanding aggressive behavior neurobiological complexity.
Keywords: Rat, Raphe nuclei, Olfactory bulb, Social interaction, Resident intruder test
Introduction
Aggressiveness is considered a social behavior since it requires at least two subjects and can be either defensive or offensive. There are similarities in aggressive neurobiology among primates, hu mans, and rodents [1,2]. The aggressive spectrum displayed depends on the specie, the sex, the stimuli, and the contextual contingency [1-3]. According to the qualitative and quantitative features, aggressiveness in rodents could be considered adaptive or maladaptive [4]. In their natural environment, rodents are powerfully aggressive in defending their food resources, reproductive possibilities, and territory [5]. The Resident Vs. Intruder Test (RVI) is the most validated paradigm to measure this interaction [3].
Serotonin (5-HT) plays an essential role in social behavior. Among others, modulates several behaviors, such as copula, postnatal care, adolescent social playing, and maternal and territorial aggressiveness [5-9]. Many systems are involved in aggressive behavior Chamero, et al., (2011), Carrillo, et al., (2009), Duke, et al., (2013) Although, serotonergic deficiency hypothesis establishes an inverse relationship between the serotonergic system activity and aggressive behavior [1,2,10-13] Miczek, et al., (2004). In rodent models, there are several ways to generate aggressiveness by decreasing 5-HT: neurotoxicity, olfactory bulbectomy, social isolation, and pharmacological depletion [14-17]. Para-chlorophenylalanine (pCPA) is an irreversible TPH enzyme inhibitor. An intraperitoneal pCPA administration causes an acute decrease within the central serotonergic system [14] Jáquier, et al., (1967). It has been used to generate aggression models [18,19] Jáquier et al., (1967).
Serotonin-producing neurons in the raphe complex project their axons to the cortex, amygdala, hippocampus, basal ganglia, thalamus, hypothalamus, and Olfactory Bulb (OB) [20] Mazerolle, et al., (2016). OB is an important serotonergic innervated structure [21-23], and the primary sensory information used by rodents to start social interactions, such as aggressive behavior, is olfaction [24]. Moreover, [25] showed that 5-HT synthesis inhibition in OB rats caused increased aggressiveness, whereas enhancement transmission suppressed aggressive behavior. The first sensory center that processes odor information is the OB, a structure considered when studying territorial aggression [26]. Nonetheless, the relationship between OB and aggression in a pCPA 5-HT depletion model has not been studied.
5-HT is synthesized through the Tryptophan Hydroxylase (TPH) enzyme [27]. TPH gene has two isoforms [28] while the TPH1 isoform is mainly expressed outside the blood-brain barrier, the TPH2 isoform is expressed in the cerebral tissue, and its mRNA expression has so far been primarily described in raphe complex neurons [28-30] Patel, et al., (2004). A human brain post-mortem study showed TPH2 expression in the cortex, thalamus, hypothalamus, hippocampus, amygdala, cerebellum, and raphe nuclei [31]. A study using catfishes [32] reported TPH2 mRNA expression in OB. Furthermore, Patel, et al., (2004) showed that TPH2 mRNA expression in rat brains revealed a weak signal in the OB.
In OB, principal neurons and local interneurons have GABAA receptors with different subunit components [33]. Remarkably, the presence of GABAA receptor subunit α1mRNA in the OB has been described [34] and is the most common GABAAα subunit in adult rodent neurons [35]. In addition, it has been reported that 5-HT depletion could affect GABAA receptor expression in different brain areas [36,37]. We aimed to evaluate aggressive behavior and serotonergic activity in the OB, TPH2, and GABAA α1 mRNA expression in the OB after a single pCPA i.p administration in male rats.
Methods and Materials
Subjects and Housing
Animals were housed in a temperature-controlled animal room (22+/- 2°C) on a 12 h light/dark cycle (light on from 07:00 to 19:00), with artificial light (60-70lumens). Food and water were available ad libitum.
RVI test requires a resident subject and an intruder. Residents were Sprague-Dawley male rats of 60days and 350g (average weight). Intruders were Sprague-Dawley male rats of 50days and 280g (average weight). Resident subjects were divided into two groups (n=10). Experimental subjects were injected with pCPA and returned to their home cage for six days; control subjects were injected with a vehicle and returned to their home cage simultaneously. After the RVI test, residents were euthanized, and OB was removed for posterior analysis [38]. Intruder subjects remained housed in groups of 3 until the test and were euthanized.
Experimental Design
Between 15:00h and 18:00h, animals from the experimental groups received a single pCPA (Sigma Aldrich, 2015) i.p. injection (dose 300mg/kg), solved in sterile saline. After pCPA administration, residents were housed individually in their homecages until RVI test day, performed six days later. Then animals were euthanized, and OB was obtained for neurochemical and genetic analysis.
Behavioral Testing
To assess aggressive behaviors, RVI was used. RVI test was applied according to [38] with modifications. Briefly, the RVI test consists of the interaction of two subjects: the experimental animal, named the resident, and the interaction animal, named the intruder. RVI test was performed six days after pCPA administration, between 15:00h and 18:00h [39]. To verify that residents were heavier than intruders, the animal’s weights before the test. The test was carried out in a wooden box with wood chips from the resident’s home cage. The box was cleaned after each test with ethanol (10%) [40]. All the tests were recorded with the Everio G-series GZMG330 JVC camera from above the field. Behavioral analysis was hand scored watching the videos in blind.
RVI test total time was 900 seconds. RVI test total time was divided into two phases, the adaptation phase, and the interaction phase. The first 300 seconds were considered the resident adaptation phase to the environment. Six hundred seconds remaining were considered the interaction phase. At the beginning of the interaction phases, an intruder was placed on the opposite side of the resident at the end of the adaptation phase, and the interaction behaviors were measured. We evaluated the animal locomotor activity as the travel distance during the adaptation phase. In the interaction phase, we evaluated four main groups of behaviors: 1-nonsocial activity, as time grooming, sniffing, and sitting; 2-social activity as the time of heterogrooming and heterosniffing; 3-aggressive behavior, as an event of the chase, moving towards, upright posture and false mount, bite, clinch, clinch attack, lateral threat and kept down were evaluated in the resident during interaction phase; and 4-aggressive latency, as the time preceding the first aggressive behavior. The criteria to discontinue resident interactions with the intruders were bite to delicate body parts, e.g., belly, throat, and paws.
RNA Extraction and Real-Time PCR Analysis
Total RNA from all left and right OB tissue was extracted using the TRIzol™ reagent, according to the manufacturer specifications (Invitrogen-Life Technologies, Buenos Aires, Argentina.). mRNA integrity samples were confirmed by 1% agarose gel electrophoresis and staining with Sybr Gold™ (Invitrogen-Life Technologies, Buenos Aires, Argentina). 10μg of total RNA was reverse transcribed at 37℃ using random hexamer primers and Moloney murine leukemia virus retrotranscriptase (Invitrogen-Life Technologies, Buenos Aires, Argentina) in a 20μL reaction mixture. The RNA was first denatured at 70℃ for 5min in the presence of 2.5μg of random hexamer primers (Invitrogen). For the subsequent RT reaction, the following mixture was added: RT buffer [50mM Tris-HCl (pH 8.4), 75 mM KCl, 3mM MgCl2], 0.5mM dNTPs, 5mM DTT, 200 units M-MLV Reverse Transcriptase (Invitrogen). The reaction was incubated at 37℃ for 50 min.; next, the reaction was inactivated by heating at 70℃ for 15min. The cDNA was stored at-20℃. The mRNA levels of TPH2 and GABAAα1 were estimated by RT real-time PCR with a Corbett Rotor-Gene 6000 Real-Time Thermocycler (Corbett Research Pty Ltd (Sydney, Australia) using rat-specific primers and reaction conditions described in (Table 1).
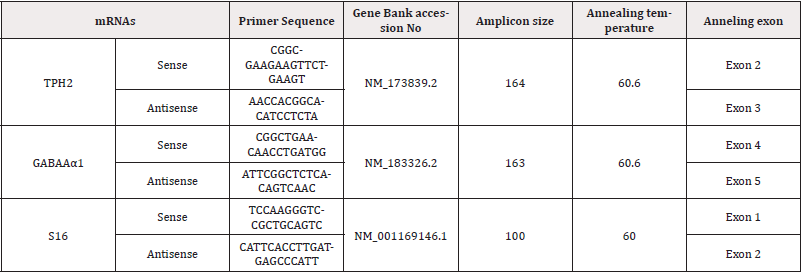
Table 1: Real-Time PCR Primer Design. Specific primers for rat gene sequence amplification were selected for real-time PCR assay.
The PCR reactions were performed using a Corbett Rotor-Gene 6000 Real-Time Thermocycler using Eva-GreenTM (Biotium, Hayward, CA) in a final volume of 20μL. The reaction mixture consisted of 2μL of 10×PCR Buffer, 1μL of 50mM MgCl2, 0.4μL of 10 mM dNTP Mix (Invitrogen), 1μL of 20× Eva Green, 0.25μL of 5U/μL Taq DNA Polymerase (Invitrogen) 0.1μL of each 2.5mM primer (forward and reverse. primers) and 10μL of diluted cDNA. The PCR reactions were performed under the conditions described in Table 1. Melt curve analysis was used to determine whether a specific amplified product was generated. Real-time quantification was monitored by measuring the increase in fluorescence caused by the binding of EvaGreen dye to double-strand DNA at the end of each amplification cycle. According to the manufacturer protocol, the relative expression was determined using the Comparative Quantitation method of normalized samples about the expression of a calibrator sample [41]. Each PCR run included a no-template control and a sample without reverse transcriptase. All measurements were performed in duplicate. The reaction conditions and quantities of cDNA added were calibrated such that the assay response was linear concerning the amount of input cDNA for each pair of primers. RNA samples were assayed for DNA contamination by performing the different PCR reactions without prior reverse transcription. Relative levels of mRNA were normalized to the S16 reference gene. The real-time PCR products were analyzed on 2% agarose gels containing 0.5 mg/mL ethidium bromide. A unique band of the approximately correct molecular weight corresponded with a unique peak in melt curve analysis. The Real-Time PCR reactions were carried out for 40 cycles with an initial step of 5min at 95ºC followed by a threestep scheme: 30 s at 95ºC, 30 s at the annealing temperature shown above for each primer pair, and a final step at 72ºC for 30 s. Primer’s design was done with Beacon Designer 7.9 software.
5-HT and 5-HIAA Content Determination by HPLC in the Olfactory Bulb
All OB tissue homogenization was performed according to [42]. Briefly, the tissue was collected in 400μl of 0.2N perchloric acid and then homogenized in a glass-glass homogenizer. The homogenate was centrifuged at 12000×g for 15min at 4℃ (Hermle LaborTechnik GmbH, model Z233MK-2), and the supernatant was injected into a High-Performance Liquid Chromatography (HPLC) instrument coupled to electrochemical detection to measure 5-HT, 5-HIAA. The pellet was resuspended in 1N NaOH for protein quantification by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc., Richmond, CA, USA) using bovine serum albumin as standard. 5- HT and 5-HIAA were expressed as picograms per milligram of total protein. 10μl of each supernatant were injected into the HPLC system with the following setting: A isocratic pump (model PU- 2080 Plus, Jasco Co. Ltd., Tokyo, Japan), a UniJet microbore column (MF-8912, BAS, West Lafayette, IN, USA), and an amperometric detector (set at 650mV, 0.5nA; model LC-4C, BAS, West Lafayette, IN, USA). The mobile phase, containing 0.05 M NaH2PO4, 1.0mM 1-octane sulfonic acid, 0.27mM EDTA, 1.0%(v/v) tetrahydrofuran, and 4.0%(v/v) acetonitrile (CH3CN) (pH adjusted to 2.6) was pumped at a flow rate of 100μl/min. The level of neurotransmitters and metabolites was assessed by comparing the sample’s respective peak area and elution time with a reference standard. The quantification was performed using a calibration curve for each neurotransmitter (Program Chrom Pass, Jasco Co. Ltd., Tokyo, Japan). Under these experimental conditions, retention times were 33.3 for 5-HT and 25.6 for 5-HIAA. Standards, EDTA, and 1-octane sulfonic acid were purchased from Sigma-Aldrich, Inc. (St Louis, MO, USA), and all other reagents were of analytical grade.
Statistical Analysis
All data were analyzed by t-test. The Shapiro-Wilks test was previously performed on each group to determine normal distribution. The significance level was set at p <0.05 for all statistical tests. Data were expressed as means±SEM of 10 rats per experimental group. All data were analyzed using Statistic’s software application (Stat Soft, Krakow, Poland).
Results
Behavioral Assays
The pCPA effect in behavioral evaluation. We found that pCPA single administration did not affect travel distance (p= 0.3296; t= 1.004; df=18) Table 2 compared to the control group. Moreover, pCPA did not affect non-social activity (p= 0.1746; t= 1.417; df= 18) and social activity (p= 0.1957; t= 1.347; df= 18) (Table 2).
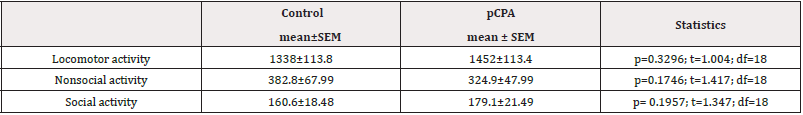
Table 2: pCPA effects on locomotor, Non-social activity, and social activity. Locomotor activity results are expressed as mean ± SEM of distanced travel in centimeters. Non-social and social activity results are expressed in mean ± SEM of time in seconds: control (N=10) and pCPA (N=10).
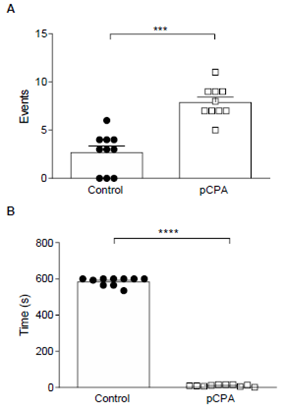
Figure 1: A- pCPA effects in Aggressive Behavior. Results are expressed in the mean ± SEM of several events. B- pCPA effects in Aggressive Latency. Results are expressed in mean ± SEM of latency during the first aggressive behavior.
Note*: Control (N=10) and pCPA (N=10). ***p <0.001; ****p <0.0001 for “t” Test.
However, pCPA induced a significant increase in aggressive behavior concerning the control group (p <0.001) (Figure 1-A); and significantly decreased aggressive latency (p <0.0001) (Figure 1-B).
5-HT and 5-HIAA Content Determination by HPLC in the Olfactory Bulb
The pCPA effect in neurochemical assays in the OB. We found that pCPA single administration decreased significantly 5-HT concentration (p <0.01) and serotonergic turnover (5-HIAA/5-HT) (p <0.05) in OB; 5-HIAA concentration was not affected (p= 0.7982; t= 0.2591, df= 18) (Table 3).
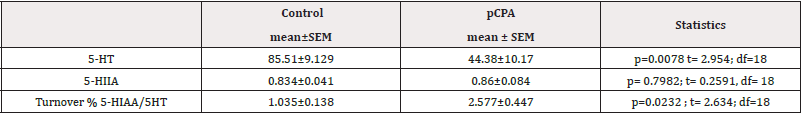
Table 3: pCPA effects in 5-HT, 5-HIAA, and turnover %5-HIAA/5-HT. Content determination by HPLC in the OB 5-HT and 5-HIAA results are expressed in ± SEM of pg/mg protein. Turnover 5-HIAA/5-HT results are expressed in mean ± SEM of the 5-HIAA/5-HT pg/mg protein percentage ratio: control (N=10) and pCPA (N=10).
Real-Time PCR Analysis
The pCPA effect on TPH2 and GABAAα mRNA expression in the OB. We found that pCPA single administration significantly increased (p<0.05) TPH2 mRNA expression in OB (Figure 2-A). Also, it significantly increased GABAAα1 mRNA expression (p<0.05) in OB (Figure 2-B).
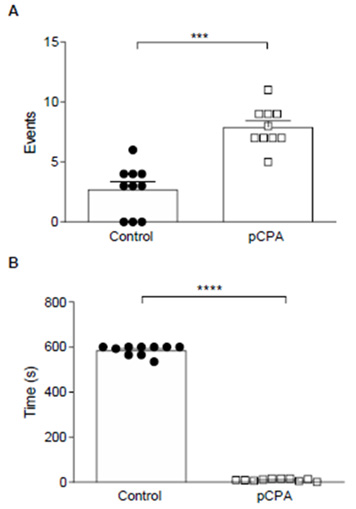
Figure 2: A- pCPA effects in TPH2 gene expression in the OB. Results are expressed in mean ± SEM of TPH2 relative expression units. B- pCPA affects GABAAα1 mRNA expression in the OB.
Note*: Results are expressed in mean ± SEM of GABAAα1 relative expression units. Control (N=10) and pCPA (N=10). *p <0.05 for t-Test.
Discussion
The initiation, maintenance, and termination of aggressive behavior activate complex neurobiological circuits, among which the serotonergic system is intensely involved [2,3]. In the current study, using an RVI paradigm, we studied aggression behavior induced by pCPA and the relation with serotonergic activity, TPH2, and GABAAα1 mRNA expression in the OB. It is well-accepted that discarding locomotor activity alterations is necessary when evaluating social interaction. [3,43]. Alocomotor activity alteration in the resident could prevent the correct measurement of aggressive behavior, particularly the aggression latency parameter. Interestingly, 5,7- dihydroxytryptamine lesions, which deplete central 5-HT, did not affect locomotor activity [44] Vergner, et al., (1988). Nonetheless, previous works utilizing pCPA 1000 mg/kg doses found a substantial decrease in locomotor activity [45,46]. Significantly reduced locomotor activity was also seen following chronic treatment with a lower dosage of pCPA (100 mg/kg) [18,19]. Our data show that pCPA i.p. did not change locomotor activity. An acute administration of pCPA at lower doses does not affect this parameter.
According to [3,38,] species-specific qualitative and quantitative aggression parameters must be considered to establish an animal aggressive behavior model. Therefore, we considered parameters that reconciled both characteristics when evaluating behaviors. We measured aggressive behavior as a quantitative parameter and aggressive latency as a qualitative one [43,47]. Show decreased aggressive latency as a parameter of des adaptative aggression and nonaggressive behaviors in mice treated with pCPA. Our data show that administering pCPA increased aggressive behavior and decreased aggressive latency. These findings are consistent with others in which central 5-HT depletion caused an aggression increase Valzelli, et al., (1981), Vergner, et al., (1986). indicating that our model, with pCPA, was adequate to induce aggression, both in a qualitative and quantitative sense. Furthermore, we did not find changes in non-social activity and social activity after the pCPA administration. 5-HT low levels might affect social interactions, e.g., in studies where social isolation produces aggressive behavior models [48] or maternal aggression models [49]. Thus, this result could be explained by our model and experimental design.
Serotonergic innervation originating in the raphe nuclei towards the different brain structures has one of its main synaptic centers in the OB [23] Locki, et al., (1985). Our results show that after pCPA administration, the concentration of 5-HT in the OB was significantly decreased, pointing to higher aggressive behavior. Our model provides evidence for the serotonergic deficiency hypothesis and aggression [1,2,11,12] Miczek, et al., (2004). In addition, 5-HT modification has been associated with changes in its major metabolite, 5-HIAA [50]. In humans [51] Sharma, et al., (2021) and monkeys [52], higher aggressiveness and low cerebrospinal 5-HIAA levels were associated. Furthermore, mutual decreased 5-HT, and 5-HIAA were observed in models of pCPA aggression [18,19]. However, the decrease in 5-HT concentration, following local or systemic administration of substances that affect their release from nerve terminals, does not always affect 5-HIAA concentration similarly [53]. In our model, pCPA administration did not cause significant modifications in the concentration of 5-HIAA. Probably, systemic depletion did not affect the metabolite as well as 5-HT because others [45,46] administered higher doses or performed a chronic treatment [18,19]. Under previously reported data, we also showed that serotonergic metabolism (5-HIAA/5-HT) decreased in treated animals [19,24]. These findings suggest that pCPA may cause alterations in the OB serotonergic innervation, enhancing aggression in our model.
Since pCPA animals exhibited increased aggression and 5-HT decreased, we hypothesized that differences in a critical serotonergic gene, TPH2 accompany these differences. TPH2 mRNA expression is frequently found in raphe complex neurons in rodents [28- 30] Patel, et al., (2004), and a shallow expression has been found in other rats’ brain areas, Patel, et al., (2004). Interestingly, pCPA animals showed increased TPH2 mRNA expression in the OB. Therefore, our results provide evidence for the TPH2 mRNA expression presence in the OB. It has also been found that after postnatal programming with pCPA, TPH2 mRNA expression decreases in raphe nuclei [54-72]. In this way, despite our opposite results, they could indicate a compensatory mechanism in a brain area receiving 5-HT depleted innervation.
In addition, pCPA decreases the protein expression of GABAA α1 receptors [37], and GABAAα1 is expressed in OB [33]. Interestingly, our results showed a higher expression of the GABAAα1 subunit after pCPA administration. These results may indicate increased levels of GABAAα1 subunit mRNA in OB due to its high synthesis demand caused by the serotonergic decrease due to pCPA administration.
Conclusion
In this work, we studied aggressive behavior in a rat male model. We concluded that a single and acute pCPA administration produces an increase in aggressive behavior, without affecting locomotion, non-social and social activity. We study the OB, a highly innervated serotonergic and GABAergic structure. The pCPA single and acute administration affects the serotonergic activity in OB. We verify TPH2 mRNA expression presence in OB. Moreover, increased GABAAα1 subunit expression mRNA in the OB may suggest a high synthesis demand as an alternate result in the OB serotonergic function. Thus, our data provide evidence for the serotonergic deficiency hypothesis of aggression and show OB as a relevant structure to understanding neurobiological complexity in aggressive behavior.
Compliance with Ethical Statement
All procedures were made following the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and the EU (Eighth Edition 2011) and approved by the Comité Institucional para el Cuidado y Uso de Animales de Laboratorio de la Universidad Nacional de Cuyo (CICUAL UNCuyo) (Aval 82/2016), Mendoza, Argentina.
Limitations
The present study does not include other brain areas related to aggressive behavior.
Data Accessibility Statement
The authors confirm that all data underlying the findings are available without restriction. All relevant data are included in the paper.
Conflict of Interests
The authors declared no potential conflict of interest concerning this article’s research, authorship, and publication.
References
- Takahashi A, Quadros IM, de Almeida RM, Miczek KA (2011) Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology 213(2-3): 183-212.
- Niederkofler V, Asher TE, Okaty BW, Rood BD, Narayan A, et al. (2016) Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior. Cell Rep 17(8): 1934-1949.
- Takahashi A, Miczek KA (2014) Neurogenetics of Aggressive Behavior-Studies in Rodents. Curr Top Behav Neurosci 17: 3-44.
- Muroy SE, Long KL, Kaufer D, Kirby ED (2016) Moderate Stress-Induced Social Bonding and Oxytocin Signaling are Disrupted by Predator Odor in Male Rats. Neuropsychopharmacology 41(8): 2160-2170.
- Takahashi A, Quadros IM, De Almeida RM, Miczek KA (2012) Behavioral and pharmacogenetics of aggressive behavior. Current topics in behavioral neurosciences 12: 73-138.
- Berger M, Gray J A, Roth B L (2009) The expanded biology of serotonin. Annual review of medicine 60: 355-366.
- Buhot M C, Martin S, Segu L (2000) Role of serotonin in memory impairment. Annals of Medicine 32(3): 210-221.
- Mittal R, Debs L H, Patel AP, Nguyen D, Patel K et al. (2017) Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J cellular physiol 232(9): 2359-2372.
- Strüder HK, Weicker H (2001) Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part II. Int j sports med 22(7): 482-497.
- Cervantes M C, Delville Y (2009) Serotonin 5-HT1A and 5-HT3 receptors in an impulsive-aggressive phenotype. Behavioral neuroscience 123(3): 589-598.
- Kravitz EA, Huber R (2003) Aggression in invertebrates. Curr opin neurobiol 13(6): 736-743.
- Mongillo DL, Kosyachkova EA, Nguyen TM, Holmes MM (2014) Differential effects of chronic fluoxetine on the behavior of dominant and Subordinate naked mole rats. Behav brain Res 258: 119-126.
- Van Erp AM, Miczek KA (2000) Aggressive behavior, increased accumbens dopamine, and decreased cortical serotonin in rats. The Journal of Neuroscience the official journal of the Society for Neuroscience 20(24): 9320-9325.
- Hritcu L, Clicinschi M, Nabeshima T (2007) Brain serotonin depletion impairs short-term memory but not long-term memory in rats. Physiology & behavior 91(5): 652-657.
- Jéquier E, Lovenberg W, Sjoerdsma A (1967) Tryptophan hydroxylase inhibition: the mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Molecular Pharmacology 3(3): 274-278.
- Koe BK, Weissman A (1966) p-Chlorophenylalanine: a specific depletion of brain serotonin. J Pharmacol Exp Ther 154: 499-516.
- Vergnes M, Depaulis A, Boehrer A, Kempf E (1988) Selective increase of offensive behavior in the rat following intrahypothalamic 5,7-DHT-induced serotonin depletion. Behavioral brain research 29(1-2): 85-91.
- Keleta Y B, Lumia A R, Anderson G M, McGinnis M Y (2007) Behavioral effects of pubertal anabolic androgenic steroid exposure in male rats with low serotonin. Brain Research 1132(1): 129-138.
- Kubala KH, McGinnis MY, Anderson GM, Lumia AR (2008) The effects of an anabolic androgenic steroid and low serotonin on social and non-social behaviors in male rats. Brain Res 1232: 21-29.
- Lesch KP, Waider J (2012) Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76(1): 175-191.
- Huang Z, Thiebaud N, Fadool D A (2017) Differential serotonergic modulation across the main and accessory olfactory bulbs. The Journal of Physiology 595(11): 3515-3533.
- McLean JH, Shipley MT (1987) Serotonergic afferents to the rat olfactory bulb: Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci 7(10): 3016-3028.
- Steinfeld R, Herb JT, Sprengel R, Schaefer AT, Fukunaga I (2015) Divergent innervation of the olfactory bulb by distinct raphe nuclei. J Comp Neurol 523(5): 805-813.
- Guillot P V, Chapouthier G (1996) Olfaction, GABAergic neurotransmission in the olfactory bulb, and intermale aggression in mice: modulation by steroids. Behavior genetics 26(5): 497-504.
- Lucki I (1998) The spectrum of behaviors is influenced by serotonin. Biol psychiatry 44(3): 151-162.
- Bester Meredith JK, Burns JN, Dang MN, Garcia AM, Mammarella GE, et al. (2022) Blocking olfactory input alters aggression in male and female California mice (Peromyscus californicus). Aggressive behavior 48(3): 290-297.
- Khan IA, Thomas P (2004) Aroclor 1254 inhibits tryptophan hydroxylase activity in the rat brain. Archives of toxicology 78(6): 316-320.
- Walther DJ, Bader M (2003) A unique central tryptophan hydroxylase isoform. Biochem pharmacol 66(9): 1673-1680.
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci 22(4): 895-901.
- Pelosi B, Pratelli M, Migliarini S, Pacini G, Pasqualetti M (2015) Generation of a Tph2 Conditional Knockout Mouse Line for Time- and Tissue-Specific Depletion of Brain Serotonin. PloS One 10(8): e0136422.
- Zill P, Büttner A, Eisenmenger W, Möller HJ, Ackenheil M, et al. (2007) Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J Psychiatr Res 41(1-2): 168-173.
- Raghuveer K, Sudhakumari CC, Senthilkumaran B, Kagawa H, Dutta Gupta A, et al. (2011) Gender differences in tryptophan hydroxylase-2 mRNA, serotonin, and 5-hydroxytryptophan levels in the brain of catfish, Clarias gariepinus, during sex differentiation. Gen Comp Endocrinol 171(1): 94-104.
- Panzanelli P, Perazzini AZ, Fritschy JM, Sassoè Pognetto M (2005) Heterogeneity of gamma-aminobutyric acid types A receptors in mitral and tufted cellsof the rat's main olfactory bulb. J Comp Neurol 484(1): 121-131.
- Zhang JH, Araki T, Sato M, Tohyama M (1991) Distribution of GABAA- receptor alpha 1 subunit gene expression in the rat forebrain. Brain research. Mol Brain Res 11(3-4): 239-247.
- Bosman L W, Rosahl T W, Brussaard A B (2002) Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. The Journal of Physiology 545(1): 169-181.
- Si Y, Wei W, Chen X, Xie X, Guo T, et al. (2022) A comprehensive study on the relieving effect of Lilium brownii on the intestinal flora and metabolic disorder in p-chlorphenylalanine induced insomnia rats. Pharm biol 60(1): 131-143.
- Wang M, Li N, Jing S, Wang C, Sun J, et al. (2020) Schisandrin B exerts hypnotic effects in PCPA-treated rats by increasing hypothalamic 5-HT and γ-aminobutyric acid levels. Experimental and therapeutic medicine 20(6): 142.
- Koolhaas JM, Coppens CM, Buwalda B, Meerlo P, Timmermans PJ (2013) The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J vis exp (77): e4367.
- Corthell JT, Stathopoulos AM, Watson CC, Bertram R, Trombley PQ, et al. (2013). Olfactory bulb monoamine concentrations vary with the time of day. Neuroscience 247: 234-241.
- Casas S, García S, Cabrera R, Nanfaro F, Escudero C, et al. (2011) Progesterone prevents depression-like behavior in a model of Parkinson's disease induced by 6- hydroxydopamine in male rats. Pharmacology biochemistry and behavior 99(4): 614-618.
- Pfaffl M W (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids Res 29(9): e45.
- Chi JD, Odontiadis J, Franklin M (1999) Simultaneous determination of catecholamines in rat brain tissue by high-performance liquid chromatography. Journal of chromatography. B: Biomedical sciences and applications 731(2): 361-367.
- Miczek KA, de Boer SF, Haller J (2013) Excessive aggression as a model of violence: a critical evaluation of current preclinical methods. Psychopharmacology 226(3): 445-458.
- Hole K, Johnson GE, Berge OG (1977) 5,7-Dihydroxytryptamine lesions of the ascending 5-hydroxytryptamine pathways: habituation, motor activity, and agonistic behavior. Pharmacology, biochemistry, and behavior 7(3): 205-210.
- Dringenberg HC, Hargreaves EL, Baker GB, Cooley RK, Vanderwolf CH, et al. (1995) p-chlorophenyl alanine-induced serotonin depletion: reduction in exploratory locomotion but no obvious sensory-motor deficits. Behavioral brain research 68(2): 229-237.
- Matte AC, Tornow H (1978) Parachlorophenylalanine produces dissociated effects on aggression, emotionality, and motor activity. Neuropharmacology 17(8): 555-558.
- Näslund J, Studer E, Pettersson R, Hagsäter M, Nilsson S, et al. (2015) Differences in Anxiety-Like Behavior within a Batch of Wistar Rats Are Associated with Differences in Serotonergic Transmission, Enhanced by Acute SRI Administration and Abolished By Serotonin Depletion. Int j Neuropsychopharmacol 18(8): pyv018.
- Goodell DJ, Ahern MA, Baynard J, Wall VL, Bland ST, et al. (2017) A novel escapable social interaction test reveals that social behavior and mPFC activationduring an escapable social encounter are altered by post-weaning social isolation and are dependent on the aggressiveness of the stimulus rat. Behavioral brain research 317: 1-15.
- Toth M, Tulogdi A, Biro L, Soros P, Mikics E, et al. (2012) The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behavioral brain research 233(1): 120-129.
- Stenfors C, Ross SB (2004) Changes in extracellular 5-HIAA concentrations as measured by in vivo microdialysis technique in relation to changes in the 5-HT release. Psychopharmacology 172(2): 119-128.
- Stanley B, Molcho A, Stanley M, Winchel R, Gameroff MJ, Parsons B, et al. (2000) Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry 157(4): 609-614.
- Zajicek KB, Price CS, Shoaf SE, Mehlman PT, Suomi SJ, et al. (2000) Seasonal variation in CSF 5-HIAA concentrations in male rhesus macaques. Neuropsychopharmacology 22(3), 240-250.
- Kalén P, Strecker RE, Rosengren E, Björklund A (1988) Endogenous release of neuronal serotonin and 5-hydroxyindoleacetic acid in the caudate-putamen of the rat as revealed by intracerebral dialysis coupled to high-performance liquid chromatography with fluorimetric detection. Journal of Neurochemistry 51(5): 1422-1435.
- Trujillo V, Valentim Lima E, Mencalha R, Carbalan Q, Dos Santos RC, et al. (2021) Neonatal Serotonin Depletion Induces Hyperactivity and Anxiolytic-like Sex-Dependent Effects in Adult Rats. Molecular neurobiology 58(3): 1036-1051.
- Auerbach SB, Minzenberg MJ, Wilkinson LO (1989) Extracellular serotonin and 5-hydroxy indole acetic acid in the hypothalamus of the unanesthetized rat measured by in vivo dialysis coupled to high-performance liquid chromatography with electrochemical detection: dialysate serotonin reflects neuronal release. Brain Research 499(2): 281-290.
- Fibiger HC, Campbell BA (1971) The effect of para-chlorophenylalanine on spontaneous locomotor activity in the rat. Neuropharmacology 10(1): 25-32.
- Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer A T, et al. (2012) Two distinct channels of olfactory bulb output. Neuron 75(2): 320-329.
- Harvey JD, Heinbockel T (2018) Neuromodulation of Synaptic Transmission in the Main Olfactory Bulb. Int J Environ Res Public Health 15(10): 2194.
- Hernández Vázquez F, Garduño J, Hernández López S (2018). GABAergic modulation of serotonergic neurons in the dorsal raphe nucleus. Reviews in neurosciences, 30(3): 289-303.
- Kohlert JG, Mangan BP, Kodra C, Drako L, Long E, Simpson H (2012) Decreased aggressive and locomotor behaviors in Betta splendens after exposure to fluoxetine. Psychol Rep 110(1): 51-62.
- Linster C, Fontanini A (2014) Functional neuromodulation of chemosensation invertebrates. Curr opin neurobiol 29: 82-87.
- Miczek KA, Fish EW, De Bold JF,De Almeida RM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine, and gamma-aminobutyric acid systems. Psychopharmacology 163(3-4): 434-458.
- Miguez J, Martin F, Aldegunde M (1991) Differential effects of pinealectomy on amygdala and hippocampus serotonin metabolism. J pineal Res 10(2): 100-103.
- Moeller FG, Dougherty DM, Swann AC, Collins D, Davis CM, et al. (1996) Tryptophan depletion and aggressive responding in healthy males. Psychopharmacology 126(2): 97-103.
- Muzerelle A, Scotto Lomassese S, Bernard JF, Soiza Reilly M, Gaspar P (2016) Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct Funct 221(1): 535-561.
- Popova NK, Gilinsky MA, Amstislavski TG, Morosova EA, Seif I, et al. (2001) Regional serotonin metabolism in the brain of transgenic mice lacking monoamine oxidase A. J Neurosci Res 66(3): 423-427.
- Scott JW, Wellis DP, Riggott MJ, Buonviso N (1993) Functional organization of the main olfactory bulb. Microsc Res Tech 24(2): 142-156.
- Ogawa S, Tsuchimine S, Kunugi H (2018) Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historical evidence. Journal of psychiatric research 105: 137-146.
- Urban NN, Arevian AC (2009) Computing with dendrodendritic synapses in the olfactory bulb. Ann N Y Acad Sci 1170: 264-269.
- Vergnes M, Kempf E (1982) Effect of hypothalamic injections of 5,7- dihydroxytryptamine on elicitation of mouse-killing in rats. Behav Brain Res 5(4): 387-397.
- Yu HL, Chen ZJ, Zhao JW, Duan SR, Zhao JK, et al. (2019) Olfactory Impairment and Hippocampal Volume in a Chinese MCI Clinical Sample. Alzheimer Dis Assoc Disord 33(2): 124-128.
- Zepf FD, Stadler C, Demisch L, Schmitt M, Landgraf M, et al. (2008) Serotonergic functioning and trait-impulsivity in attention-deficit/hyperactivity-disordered boys (ADHD): influence of rapid tryptophan depletion. Hum psychopharmacol 23(1): 43-51.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.