Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Antimalarial Assessment of Artesunate/Ketoconazole in a Mouse Model
*Corresponding author: Elias Adikwu, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria.
Received: June 06, 2023; Published: July 12, 2023
DOI: 10.34297/AJBSR.2023.19.002601
Abstract
Malaria is a common cause of death in the tropics. Drug repurposing is a quick and effective modality for the discovery of antimalarial drugs. Artesunate (AS) is combined with other antimalarial drugs for malaria treatment. Ketoconazole (KT) is an antifungal drug with possible antiplasmodial activity. This study evaluated the antiplasmodial effect of AS/KT on a mouse model infected with Plasmodium berghei. Sixty Swiss albino mice of both sexes weighing 30-35g randomly grouped into n=5/ group were used. The mice were inoculated with Plasmodium berghei (1x107) intraperitoneally. Thereafter, using the curative and suppressive protocols, the mice were orally treated with AS (12mg/kg/day), KT (7mg/kg/day) and AS/KT, respectively. Chloroquine (CQ) (10mg/kg/day) served as the standard control. After treatment, blood samples were evaluated for percentage parasitemia, hematological and liver function parameters. The mice were observed for mean survival time and liver tissues were histologically assessed for changes. In the curative, and suppressive tests, AS/KT significantly (p<0.05) decreased percentage parasitemia when compared to AS or KT. In the curative test, AS, KT and AS/KT produced 83.21%, 71. 84% and 92.11% parasitemia inhibitions, respectively while CQ produced 90.63% parasitemia inhibition. AS/KT significantly (p<0.05) prolonged mean survival time in the curative test when compared to AS or KT. AS/KT significantly (p<0.05) restored hematological parameters (hemoglobin, red blood cells, packed cell volume and white blood cells) when compared to AS or KT. AS/KT restored liver histology in the parasitized mice. AS/KT shows promising antiplasmodial activity.
Keywords: Artesunate, Ketoconazole, Combination, Malaria, Mice
Introduction
About 3.2 billion people globally are at risk of malaria with greater than 200 million cases of malaria infection reported yearly. According to the World Health Organization (WHO) in 2015, there were 214 million cases of malaria and 438,000 malaria-related mortality, primarily in sub-Saharan Africa [1]. Vector control programme and antimalarial drug use have been the main approaches for the control of malaria infection [2]. The major set-backs to the aforementioned approaches are cost and the development of resistance by malaria parasites [3]. Hence the urgent need to develop novel and cost-effective antimalarial drugs as well as synergistic partners for artemisinins cannot be overemphasized. The reliance on the traditional drug development methods to deliver on this goal have been associated with significant implications on both cost and time.
Drug repurposing or repositioning connotes the use of an existing drugs in diseases other than those it was originally used for. It offers a route to significantly shorten the traditional drug development pipelines [4]. It affords attractive, alternate and valid paradigm for drug discovery [5,6]. For diseases like malaria, drug repositioning may not only deliver novel candidates, but also provide partner drugs for combinatorial regimens with artemisinins, thereby increasing longevity of these highly effective and affordable frontline drugs [7,8].
Artesunate is an antimalarial drug that is a semi-synthetic, and water-soluble, artemisinin derivative. It displays high and fast antiplasmodial activity with a spectrum of specific action on the various stages of malaria parasite. It vividly and rapidly kills circulating ring-stage parasites and prevents their maturation and sequestration in organs such as the brain and liver [9]. It has been used in combination with other antimalarial drugs such as amodiaquine, mefloquine, sulfadoxine/pyrimethamine and pyronaridine for the treatment of malaria with outstanding results [10]. It is imperative for its combination with new antimalarial drugs to be explored.
The effectiveness of some antifungal drugs against malaria parasites have been documented in some studies [11]. Ketoconazole, an antifungal that inhibits ergosterol formation has been reported to have notable antimalarial activity [12]. In-vitro studies reported that ketoconazole in combination with α/β arteether showed increased antimalarial activity against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum strains as well as with the multidrug-resistant Plasmodium yoelii nigeriensis [13]. In the absence of in-vivo studies, this study investigated the potential of repurposing ketoconazole in combination with artesunate as an antimalarial drug in Plasmodium berghei-infected mice.
Methods
Animals
Sixty (60) adult Swiss albino mice (30-35g) of both sexes were obtained from the animal house of the Department of Pharmacology, Faculty of Basic Clinical Sciences, University of Port Harcourt, Rivers State, Nigeria. The mice were kept under standard conditions (temperature 24˚C± 1˚C; 12:12 days/night; relative humidity of 60%±5%) and were housed in wood-shaving-bedded standard plastic cages. The study was performed in the Department of Pharmacology/ Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria.
Ethical Considerations and Approval
Ethical approval was granted by the Research Ethics Committee of the Department of Pharmacology/Toxicology, Faculty of Pharmacy, Niger Delta University, Nigeria. The mice were handled using the guidelines of the National Research Council [14].
Drugs and Chemicals
Chloroquine (Evans, Nigeria), artesunate (Mekophar, Vietnam), ketoconazole (Medreich, India), and methanol (JHD Sci-Tech. Co. Ltd, China) were used.
Plasmodium Parasite
Chloroquine (CQ) sensitive Plasmodium berghei sourced from the National Institute of Medical Research, Yaba, Lagos State, Nigeria was used. It was preserved by the inoculation of a fresh mouse from a donor mouse intraperitoneally every four days.
Inoculation of Mice with Parasite
Mouse infected with Plasmodium berghei with a parasitemia level of 35% served as the donor blood sample was collected using an ethylene diamine tetra acetic acid bottle, retro-orbitally, and percentage parasitemia and red blood cells (RBCs) count were determined. Preparation of inoculum was done by diluting the blood with normal saline which was administered intraperitoneally. Approximately 1×107 parasitized RBCs were present in 0.2mL of the blood solution.
Evaluation of Antiplasmodial Activity
Curative Test
The method explained by Adikwu, et al., 2022 [15] was used. The mice were infected with RBCs containing Plamodium berghei (1×107) intraperitoneally. The mice were grouped into 6 of n=5/ group and allowed for 3 days. Thereafter, the mice were orally treated with AS (12mg/kg/day), KT (7mg/kg/day), and AS/KT respectively for 4 days. The standard control was orally treated with CQ (10mg/kg/day) while the parasitized and normal controls were orally treated daily with normal saline. Tail blood samples were obtained and thin blood films were produced on microscope slides. The slides were fixed in methanol allowed to dry and stained with 10% Giemsa stain. The slides were viewed with the aid of a microscope and the percentage parasitemia and inhibitions were calculated as shown below.
Suppressive Test
The protocol explained by Adikwu, et al., 2022 [15] was used. The mice were intraperitoneally infected with RBCs containing Plasmodium berghei (1×107) intraperitoneally. The mice were randomly grouped into 6 of 5 mice /group. The mice were allowed for 2 hours, thereafter, they were orally treated with AS (12mg/kg/day), KT (7mg/kg/day), and AS/KT respectively for 4 days. The standard control was orally treated with CQ (10mg/kg/day) while the parasitized and normal controls were treated daily with normal saline. Tail blood samples were obtained, and thin blood films were prepared and processed as explained above. The percentage parasitemia and inhibitions were calculated as shown below.
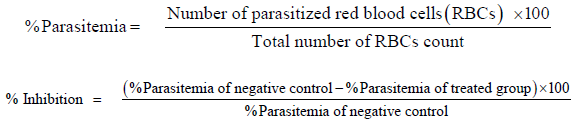
Evaluation of Mean Survival Time
The mice in the curative group were observed for mortality in days which was calculated as mean survival time (MST) using the formula below
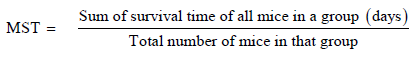
Analyses of Hematological and Liver Biochemical Parameters
On the final day of treatment, blood samples were collected in heparinized sample containers from the mice used for the curative study. The samples were assessed for Alanine Amino Transferase (ALT), Aspartate Amino Transferase (AST), Alkaline Phosphatase (ALP), Red Blood Cells (RBCs), Hemoglobin (Hb), White Blood Cells (WBCs) and Packed Cell Volume (PCV) using an auto analyzer.
Liver Histology
After the observation of mean survival time, liver samples were obtained from the mice used for curative study and stored in formalin saline for 24hr. There after, the samples were processed and sectioned (3μcm thick) using a microtome. The sectioned liver tissues were stained on slides with hematoxylin and eosin and viewed with the aid of a microscope. The relevant sections were photographed.
Data Analysis
Data was analyzed using the One-way Analysis of Variance (ANOVA) followed by Turkey’s post hoc test. Data was presented as mean± standard error of mean (mean± SEM). A P< 0.05 was considered significant.
Results
Curative Effect of Artesunate/Ketoconazole on Plasmodium Berghei-Infected Mice
Treatment with AS/KT produced significant daily parasitemia reductions (p<0.05) when compared to treatment with AS and KT individually. On day 4, AS/KT produced 3.51±0.38 % parasitemia, while AS, KT and CQ produced 7.47±0.22%, 12.53±0.47% and, 4.71±0.34% parasitemia, respectively. AS/KT produced 92.11 % inhibition whereas AS, KT and CQ produced 83.21% 71.84% and 90.63% inhibitions, respectively. It was observed that AS/KT increased MST significantly (p<0.05) when compared to AS and KT, respectively (Table 1).
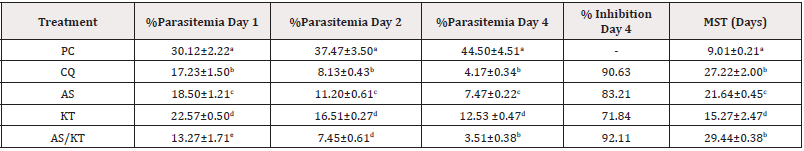
Table 1: Curative effect of artesunate/ketoconazole on Plasmodium berghei-infected mice.
Note*: Data expressed as mean ± standard error of mean, n=5, PC: Parasitized Control, CQ: Chloroquine (Standard), AS: Artesunate, KT: Ketoconazole. MST: Mean Survival Time, Values with different superscripts down the column differ significantly at p<0.05 (ANOVA).
Suppressive Effect of Artesunate/Ketoconazole on Plasmodium Berghei-Infected Mice
It was observed that in the suppressive study, treatment with AS/KT significantly (p<0.05) decreased percentage parasitemia when compared to AS and KT respectively. Treatment with AS/KT produced 1.12±0.01% parasitemia when compared to 3.00±0.50%, 5.01±0.23% and 1.33±0.07% parasitemia produced by AS, KT and CQ, respectively. AS/KT produced inhibition which represents 94.45% whereas inhibitions which represent 85.13%, 75. 16% and 93.41% were produced by AS, KT and CQ respectively (Table 2).
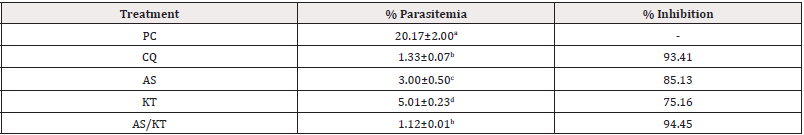
Table 2: Suppressive effect of artesunate/ketoconazole on Plasmodium berghei-infected mice.
Note*: Data expressed as mean ± standard error of mean, n=5, PC: Parasitized control, CQ: Chloroquine (Standard), AS: Artesunate, KT: Ketoconazole. MST: Mean Survival Time, Values with different superscripts down the column differ significantly at p<0.05 (ANOVA).
Effect of Artesunate/Ketoconazole on Hematological and Liver Parameters of Plasmodium Berghei-Infected Mice
Significantly (p<0.05) decreased RBCs, PCV and Hb and significantly (p<0.05) increased WBCs were observed in Plasmodium berghei-infected mice when compared to the normal control. On the other hand, AS/KT significantly increased RBCs, PCV and Hb and significantly decreased WBCs when compared to AS or KT at p<0.05. The effects of AS/KT on RBCs, PCV, Hb and WBCs were not statistically (p>0.05) different from CQ. Furthermore, AS/KT had no significant (p>0.05) effects on serum ALT, AST and ALP levels when compared to AS or KT (Tables 3,4).
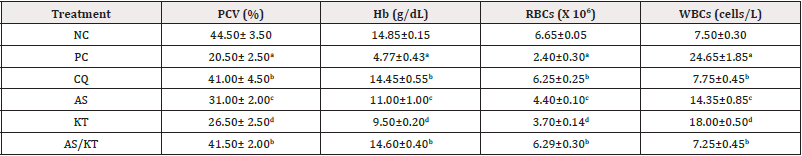
Table 3: Effect of artesunate/ketoconazole on hematological parameters on Plasmodium berghei-infected mice.
Note*: Data expressed as mean ± standard error of mean, n=5, NC: Normal Control, PC: Parasitized Control, CQ: Chloroquine (Standard), AS: Artesunate, KT: Ketoconazole. RBCs: Red Blood Cells, WBCs: White Blood Cells, PCV: Packed Cell Volume, Hb: Hemoglobin; Values with different superscripts down the column differ significantly at p<0.05 (ANOVA).

Table 4: Effect of artesunate/ketoconazole on liver biochemical parameters of parasitized mice.
Note*: Data expressed as mean ± standard error of mean, n=5, NC: Normal Control, PC: Parasitized Control, CQ: Chloroquine (Standard), AS: Artesunate, KT: Ketoconazole. AST: Aspartate Aminotransferase, ALT: Alanine Aminotransfease, ALP: Alkaline Phosphatase.
Effect of Artesunate/Ketoconazole on Liver Histology
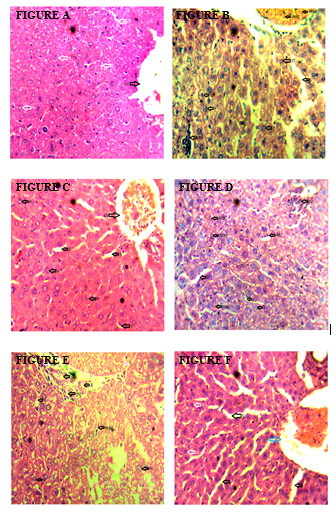
Figure A: Liver of the control mice, Figure B: Liver of parasitized control mice, Figure C: Liver of parasitized mice treated with chloroquine, Figure D: Liver of parasitized mice treated with artesunate, Figure E: Liver of parasitized mice treated with ketoconazole, Figure F: Liver of parasitized treated with artesunate/ketoconazole.
Note*: H: Hepatocytes, S: Sinusoids, NCV: Normal Central Vein, CV: Central Vein Congestion, INF: Inflammatory Cells, ST: Steatosis, PRBC: Parasitized Red Blood Cells.
The liver of the control mice showed normal hepatocytes, sinusoids and central vein (Figure A), while the liver of the parasitized control mice showed steatosis, inflammatory cells, and parasitized red blood cells (Figure B). The liver of parasitized mice treated with chloroquine (Figure C) and artesunate (Figure D) showed normal sinusoids, hepatocytes and congested central vein. The liver of parasitized mice treated with ketoconazole showed parasitized red blood cells, inflammatory cells and normal hepatocytes and sinusoids (Figure E) whereas the liver of parasitized mice treated with artesunate/ketoconazole showed normal hepatocytes, sinusoids and central vein congestion (Figure F).
Discussion
The vacuum in the market for new antimalarial drugs and the paucity of affordable alternatives in the developmental pipeline, make it imperative that very fast drug developmental methods are urgently established to avoid the imminent consequences of drug failure [4]. The aforementioned can be achieved through the repurposing of existing clinically used drugs by taking advantage of their combinations with artemisinins. This work provides an in-depth assessment of the antimalarial activity of KT in partnership with AS in parasitized mice. The current study used the most common mouse models of malaria which employ rodent-specific parasite species such as Plasmodium berghei that cause immune responses and distinct pathologies and model different manifestations of human diseases including malaria [16]. This study used the two in-vivo models frequently employed for the antimalarial screening of new compounds which are 4 days suppressive and curative tests that evaluate the suppressive and curative capabilities of candidate drugs on early and established infections respectively [17]. The two animal models were also used, because they permit possible pro-drug effect and the activity of the host defense system in infection eradication [18]. The observation in the current study showed notable curative antiplasmodial activity of AS/KT characterized by daily reductions in percentage parasitemia. The observed curative antiplasmodial activity of AS/KT was at par with the standard control. Also, AS/KT produced very visible suppressive antiplasmodial activity marked by reduced percentage parasitemia, which was the same with the effect produced by the standard control.
Severe malaria manifests a variety of clinical syndromes which depends on the properties of the host and the parasite. There is now budding evidence, from both human and mouse studies of malaria, which showed that anemia is not only related to hemolysis of infected and clearance of uninfected RBCs, but also due to inadequate erythroid response by the infected host [19]. Rodent malaria species infected with Plasmodium species have been used to investigate the contribution of various aspects of anemia in malaria. Studies used hematological markers, which include PCV, Hb and RBCs for assessing malaria related anemia while WBCs, total and differential counts are determinants of the severity of infections [20]. In this study, AS/KT remarkably alleviates anemia in the parasitized mice marked by conspicuous increased RBCs, PCV, and Hb. It also alleviates the severity of malaria infection as characterized by decreased WBCs. Interestingly, the propensity of AS/KT to alleviate anemia is comparable to the standard control. Malaria is a significant cause of mortality worldwide. One of the significant goals of antimalarial drug use is the prevention or reduction of malaria related death. Experimentally, in malaria studies, MST in measured to ascertain the proficiencies of drug candidates to reduced or prevent malaria related death [21]. The present study observed that AS/KT remarkably prolonged MST in the treated mice. Its impact on MST was comparable to the standard control. At present, monitoring of malaria-associated liver injury is heterogeneous, with the measurement of biochemical liver function tests which involves liver enzymes (AST and ALT) being the primary determinant for liver injury [22]. Also, the measurement of the aforementioned indices is imperative due to the potential of some antimalarial drugs to cause liver injury [23,24]. The current study observed that treatment with AS/KT had no deleterious effects on serum AST, ALT and ALP levels.
Liver involvement in severe malaria infection especially in Plasmodium falciparum infection is a significant cause of mortality in humans characterized by deleterious liver morphological changes [25]. After mortality, this study histologically examined the liver of mice in the control and treated groups. Steatosis, inflammatory cell infiltrations, and PRBCs were conspicuous in the liver of the death parasitized control. The observation correlates with similar changes in the liver of patients who died of malaria reported by Viriyavejakul, et al., 2014 [25]. Interestingly, the afore mentioned changes were absent in the liver of AS/KT treated mice except for the observed central vein congestion. The study shows that KT augmented the antiplasmodial activity of AS. The mechanism of the antiplasmodial action of KT is not known, but its antifungal effect involves the inhibition of the formation of ergosterol in cell membrane. Studies have shown that KT is a primary inhibitor of liver CYP 3A4. It may have increased the antiplasmodial activity of AS by inhibiting CYP 3A4, which is responsible for the metabolism of AS thereby prolonging the plasma concentration of AS [13]. Conclusion: This study showed that AS/KT exhibited remarkable suppressive and curative antiplasmodial activities. It is suggested that KT can be repurposed in combination with AS for the treatment of malaria.
Funding
None.
Acknowledgement
None.
Conflicts of Interest
None.
References
- (2015) World Health Organization. World Malaria Report. Geneva, Switzerland: WHO Press.
- Rappuoli R, Aderem A (2011) A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 473(7348): 463-469.
- RTS, SC (2015) Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. The Lancet 386(9988): 31-45.
- Matthews H, Usman Idris M, Khan F, Martin Read, Niroshini Nirmalan (2013) Drug repositioning as a route to anti-malarial drug discovery: preliminary investigation of the in vitro anti-malarial efficacy of emetine dihydrochloride hydrate. Malar J 12: 359.
- Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 3(8): 673-683.
- Ekins S, Williams AJ, Krasowski MD, Freundlich JS (2011) In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today 16(7-8): 298-310.
- Nzila A, Ma Z, Chibale K (2011) Drug repositioning in the treatment of malaria and TB. Future Med Chem 3(11): 1413-1426.
- Burrows JN, Leroy D, Lotharius J, Waterson D (2011) Challenges in antimalarial drug discovery. Future Med Chem 3: 1401-1412.
- Marijon A, Bonnot G, Fourier A, Coralie Bringer, Adeline Lavoignat, et al. (2014) Efficacy of intranasal administration of artesunate in experimental cerebral malaria. Malar J 13: 501.
- Koko VS, Warsame M, Vonhm B, Moses K Jeuronlon, Didier Menard, et al. (2022) Artesunate-amodiaquine and artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Liberia: in vivo efficacy and frequency of molecular markers. Malar J 21(1): 134.
- Crowley PD, Gallagher HC (2014) Clotrimazole as a pharmaceutical: past, present and future. J appl microbiol 117(3): 611-617.
- Deutsch PH, Quintiliani R (1954) Ketoconazole. Connecticut medicine 48(4): 216-221.
- Tripathi R, Rizvi A, Pandey SK, Dwivedi H, Saxena JK (2013) Ketoconazole, a cytochrome P450 inhibitor can potentiate the antimalarial action of α/β arteether against MDR Plasmodium yoelii Nigeriensis. Acta Trop 126(2): 150-155.
- (2011) National Research Council. Guidance for the description of animal research in scientific publications.
- Ryley JF, Peters W (1970) The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol 64(2): 209-222.
- Huang BW, Pearman E, Kim CC (2015) Mouse Models of Uncomplicated and Fatal Malaria. Bio Protoc 5(13): e1514.
- Adikwu E, Nworgu CO, Ajeka SI (2023) Antimalarial activity of amodiaquine-moxifloxacin: A study in mice. Arch Curr Med Res 4(1): 1-6.
- Mekonnen LB (2015) In vivo antimalarial activity of the crude root and fruit extracts of Croton macrostachyus (Euphorbiaceae) against Plasmodium berghei in mice. J Tradit Complement Med. 5(3): 168-173.
- Lamikanra AA, Brown D, Potocnik A, Casals Pascual C, Langhorne J, et al. (2007) Malarialanemia:ofmiceandmen Blood 110 (1): 18-28.
- Adikwu E, Ajeka SI, Nworgu CO (2022) Antiplamodial effect of sulfadoxine/pyrimethamine/clindamycin: A study in parasitized mice. Bulletin of Biotechnology 3(2): 32-38.
- Adikwu E, Ajeka SI, Nworgu CO (2022) Amodiaquine-Azithromycin Eradicates Blood and Liver Stages of Plasmodium berghei Infection in Mice. Am J Biomed Sci 14(3): 136-145.
- Woodford J, Shanks GD, Griffin P, Chalon S, McCarthy JS (2018) The dynamics of liver function test abnormalities after malaria infection: a retrospective observational study. Am J Trop Med Hyg 98(4): 1113-1119.
- Wielgo Polanin R, Lagarce L, Gautron E, Diquet B, Lainé Cessac P (2005) Hepatotoxicity associated with the use of a fixed combination of chloroquine and proguanil. Int J Antimicrob Agents 26(2): 176-178.
- Alkadi HO (2007) Antimalarial drug toxicity: A review. Chemotherapy 53(6): 385-391.
- Viriyavejakul P, Khachonsaksumet V, Punsawad C (2014) Liver changes in severe Plasmodium falciparum malaria: histopathology, apoptosis and nuclear factor kappa B expression. Malar J 13: 106.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.