Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Porcine Placenta Peptide Increases the Proliferation and Migration of Human Gingival Epithelial Cells and the Secretion of Epidermal Growth Factor
*Corresponding author: Mahmoud Rouabhia, Oral Ecology Research Group, Faculty of Dentistry Laval University, Quebec City, Quebec, Canada.
Received: May 22, 2023; Published: May 25, 2023
DOI: 10.34297/AJBSR.2023.18.002536
Abstract
Placenta tissue contains multiple biologically active substances, such as amino acids, trace elements, hormones, and cytokines required for fetal growth. The role of the placenta as a health improvement product can also be as placenta extracts containing different peptides. The beneficial use of placenta membrane has been described with periodontal tissue regeneration. These positive effects could be due to the presence of biological mediators in the placenta membrane and peptides. We explored the effect of a specific placenta extract (molecular weight cutoff of 30kDa) housing five small peptides, LSSPATLNSR, ASISLPR, ILLEVNNR, ESLITLIEK, and QPLLLDDR, on human gingival epithelial cell behavior. Results show that placenta peptide at 0.1-100μg/mL significantly increased the proliferation of epithelial cells at 48 and 96h, and at 10 and 100μg/mL accelerated cell migration, contributing to faster wound closure than that in the control. Cell proliferation and migration were supported by increased Epidermal Growth Factor (EGF) secretion and a decrease in metalloproteinase MMP-2. This is the first study to report a novel biological activity of placenta peptide as a wound-healing contributor in human gingival epidermal cells.
Keywords: placenta peptide extract, gingival cells, growth, migration, EGF, MMP-2
Introduction
The placenta is a vital tissue for fetal and maternal health throughout gestation, providing an interface for the exchange of nutrients and waste. The placenta is also a source of hormones and immune factors that facilitate pregnancy maintenance and fetal growth [1]. One of the main roles of the placenta is the establishment of an immune barrier [2]. Placenta tissue expresses angiotensin II (Ang II), renin, and Angiotensin-Converting Enzyme (ACE) [3]. Ang II has been shown to modulate the production of Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PlGF) in vascular endothelium and smooth muscle cells via activating AT1R [4]. In normal pregnancy, both VEGF and PlGF can be found in the trophoblast as well as the maternal decidua. VEGF and PlGF are involved in the development of the placental vasculature by controlling the growth of blood vessels in the chorionic villi [5].
The placenta contains multiple biologically active substances, such as amino acids, trace elements, hormones, and cytokines, suggesting its use as a wound-coverage biological membrane [6-8]. The role of the placenta as a health improvement product can also be as Placenta Extracts (PE). Indeed, human placenta extracts have been shown to display anti-inflammatory and antioxidant properties [9]. Human placenta generated nitic oxide in mouse peritoneal macrophages and suppressed Lipopolysaccharide (LPS)-induced proinflammatory protein expression and reduced cell death by 24% [10].
The primary bioactive components of the placenta are placental peptides [11] that prevent the generation of reactive oxygen species and tumor necrosis factor α (TNF-α) [12]. Porcine placenta peptides have been found to improve endothelial cell viability, proliferation, migration, and angiogenesis by exhibiting angiogen ic growth factor recovery and inhibiting endothelial cell apoptosis [13]. As an active biological agent, porcine placenta could thus serve as an alternative for the treatment of diabetic wounds [14]. Porcine placenta reportedly improved immunity by modulating the expression of cytokines, including interleukin-2 (IL-2) and IFN-γ, and by increasing the phagocytic rate of macrophages and the conversion rate of T cells [15]. In the liver of non-alcoholic steatohepatitis mice fed a high-cholesterol, high-fat diet, placenta peptide extracts were shown to decrease lipid accumulation and peroxidation, insulin resistance, inflammatory and stress signaling, and fibrogenesis [16].
The alcalase enzyme reportedly hydrolyzed the placenta, releasing glycine, glutamic acid, and histidine within the peptide sequence [17,18]. The peptides released from the porcine placenta and processed through ultrafiltration had a Molecular Weight (MW) cutoff of 30kDa and led to the identification of the following peptide sequences: LSSPATLNSR, ASISLPR, ILLEVNNR, ESLITLIEK, and QPLLLDDR. These are the most abundant peptide sequences with a tendency to control antioxidant activity, anti-diabetic activity, and ACE inhibition [18]. Some other peptides containing tyrosine, phenylalanine, and tryptophan have been reported as controlling oxidative stress [13,19]. Several in vitro studies have proven that crude porcine placenta contains relaxing growth factors that improve a variety of bioactivities [20,21].
The beneficial use of placenta membranes has also been reported in dentistry as improving clinical periodontal parameters [22,23]. These beneficial effects on periodontal tissue regeneration could be due to biological mediators in the placenta membrane and the placenta peptides [24]. The Ultrafiltration (UF) from 100kDa to 30kDa cutoff enabled the isolation of different permeates. The one obtained with a 30kDa cutoff allowed for the isolation of UF4, consisting of LSSPATLNSR, ASISLPR, ILLEVNNR, ESLITLIEK, and QPLLLDDR. UF4 displays radical scavenging activities (DPPH●/ ABTS●+), ferric-reducing power, β-carotene bleaching inhibitory activity, nitric oxide scavenging activity, angiotensin-converting enzyme inhibition, and anti-α-amylase/α-glucosidase activity, with high antioxidant stability demonstrated in an in vitro gastrointestinal tract model [24]. These studies suggest the possible positive behavior of UF4 in promoting wound healing. We thus examined the effect of UF4 on gingival epithelial cell adhesion and proliferation as well as on gingival epithelial cell migration and the secretion of EGF and MMP-2.
Materials and Methods
Placenta Peptide Extraction and Purification
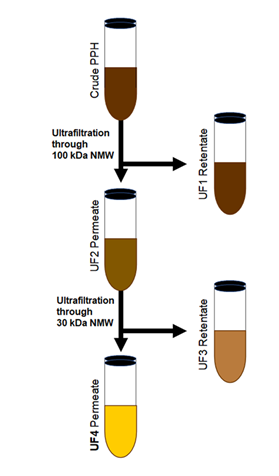
Figure 1: Schema summarizing the process to obtain UF4. The crude porcine placenta hydrolysate was Ultrafiltered (UF) through a 100kDa cutoff filter leading to a UF1 retentate, and an UF2 permeate UF2. The UF2 was ultrafiltered through a 30kDa cutoff filter, resulting in a retentate UF3 and a permeate UF4. The UF4 was used to perform the experiments.
Porcine placenta hydrolysis was created using the previously described method [24]. The ground porcine placenta was mixed with a 0.05M of glycine-NaOH buffer solution to achieve a protein concentration of 10% (w/w of placenta protein). The pH of the solution was then adjusted to 9.5 prior to incubation in a shaking water bath at 50℃. Alcalase was then added at a 10% concentration (based on porcine protein concentration) with constant stirring (120rpm) until the Degree of Hydrolysis (DH) reached 30%. After collecting the samples, the process was completed by heating the solution for 10 min at 95℃. The solution was then centrifuged at 4500rpm for 20 min (RC-5B Plus Centrifuge, Sorvall, Norwalk, CT, USA). Polysulfone hollow fiber membranes with MW cutoffs of 100kDa and 30kDa were used to separate the supernatant by means of a Büchner funnel filtering kit and a Rocker 400 vacuum pump (Rocker Scientific Co., Ltd., New Taipei, Taiwan). In the first step, 100kDa filtering was used to collect permeated fractions (Figure 1). The permeated fraction was subsequently passed through the 30kDa MW cutoff to obtain the permeated fraction (UF30P). The UF30P sample was then purified using a C18 Zip Tip (Merck Millipore, Darmstadt, Germany). The peptide samples were examined by LC-MS/MS using an Ultimate 3000 Nano/Capillary LC System (Thermo Scientific, UK) linked to a hybrid quadrupole Q-Tof impact II™ (Bruker Daltonics) connected to a nano-captive spray ion source, which enabled us to identify the highest peptides present in the UF4, namely, LSSPATLNSR, ASISLPR, ILLEVNNR, ESLITLIEK, and QPLLLDDR.
Gingival Epithelial Cells
The immortalized human epithelial cell line, GMSM-K, developed by Valerie Murrah (Department of Diagnostics Sciences and General Dentistry, University of North Carolina at Chapel Hill, NC, USA) [25], was used in this study. The cells were grown in a Dulbecco- Vogt modification of Eagle’s and Ham’s F12 media (Invitrogen Life Technologies, Burlington, ON, Canada) supplemented with 10% fetal calf serum and growth factors, including 24.3μg/mL of adenine, 10μg/mL of human epidermal growth factor, 0.4μg/mL of hydrocortisone, 5μg/mL of bovine insulin, 5μg/mL of human transferrin, and 2×10-9 M of 3,3′,5′-triiodo-L-thyronine. When the epithelial cells reached 80% confluence, they were used to perform our different experiments.
Effect of Placenta Peptide UF4 on Gingival Epithelial Cell Morphology and Adhesion
Gingival epithelial cells (2×105) were seeded into 6-well tissue culture plates in one ml of culture medium containing peptide UF4 at 0, 0.1, 1, 10, or 100mg/mL. The cells were incubated in a 5% CO2 humid incubator at 37°C for 24h, after which time the medium was changed to eliminate nonadherent cells; subsequently, photos were taken under an inverted optical microscope. To quantitatively analyze the effect of placenta peptide UF4 on gingival epithelial cell adhesion, we performed a thiazolyl tetrazolium salt (MTS) colorimetric assay (ab197010, Abcam, Cambridge, UK). Each experiment was repeated in triplicate, with three different experiments.
Effect of Placenta Peptide UF4 on Gingival Epithelial Cell Proliferation
The effect of this peptide on gingival epithelial cell proliferation was evaluated at 48 and 92h. Cells were seeded (2×104) into 6-well culture plates and cultured for 24h to allow for adequate adhesion. Thereafter, the medium was refreshed, and UF4 at various concentrations (0.1, 1, 10, and 100ug/mL) was added or not to the culture. The cells were incubated thereafter in a 5% CO2 humid incubator at 37°C. The medium and the UF4 were changed every other 24h. Following 48 and 96h of stimulation or not with UF4, cell proliferation was assessed by MTS colorimetric assay, as we previously reported [26]. The experiments were repeated four independent times, and the results were presented as the mean ±SD.
Effect of Placenta Peptide UF4 on Gingival Epithelial Cell Migration
Gingival epithelial cells were seeded (2×105) into wells of 6-well plates and cultured until they reached confluence. Crossed scratch wounds were created on each confluent monolayer using a 200μL sterile pipette tip perpendicular to the bottom of the dish. The culture medium was then refreshed with new medium supplemented with placenta peptide UF4 at various concentrations (0.1, 1, 10, and 100ug/mL), with the cells incubated in a CO2-humid atmosphere at 37°C. Cell migration was monitored using an inverted optical microscope and was photographed at different time points up to 48h. Wound closure (cell migration) was then analyzed using NIH ImageJ (32 bit) public-domain image processing software to measure the noncovered surface between the opposite edges of the wound as a function of time. The UF4-stimulated and non-stimulated cell cultures were compared, with the difference considered significant when p≤0.05. Results were reported as the mean ±SD, n=5.
Effect of Placenta Peptide UF4 on EGF And MMP-2 Secretion by Gingival Epithelial Cells
Cells were seeded (2×105) into wells of 6-well plates and cultured in a 5% CO2 humid atmosphere at 37ᵒC for 48h, after which time the cells were stimulated with placenta peptide UF4 at different concentrations (0, 0.1, 1, 10, or 100mg/mL) for 24h. At the end of this stimulation period, the culture medium of each well was collected and used to measure MMP-2 and EGF concentrations. The levels of these mediators were measured using ELISA kits (Cedarlane Canada, Burlington, ON, Canada), and absorbance was measured using a Microplate Reader Model 680 (Bio-Rad, Philadelphia, PA, USA). The sensitivity of the MMP-2 ELISA kit is 0.033ng/ mL, and that of the EGF ELISA kit is 0.7pg/mL. Four measurements were performed for each condition (n=4).
Statistical Analyses
Each experiment was performed at least three times, with the experimental values expressed as means ±SD. The statistical significance of the differences between the control (absence of placenta peptide) and test (presence of placenta peptide) values were determined by two-way ANOVA. Posteriori comparisons were performed using Tukey’s method, while normality and variance assumptions were verified using the Shapiro-Wilk test and the Brown and Forsythe test, respectively. All the assumptions were fulfilled. The p values were declared significant when ≤0.05. The data were analyzed using GraphPad INSTAT version 3 software.
Results and Discussion
Placenta Peptide UF4 Increased Gingival Epithelial Cell Morphology and Adhesion
Placenta peptide UF4 promoted gingival epithelial cell proliferation. In the first set of experiments, we evaluated the effect of UF4 on gingival epithelial cell adhesion, showing that this peptide had no negative effect on cell shape and adhesion. Figure 1A shows adherent cell density and shape in both the UF4-exposed and nonexposed cultures. Quantitative measurement using MTS colorimetric assays confirmed the non-toxic effect of UF4 on cell adhesion Figure 1B. Indeed, this peptide promoted the adhesion of gingival epithelial cells better than did the control, as the absorbance went from 0.82±0.01 in the control to 1.02±0.09 with 0.1μg/mL of UF4, 0.04±0.03 with 1 and 10μg/mL, and 0.95±0.03 with 100μg/mL of the peptide.
Cell adhesion is the first step in preparing the cell for proliferation [27,28]. We therefore evaluated the effect of UF4 on gingival epithelial cell growth (Figure 2), showing a significant increase of viable cells in the UF4-treated culture, compared to what was observed in the control. At 48h of stimulation, absorbance Figure 2A went from 0.66±0.07 in the control to 0.75±0.02 with 0.1μg/ mL, 0.83±0.06 with 1μg/mL, 0.91±0.03 with 10μg/mL, and finally 1.11±0.1 with 100μg/mL of UF4. The differences were significant (p˂0.05) between the UF4-stimulated conditions and the non-stimulated control and when comparing the UF4 concentrations to each other. A similar observation was made following the exposure of gingival epithelial cells to UF4 for 96h Figure 2B. For example, absorbance increased from 1.33±0.03 in the control to 2.07±0.08 with 100μg/mL of UF4. Previous studies using porcine placenta extract report that these peptides attenuated high glucose-induced intracellular ROS overproduction, which improves the viability, proliferation, migration, and angiogenesis of endothelial cells [14]. Furthermore, porcine placenta extract also increased Ki-67 expression and promoted splenocyte proliferation [29]. These probing data and our results suggest that porcine placenta peptide could promote wound healing.
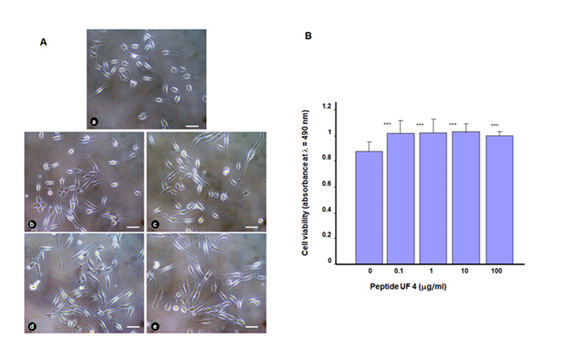
Figure 2: Cell morphology and distribution after stimulation with placenta peptide UF4. Gingival epithelial cells were seeded in the presence of UF4 and cultured for 24h. (A) Adherent cells were observed under an optical microscope and photographed. Photos are representative of four independent experiments. (a) nonstimulated control; (b) with UF4 at 0.1mg/ml; (c) UF4 at 1mg/ml; (d) UF4 at 10mg/ml, and (e) UF4 at 100mg/ ml. Scale bar = 20μm. (B) Adherent cells were assessed by MTS assay, n=4. ***p˂0.001 as a statistical difference when comparing the UF4- stimulated and nonstimulated (Ctrl) cells.
Placenta Peptide Increased Gingival Epithelial Cell Migration
Because UF4 was found to promote gingival epithelial cell adhesion and proliferation, this could improve cell migration after a wound. We thus investigated this hypothesis using a cell scratch assay and found that exposure of the wound culture to UF4 promoted cell migration leading to wound closure. As shown in Figure 3, the distance separating the wound edges decreased with time. This decrease was more noticeable in the UF4-treated culture than it was in the control. Our results support other observations that human placenta crude extract stimulates endothelial cell growth and motility [30]. The cell migration follow-up using an optical microscope was quantitatively confirmed by measuring the distance separating the two edges at different times post-scratch (Figure 3).
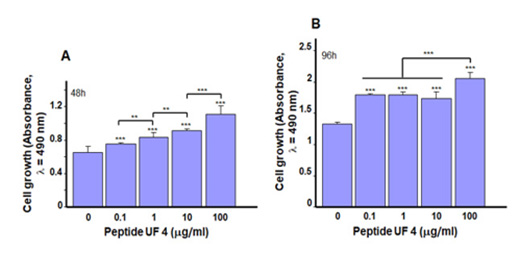
Figure 3: Placenta peptide UF4 promoted gingival epithelial cell proliferation. Cells were stimulated for 48 and 96h with various concentrations of UF4 then cell growth was assessed by colorimetric assay. (A) After 48h (B) After 96h of stimulation (n=5). **p˂0.01, ***p˂0.001. Free asterisks refer to the statistical difference when comparing the UF4-stimulated and non-stimulated (Ctrl) cells. Bars with asterisks show the comparison of the different peptide concentrations.
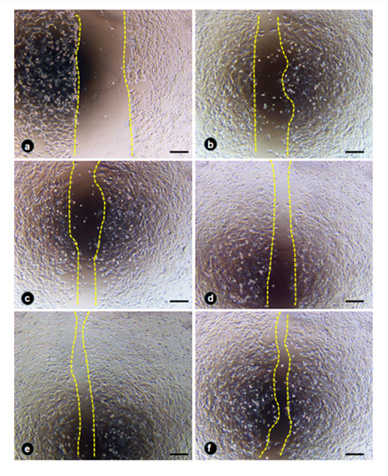
Figure 4: Placenta peptide UF4 promoted gingival epithelial cell migration. After the wound, UF4 was added to the culture medium, and the cell migration was observed and photographed at 24h post-wound. (a) photo immediately after wound; (b) nonstimulated control; (c) UF4 at 0.1mg/ml; (d) UF4 at 1mg/ml; (e) UF4 at 10mg/ml; and (f) UF4 at 100mg/ml. Photos are representative of four independent experiments (n = 4). Scale bar = 20 mm. (AU) = arbitrary unit; (UF4) = ultrafiltration 4.
Figure 4 shows a significant (p˂0.05) decrease of the uncovered area following stimulation with UF4, particularly at 10 and 100μg/mL. Of interest here is that when comparing the effects of each UF4 concentration to each other, the high amounts of UF4 (10 and 100μg/mL) promoted cell migration better than did the low amounts (0.1 and 1μg/mL) (Figure 4).
Our overall cell migration results demonstrate that placenta peptide had a positive effect on cell migration, thus suggesting their potential to promote wound healing [31,32]. These results agree with those reporting on human placenta extract and endothelial cell migration [30].
Placenta Peptide Promoted the Secretion of EGF by Gingival Epithelial Cells
Proliferation and migration are two cellular events that involve different mediators [33,34]. Growth factors such as EGF play an active role in stimulating cells to proliferate [35,36]. Because UF4 was found to increase gingival epithelial cell proliferation and migration, we proceeded to evaluate the secretion of EGF by gingival epithelial cells after stimulation with UF4. Our results (Figure 5) indicate a significant (p<0.05) increase of secreted EGF, as the concentration went from 310±0.4pg/mL in the control to 314pg/ mL with 10μg/mL of UF4 and 315±0.5pg/mL with 100μg/mL of UF4. The increased level of EGF confirms its stimulating effect on gingival epithelial cell proliferation; indeed, this factor reportedly promotes the growth of different cell types [35,36]. The secretion of other growth factors, such as FGF and IGF [37,38], could also be stimulated by UF4 promoting epithelial cell proliferation and migration. Further research must therefore be conducted to confirm this hypothesis.
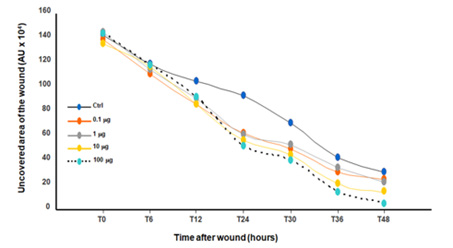
Figure 5: Quantitative measurement of the cell migration after wound then stimulation with placenta peptide UF4. Confluent gingival epithelial cell monolayers were scratched and exposed to UF4 at different concentrations. Wound closure referring to cell migration was followed at different times up to 48h (n=4). The noncovered area was measured using NIH-ImageJ software after each time. The non-stimulated and UF4-stimulated cultures were compared, as were the UF4 concentrations.
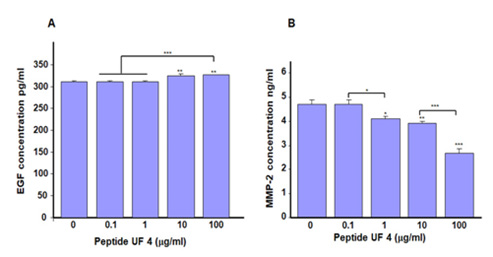
Figure 6: Placenta peptide UF4 increased EGF and decreased MMP-2 secretion by gingival epithelial cells. Cells were cultured in the presence of UF4 at various concentrations for 24h. The culture medium was then collected, filtered, and used to measure the levels of EGF. (A) and MMP-2 (B) As reported in the M&M. Results means ±SD from triplicate assays of three different experiments. *p˂0.05; **p˂0.01; ***p˂0.001. Free asterisks refer to the statistical difference when comparing the UF4-stimulated and non-stimulated (Ctrl) cells. Bars with asterisks show the comparison of the different peptide concentrations.
In addition to growth factors, such as EGF, UF4 modulates the secretion of MMP-2 by gingival epithelial cells. As shown in Figure 6, we observed a significant (p<0.01) decrease in the secretion of MMP-2 by gingival epithelial cells following stimulation by UF4 for 24h (Figure 6).
The levels of secreted MMP-2 ranged from 4.7±0.2 ng/mL in the control to 4.1±0.12 ng/mL with 1μg/mL of UF4, 3.9±0.1 ng/ mL with 10μg/mL of UF4, and finally 2.7±0.17ng/mL with 100μg/ mL of UF4. These results demonstrate the possible wound healing regulation by UF4 through MMP-2 secretion. Indeed, MMP-2 is secreted by fibroblasts as well as endothelial and epithelial cells [39]. MMP-2 is an essential cytokine that mediates type IV collagen proteolysis, an essential constituent of the basement membrane [40]. MMP-2 also contributes to the equilibrium of extracellular matrix production and degradation [41]. Of interest here is that the levels of MMP-2 decreased following the stimulation of gingival epithelial cells by UF4. Similar observations are reported with diabetic fibroblasts following exposure to electrical stimulation [42]. The decrease of MMP-2 we observed with UF4 suggests an improved Extracellular Matrix (ECM) buildup for better wound healing. Thus, UF4 could promote wound healing by decreasing proteolytic enzyme activities to reduce the degradation of the needed ECM and allowing for epithelial cell adhesion and growth. Therefore, this work supports other findings43 suggesting the potential of using placenta peptide to improve wound healing.
Conclusion
This study demonstrates that placenta peptide increases gingival epithelial cell proliferation and migration. The effect of placenta peptide could be through such growth factors as EGF, contributing to cell growth and migration. Our results also show that placenta peptide decreases the secretion of MMP-2 by gingival epithelial cells. This decrease could prevent the degradation of ECM, contributing to better cell adhesion and growth. Our overall findings suggest using placenta peptide to promote wound epithelialization.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author Contributions:
Conceptualization: W.P., M.C. and M.R.; Methodology: P.L. and M.R.; Investigation: W.P., M.C. and M.R.; Formal Analysis: P.L. and M.R.; Supervision: M.R., W.P. and M.C.; Writing (Original draft): P.L. and M.R.; Writing (review and editing): W.P. and M.C.; All authors approved the submission of the manuscript.
Funding
This work was supported by a grant from the Laval University Foundation, Émile-Beaulieu Fund, grant number FO127491. P.L. benefit from a Ph.D. studentship from the National Research Council of Thailand (NRCT) under the Ministry of Higher Education, Science, Research and Innovation, Royal Thai government.
Acknowledgement
None.
Conflict of Interest
None.
References
- Soncin F, Khater M, To C, Pizzo D, Farah O, et al. (2018) Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Dev 145(2): dev156273.
- Maltepe E, Fisher SJ (2015) Placenta: the forgotten organ. Annu Rev Cell Dev Biol 31: 523-552.
- Thapa L, He CM, Chen HP (2004) Study on the expression of angiotensin II (ANG II) receptor subtype 1 (AT1R) in the placenta of pregnancy-induced hypertension. Placenta 25(7): 637-641.
- Pan P, Fu H, Zhang L, Huang H, Luo F, et al. (2010) Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol 11: 1-9.
- J Delforce S, R Lumbers E, Morosin S Wang Y, G Pringle K (2019) The Angiotensin II type 1 receptor mediates the effects of low oxygen on early placental angiogenesis. Placenta 75: 54-61.
- Munoz Torres JR, Martínez González SB, Lozano Luján AD, Martínez Vázquez MC, et al. (2022) Biological properties and surgical applications of the human amniotic membrane. Front Bioeng Biotechnol 10: 1067480.
- Esteves J, Bhat KM, Thomas B, M Varghese J, Jadhav T (2015) Efficacy of human chorion membrane allograft for recession coverage: a case series. J Periodontol 86(8): 941-944.
- Schlanser V, Dennis A, Ivkovic K, Joseph K, Kaminsky M, et al. (2018) Placenta to the rescue: limb salvage using dehydrated human amnion/chorion membrane. J Burn Care Res 39(6): 1048-1052.
- Ghoneum M, El Gerbed HS (2021) Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer Chemother Pharmacol 88(6): 961-971.
- Yoshikawa C, Takano F, Ishigaki Y, Okada M, Kyo S, et al. (2012) Effect of porcine placental extract on collagen production in human skin fibroblasts. Gynecol. Obstet 3(6): 1-4.
- Huang J, Ling Z, Zhong H, Yin Y, Qian Y, et al. (2020) Distinct expression profiles of peptides in placentae from preeclampsia and normal pregnancies. Sci Rep 10(1): 1-11.
- Tebakari M, Daigo Y, Ishikawa H, Nakamura M, Kawashima J, et al. (2018) Anti-inflammatory Effect of the Water-Soluble Portion of Porcine Placental Extract in Lipopolysaccharide-Stimulated RAW264.7 Murine Macrophage Cells. Biol Pharm Bull 41(8): 1251-1256.
- Heo JH, Heo Y, Lee HJ, Kim M, Shin HY (2018) Topical anti-inflammatory and anti-oxidative effects of porcine placenta extracts on 2, 4-dinitrochlorobenzene-induced contact dermatitis. BMC complement med ther 18(1): 1-9.
- Nensat C, Songjang W, Tohtong R, Suthiphongchai T, Phimsen S, et al. (2021) Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC complement med ther 21(1): 1-13.
- Zhou Z, Wang D, Liu W, He L, Liang P, et al. (2022) The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods. Food Sci. Hum. Wellness 11(6): 1475-1481.
- Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, et al. (2018) A porcine placental extract prevents steatohepatitis by suppressing activation of macrophages and stellate cells in mice. Oncotarget 9(19): 15047-15060.
- Moon PD, Kim KY, Rew KH, Kim HM, Jeong HJ (2014) Anti-fatigue effects of porcine placenta and its amino acids in a behavioral test on mice. Can J Physiol Pharmacol 92(11): 937-944.
- Laosam P, Panpipat W, Chaijan M, Roytrakul S, Charoenlappanit S, et al. (2022) Molecular Structures and In Vitro Bioactivities of Enzymatically Produced Porcine Placenta Peptides Fractionated by Ultrafiltration. Food Bioproc Tech 15(3): 669-682.
- Cianfarani F, Zambruno G, Brogelli L, Sera F, Lacal PM, et al. (2006) Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am J Pathol 169(4): 1167-1182.
- Bryant-Greenwood GD (1991) Human decidual and placental relaxins. Reproduction, Reprod Fertil Dev 3(4): 385-389.
- M Failla C, Odorisio T, Cianfarani F, Schietroma C, Zambruno G, et al. (2000) Placenta growth factor is induced in human keratinocytes during wound healing. J Invest Dermatol 115(3): 388-395.
- Hamada Y, Yeh YT, B Blanchard S (2020) Amnion–Chorion Allograft Barrier Used on Root Surface for Regenerative Procedures: Case Report. Clin. adv. Periodontics 10(4): 195-199.
- Kiany F, Moloudi F (2015) Amnion membrane as a novel barrier in the treatment of intrabony defects: a controlled clinical trial. Int J Oral Maxillofac Implants 30(3): 639-647.
- Laosam P, Panpipat W, Yusakul G, Cheong LZ, Chaijan M, et al. (2021) Porcine placenta hydrolysate as an alternate functional food ingredient: In vitro antioxidant and antibacterial assessments. PLoS One 16(10): e0258445.
- P Gilchrist E, Moyer MP, J Shillitoe E, Clare N, A. Murrah V (2000) Establishment of a human polyclonal oral epithelial cell line. Oral Surg Oral Med 90(3): 340-347.
- Cui S, Rouabhia M, Semlali A, Zhang Z (2021) Effects of electrical stimulation on human skin keratinocyte growth and the secretion of cytokines and growth factors. J Biomed Mater 16(6): 065021.
- Jones M, Zha J, Martin J (2019) Humphries Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos Trans R Soc 374(1779): 20180227.
- Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol 10(1): 21-33.
- Han NR, Kim KY, Kim MJ, Kim MH, Kim HM, et al. (2013) Porcine placenta mitigates protein–energy malnutrition-induced fatigue. Nutr J 29(11-12): 1381-1387.
- Presta M, Mignatti P, Mullins DE, Moscatelli DA (1985) Human placental tissue stimulates bovine capillary endothelial cell growth, migration and protease production. Biosci Rep 5(9): 783-790.
- Tiwary SK, Shukla D, Tripathi AK, Agrawal S, Singh MK, et al. (2006) Effect of placental-extract gel and cream on non-healing wounds. J Wound Care 15(7): 325-328.
- Jash A, Kwon HK, Sahoo A, Lee CG, So JS et al (2011) Topical application of porcine placenta extract inhibits the progression of experimental contact hypersensitivity. J Ethnopharmacol 133(2): 654-662.
- Dolati S, Yousefi M, Pishgahi A, Nourbakhsh S, Pourabbas B (2020) Prospects for the application of growth factors in wound healing. Growth factors 38(1): 25-34.
- Vaseenon S, Chattipakorn N, Chattipakorn SC (2020) The possible role of basic fibroblast growth factor in dental pulp. Arch Oral Biol 109: 104574.
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5): 585-601.
- Miller Kobisher B, Suárez Vega DV, Maldonado GJV (2021) Epidermal growth factor in aesthetics and regenerative medicine: Systematic review. J Cutan Aesthet Surg 14(2): 137.
- Uluer ET, Vatansever HS, Aydede H, Ozbilgin MK (2019) Keratinocytes derived from embryonic stem cells induce wound healing in mice. Biotech. Histochem 94(3): 189-198.
- Walther M, Vestweber PK, Kühn S, Rieger U, Schäfer J, et al. (2022) Bioactive Insulin-Loaded Electrospun Wound Dressings for Localized Drug Delivery and Stimulation of Protein Expression Associated with Wound Healing. Mol Pharm 20(1): 241–254.
- Gama Sosa MA, De Gasperi R, Pryor D, Perez Garcia GS, Perez GM, et al. (2021) Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol Commun 9(1): 167.
- Page McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol 8(3): 221-233.
- Dissemond J, Augustin M, Dietlein M, Faust U, Keuthage W, et al. (2020) Efficacy of MMP-inhibiting wound dressings in the treatment of chronic wounds: a systematic review. J Wound Care 29(2): 102-118.
- Abedin Do A, Zhang Z, Douville Y, Méthot M, Bernatchez J, et al. (2022) Engineering diabetic human skin equivalent for in vitro and in vivo applications. Front. Bioeng. Biotechnol 10: 989888.
- Nagae M, Nagata M, Teramoto M, Yamakawa M, Matsuki T, et al. (2020) Effect of porcine placenta extract supplement on skin condition in healthy adult women: A randomized, double-blind placebo-controlled study. Nutrients 12(6): 1671.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.