Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cardiorespiratory Fitness Decreases High Sensitivity C-Reactive Protein and Improves Parameters of Metabolic Syndrome
*Corresponding author: Hildemar Dos Santos, Preventive Care, Loma Linda University School of Public Health, Loma Linda, USA.
Received: August 21, 2023; Published: August 28, 2023
DOI: 10.34297/AJBSR.2023.19.002658
Abstract
Aim: To evaluate the relationship between Cardiorespiratory Fitness (CRF), expressed as maximal oxygen uptake (ml.kg-1.min-1), metabolic syndrome, and hs-CRP.
Methods and Results: The relationship between Cardiorespiratory Fitness (CRF), metabolic syndrome, and hs-CRP was examined among 173 men and women. CRF was evaluated using the Bruce protocol treadmill test and measured as maximal oxygen uptake (VO2max). Participants’ physical activity status was assessed through self-report. Plasma hs-CRP levels were measured using a standardized immunoassay. The diagnostic criteria for Metabolic Syndrome (MetS) were based on guidelines established by both the World Health Organization (WHO) and the U.S. National Cholesterol Education Program Adult Treatment Panel III (ATP III).
Results: An inverse association was found between hs-CRP and VO2max (p<0.01) and between VO2max and the number of MetS criteria (p<0.01). Hs-CRP increased linearly with the number of MetS characteristics (p<0.01). Subjects engaging in 2-3hours of exercise per week had hs-CRP levels ≤2.5mg/L (p=0.018).
Conclusion: Higher VO2 max is associated with lowered hs-CRP levels and MetS criteria. Higher Hs-CRP is associated with a higher number of MetS criteria. Higher VO2 max is associated with higher reported physical activity levels. These associations confirmed the mechanisms by which CRF reduces the risk of CVD and diabetes
Keywords: C-reactive protein, Exercise, Metabolic syndrome, Atherosclerosis, Inflammation
Introduction
The leading cause of death in the U.S. is Cardiovascular Disease (CVD) [1]. Today, it is well known that insulin resistance plays an integral role in CVD etiology [2,3]. Certain CVD risk factors appear to be exacerbated by insulin resistance. According to the most recent guidelines, the Adult Treatment Panel-III (ATP-III), Metabolic Syndrome (MetS) is marked by the presence of a tally of three car diovascular disease (CVD) risk factors, including abdominal obesity, low High-Density Lipoprotein (HDL) cholesterol [<40mg/dL in men; <50mg/dL in women], elevated serum Triglycerides (TG) [>175mg/dL], elevated blood pressure, and impaired fasting glucose [4].
According to the US National Health and Nutrition Examination Survey (NHANES) conducted between 2011 and 2018, there was a notable rise in the prevalence of MetS in the United States in adults aged ≥20 years [5]. The rates were reported as 37.6% during 2011/2012 and increased to 41.8% in 2017/2018 [5]. MetS is also strongly linked to an elevated likelihood of developing conditions such as diabetes, Cardiovascular Diseases (CVDs), and various types of cancers [6]. The risk of cardiovascular disease appears to be increased by two-fold and type 2 diabetes by five-fold [7]. Hence, the ATP-III report emphasizes the significance of preventive therapies in these individuals.
Engaging in regular physical activity has been shown to have notable impacts on the immune system and may offer protective benefits against cardiovascular diseases and diabetes [8]. Physical activity is also considered an important determinant of metabolic syndrome [9]. All levels of metabolic syndrome, diabetes, and cardiovascular disease are thought to involve inflammation. Physical activity may reduce risk, at least in part, by modifying the inflammatory process. Recent studies have demonstrated an inverse relationship between inflammatory markers, such as high-sensitivity C-reactive protein (hs-CRP), and physical activity [10,11]. Elevated hs-CRP appears to be an independent predictor of both cardiovascular disease and diabetes. Recent evidence also suggests that hs-CRP is positively associated with all metabolic syndrome characteristics [12]. Based on these findings we sought to evaluate the relationship between hs-CRP, metabolic syndrome, and Cardiorespiratory Fitness (CRF), expressed as maximal oxygen uptake-VO2 max (ml.kg-1.min-1).
Materials and Methods
Study Sample
Study participants were selected from a pool of 1,072 men and women from the Center of Health Promotion (CHP) at Loma Linda University. Participants were included in this study if they:
1) Underwent a preventive medicine health exam at the CHP,
2) which included blood pressure screening and blood analysis (hs-CRP, lipid profile, and fasting blood glucose).
3) Completed Comprehensive Personal Wellness Profile (PWP) questionnaire regarding their medical history and lifestyle health practices (Wellsource Inc., Clackamas, OR).
4) Reached ≥85% of age-predicted maximal heart rate on the Bruce protocol treadmill stress test.
5) Were able to give informed consent. 173 subjects met the criteria and were included in the study.
Human Studies and Subjects
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/ or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants gave their informed consent prior to their inclusion in the study.
Clinical Measurements
Cardiorespiratory fitness level was assessed via maximal oxygen uptake (VO2max, expressed in ml.kg-1.min-1) estimated from the Bruce protocol treadmill stress test. The Personal Wellness Profile (PWP) questionnaire was used to collect information regarding the participant’s medical history and lifestyle behaviors such as smoking habits, alcohol intake patterns, eating habits, stress and coping patterns, social health, and physical activity status. Participants were asked about their current physical activity status under the headings of
1) No regular exercise program.
2) Occasionally walking for pleasure.
3) Regular exercise in work or recreation requiring modest physical activity such as golf, yard work, calisthenics, up to one hour per week.
4) Regular exercise in work or recreation requiring modest physical activity such as golf, yard work, calisthenics, more than one hour per week.
5) More active physical exercise (brisk walking, jogging, swimming). If the participant answered yes to the last question they indicated how much time was spent engaging in that activity each week.
Quest Diagnostics performed blood chemistry analyses. Plasma hs-CRP concentrations were measured with the Dade Bering BN II high-sensitivity immunoassay (Dade Behring, Inc. IL). Fasting glucose was performed on the Olympus AU5400 Analyzer (Olympus America Inc., NY). Total cholesterol and triglyceride analyses were performed on the Olympus AU5400 Analyzer, while HDL-cholesterol was performed on a Roche Analyzer (Roche Molecular Systems Inc., CA).
Participants had their resting blood pressure measured by auscultation. Standard procedures outlined by the American Medical Association were followed [13]. Weight and height were measured on a standardized physician’s balance beam scale and stadiometer. BMI (weight (kg)/height (m)2) was also calculated for each participant.
Definitions
Although the definition of Metabolic Syndrome has not been agreed upon internationally, a working definition continues to be used [4,14]. Our definition utilizes criteria set forth by both the WHO and the U.S. National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines [4,14]. According to ATP III, participants had to meet at least three of the following variables in order to be labeled as having metabolic syndrome: (1) blood pressure ≥130/85mm Hg, (2) HDL-C ≤40mg/dl for males, ≤50mg/dl for females, (3) fasting blood glucose ≥110mg/dl, (4) elevated TG ≥150mg/dl, and (5) BMI ≥30kg/m2. Due to the retrospective design of this study and the lack of waist circumference measurements, we chose to use BMI (≥30kg/m2) as our measure of obesity rather than waist circumference. The WHO definition utilizes ≥30kg/m2 as the criterion for obesity [14] (Table 1).
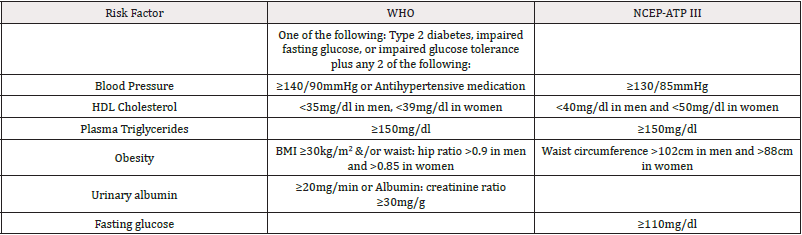
Table 1: WHO and ATP III Clinical Identification of the Metabolic Syndrome*.
*Note: Derived from Huang (2009) [14].
Statistical Methods
Any missing data variables were imputed using the maximum likelihood method (EM) in SYSTAT version 10; SPSS©2000. Because the percent body fat variable required over 100 imputations to fill in all of the missing data it was not used in any analyses. The distribution of hs-CRP, the dependent variable of greatest interest, was positively skewed. Therefore, a log transformation was applied to hs-CRP values for all analyses. Simple regression/correlation analysis was used to evaluate the relationships between log (CRP) and each of the relevant variables: VO2 max (ml.kg-1.min-1), metabolic syndrome, and physical activity status. The following relationships were explored:
1) hs-CRP and VO2 max.
2) hs-CRP and metabolic syndrome characteristics.
3) VO2 max and metabolic syndrome characteristics.
4) VO2 max and physical activity status.
Pearson Chi-square analysis was used to determine if a threshold level of physical activity was associated with hs-CRP changes.
Results
The mean age of the 173 subjects evaluated in the study was 49.5± (9.8) years. The clinical characteristics of the study participants are shown in (Table 2) (Figure 1).
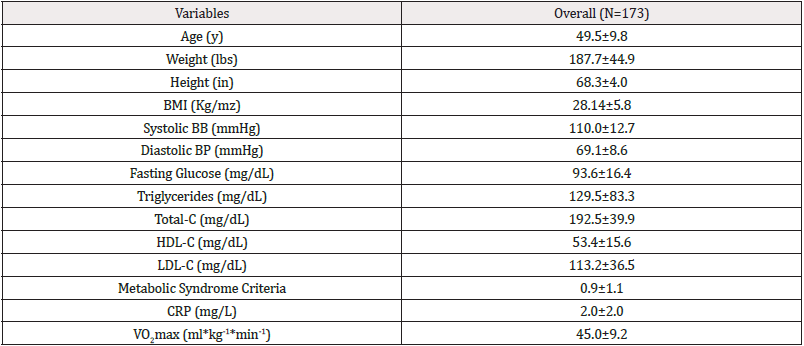
Table 2: WHO and ATP III Clinical Identification of the Metabolic Syndrome*.
*Note: BMI= Body Mass Index; HDL-C= high-density lipoprotein cholesterol; LDL-C= low-density lipoprotein cholesterol; Total-C= total cholesterol; CRP= C-reactive protein; VO2max= maximal oxygen uptake. A proportion of 8.7% of our subjects met the metabolic syndrome criteria.
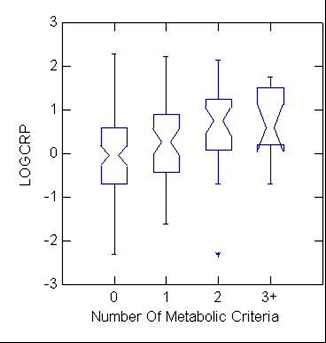
Figure 2: Distribution of log CRP levels among 173 participants according to the presence of 0, 1, 2, or ≥3 metabolic syndrome criteria. Box plots demonstrate median, 25th, and 75th percentile values of log CRP.
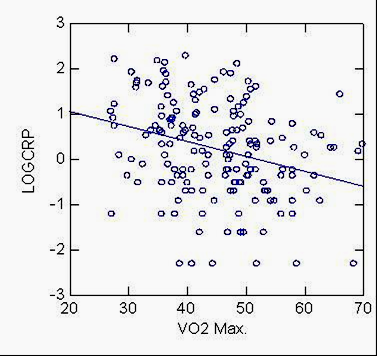
As shown in Figure 2, an inverse association was found between hs-CRP and VO2 max (p<0.01). As VO2 max increased, hsCRP decreased (Figure 2).
As shown in Figure 2, hs-CRP increased linearly with the number of metabolic syndrome criteria (p<0.01). An inverse association was also found between VO2 max and the number of metabolic syndrome criteria (p<0.01) (Not shown). VO2 max was positively related to physical activity status (p<0.01) (Not shown). Hence, fitness level was matched by the amount of reported physical activity.
Discussion
Cu Etchants Comparison
The US Department of Health and Human Services advises adults to engage in a minimum of 150 minutes of moderate-intensity aerobic activity, or 75 minutes of vigorous aerobic activity in a week to improve health and reduce the risk of chronic disease [15]. The American College of Sports Medicine (ACSM) recommends a minimum of 150 minutes per week of moderate-intensity exercise for health benefits [16]. Our findings support the US Department of Health and Human Services’ physical activity recommendations and recommendations set by the ACSM [15,16]. Subjects in our study engaging in 2-3hours of exercise per week had hs-CRP levels ≤2.5mg/L (p=0.01817), which is considered low to moderate risk.
An apparent common theme that appears to connect most of the metabolic syndrome risk factors is inflammation [17,18]. In flammation plays a role in elevated blood pressure, dyslipidemia, impaired glucose tolerance, and obesity [17,18]. The present study, in agreement with others, found a positive relationship between hs- CRP and metabolic syndrome characteristics [19,20]. We found inverse associations between hs-CRP and Cardiorespiratory Fitness (CRF) levels measured by VO2 max, and CRF levels and metabolic syndrome severity. It may be postulated that CRF reduces each of the risk factors seen in metabolic syndrome via a reduction in the inflammatory process (Figure 3).
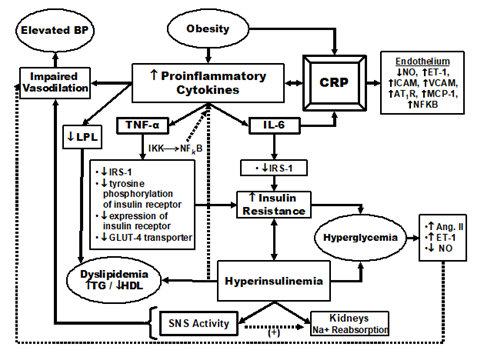
Figure 3: A model describing the relationship between inflammation and metabolic syndrome characteristics. IL-6=Interleukin-6, TNF-α=Tumor Necrosis Factor- α, CRP=C-Reactive Protein, BP=Blood Pressure, TG=Triglyceride, IGT=Impaired Glucose Tolerance, DM=diabetes mellitus, NO=Nitric Oxide, ET-1=Endothilin-1, ICAM=Intracellular Adhesion Molecule, VCAM=Vascular Cell Adhesion Molecule, AT1R=Angiotensin Type-1 Receptor, MCP-1=Monocyte Chemoattractant Protein-1, NFKB=Nuclear Factor KB, Ang. II=Angiotensin Type II, IRS-1=Insulin Receptor Substrate 1, LPL=Lipoprotein Lipase, SNS=Sympathetic Nervous System.
Several studies have found an inverse relationship between inflammatory markers and physical activity [8,10]. The proinflammatory cytokines IL-6 and tumor necrosis factor-a (TNF-α) are involved in hs-CRP production. Regular exercise training has been observed to decrease basal plasma interleukin levels, including IL-6 and TNF-a [21]. This reduction subsequently leads to the downregulation of hs-CRP production. Prescott, et al., [22] found that increased transcriptional activity of TNF-α and IL-6 results in heightened intranuclear binding of the proinflammatory transcription factor known as nuclear factor-kappa B (NF-KB). Thus, it is suggested that exercise down modulates activation of NF-KB, [23] thereby reducing TNF-α, IL-6, and CRP. This reduction in inflammation may in turn reduce risk factors seen in metabolic syndrome.
It is well-known that exercise improves blood pressure [24]. This improvement is likely due to several mechanisms, including reduced cytokines and Amyloid Precursor Protein (APP). Hypertension appears to stimulate the production of cytokines such as IL-6 and other inflammatory mediators from the endothelium. Studies indicate that heightened cytokine levels can initiate an Acute Phase Reaction (APR), resulting in endothelial cell damage [25]. Elevated cytokines along with APPs may impair endothelial- dependent vasodilation [26]. Consequently, this damage may lead to the buildup of fatty plaques, narrowing the arteries and compromising blood flow. Moreover, chronic inflammation has been linked to impaired blood vessel dilation and the development of insulin resistance [25]. Insulin resistance syndrome can stimulate the brain to secrete more catecholamines, such as Norepinephrine (NE), which can further enhance the APR and the inflammatory response [25]. This, in turn, can escalate the risk of hypertension and atherosclerosis due to the endothelial damage resulting from these events.
Another important metabolic syndrome characteristic, impaired glucose tolerance, is also improved with regular exercise. This improvement is likely due to several mechanisms, including reduced inflammation. Inflammation may activate NF-KB signaling pathways via up-regulation of TNF-α and IL-6, which stimulate hepatic CRP production [27]. TNF-α may then contribute to insulin resistance by increasing oxidation of Free Fatty Acid (FFA), stimulating additional cytokines (i.e., IL-6), inhibiting GLUT-4 transporters, harming endothelial function, and/or impairing glucose-stimulated release of insulin by B-cells [27]. Hyperglycemia induces IL-6 from macrophages and the endothelium [28]. The potential impact of CRP on insulin sensitivity and production could be attributed to its ability to modulate the innate immune response, leading to heightened systemic inflammation [29].
The ensuing hyperinsulinemia stimulates IL-6 and TNF-α. Endothelial nitric oxide synthase-mediated vasodilation becomes impaired via the inflammatory cytokines and associated abnormal FFA oxidation. Reducing inflammation may be an important mechanism by which exercise improves glycemic control. Regular exercise has been found to increase the expression of GLUT 4 transporters [30] and the expression of Insulin Receptor Substrate-1 (IRS-1) [31]. These alterations may be due in part to reductions in TNF-α and IL-6, respectively. Exercise has been found to improve glucose tolerance and both peripheral and hepatic insulin sensitivity [31]. These improvements will likely lead to reduced cytokine and APP release, further improving glycemic control.
Dyslipidemia, another risk factor seen in metabolic syndrome, is also improved by exercise. Exercise, particularly aerobic exercise, appears to increase HDL and reduce TG [32,33]. Sympathetically induced cytokines depress lipoprotein lipase (LPL) [26]. In animal models, TNF-α and IL-6 have been found to reduce LPL activity in adipose tissue [34]. Exercise appears to enhance the activity of several enzymes involved in lipid metabolism, including LPL [33]. Hence, exercise may enhance LPL activity via cytokine reduction thereby improving dyslipidemia.
The last metabolic syndrome characteristic is obesity. The beneficial impact of physical activity on this particular risk factor, as assessed through measurements such as BMI or body fat percentage, is widely recognized in scientific literature [26]. It may be that exercise reduces inflammation via a reduction in adiposity. Exercise appears to reduce visceral adipose tissue [34,35]. This may be an important mechanism by which exercise reduces inflammation. It is recognized that adipose tissue functions in part as an immune organ. It secretes many immunomodulatory factors and sends inflammatory signals known to cause insulin resistance [36].
IL-6 also induces the expression of suppressor of cytokine signaling- 3 (SOCS-3), a negative regulator of insulin signaling [29]. Rehman, et al., [29] found that IL-6 levels are elevated in obese subjects, who have increased inflammation and insulin resistance. Moreover, IL-6 may affect the function of pancreatic β-cells, which secrete insulin in response to glucose stimulation [29]. This pro-inflammatory state may contribute to insulin resistance in obese individuals as well as those with metabolic syndrome.
In adipose tissue, TNF-α reduces the expression of the insulin receptor and causes a reduction in tyrosine phosphorylation of the insulin receptor thereby interfering with insulin action [37]. TNF-α also impairs insulin signaling through serine phosphorylation that leads to the development of type 2 diabetes mellitus (T2DM) [37]. Anti-TNF-α treatment strategies have been developed to reduce the incidence of insulin resistance and T2DM [37]. The intracellular pathways activated by TNF-α involve NF-KB, which activates inflammatory target genes and inactivates the insulin receptor and IRS-1 [37]. This leads to decreased activation of phosphoinositol-3 kinase, a second messenger involved in the metabolic effects of insulin [37].
IL-6 also interferes with insulin signaling by reducing the phosphorylation of insulin receptors and insulin receptor substrate-1 (IRS-1) [29,37], which are essential for the activation of downstream pathways that mediate glucose uptake and metabolism [38,39]. As previously mentioned, exercise increases the expression of IRS-1 [34]. It may be that exercise enhances the expression of IRS-1 via a reduction in TNF-α and IL-6. Enhanced expression of IRS-1 may reduce interference in insulin’s action. It may be that exercise reduces proinflammatory cytokines and the inflammatory response via a reduction in body fat, particularly a reduction in visceral adiposity. In this context, the present study has a couple of important limitations. Inflammatory markers, such as cytokines, were not measured and waist circumference was not measured.
Conclusions
Elevated inflammation and the presence of the metabolic syndrome significantly increase the risk of CVD, diabetes, and premature mortality [12]. We postulate that exercise reduces the risk of these conditions by reducing inflammation. We found that hs-CRP and the metabolic syndrome severity were reduced in those with a higher cardiorespiratory fitness level. We also found that those engaging in 2-3 hours of physical activity per week had lower hs-CRP levels (≤2.5mg/L). Further clarification is needed to show whether exercise reduces risk factors, thereby reducing inflammation, or whether exercise reduces inflammation, thereby reducing risk factors. Regardless of which comes first, it appears that exercise is an important means of reducing risk factors seen in those individuals with metabolic syndrome. Aggressive therapeutic interventions for these individuals are imperative. The ATP-III report strongly supports the use of exercise as first-line therapy for the management of metabolic syndrome [4,14]. It would appear that our research findings add support to this recommendation.
Acknowledgments
All authors have made substantial contributions to this manuscript, which include the design, data collection and analysis, interpretation of data, drafting, and editing of the article, and final approval for publication.
Conflict of Interest Statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contribution Statement
H.D.S., M.V., and L.B. conceived the idea. M.V. wrote the first draft with contributions from L.B. who performed statistical analysis. L.B. and J.G. were responsible for the statistical methods and results. H.D.S. worked with M.V. on all the drafts focusing on the results, discussion, and conclusion. PL.C. worked on the literature review. H.D.S., W.R., and W.P. were responsible for the medical and mechanisms overview. All authors reviewed and commented on subsequent drafts and approved the final version of the manuscript.
References
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, et al. (2019) Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139(10): e56-e528.
- Adeva Andany MM, Martinez Rodriguez J, Gonzalez Lucan M, Fernandez Fernandez C, Castro Quintela E (2019) Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr 13(2): 1449-1455.
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet 375(9710): 181-183.
- Grundy SM, Stone NJ, Bailey AL, et al. (2019) 2018 AHA/ ACC/ AACVPR / AAPA/ ABC/ ACPM/ ADA/ AGS/ APhA/ ASPC/ NLA/ PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139: e1082-e1143.
- Liang X, Sun Q, Yang Y, Zhang Y, Pan L, et al. (2023) Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–18 Postgrad Med J 99(1175): 985-992.
- Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, et al. (2022) Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci 23(2): 786.
- Gami AS, Witt BJ, Howard DE, Patricia J Erwin, Lisa A Gami, et al. (2015) Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. Journal of Diabetes and its Complications 29(2): 231-240.
- Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, et al. (2019) Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 25(11): 1761-1771.
- Morales Palomo F, Ramirez Jimenez M, Ortega JF, Lopez Galindo PL, Fernandez Martin J, et al. (2017) Effects of repeated yearly exposure to exercise-training on blood pressure and metabolic syndrome evolution. J Hypertens 35(10): 1992-1999.
- Fedewa MV, Hathaway ED, Ward Ritacco CL (2017) Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med 51(8): 670-676.
- Reddy P, Lent Schochet D, Ramakrishnan N, McLaughlin M, Jialal I (2019) Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta 496: 35-44.
- Mesgari Abbasi M, Abbasalizad Farhangi M (2020) Serum concentrations of cholecystokinin, peptide YY, ghrelin and high sensitive C-reactive protein in association with metabolic syndrome ingredients in obese individuals. Acta Endocrinol (Buchar) 16(1): 37-42.
- Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, et al. (2019) Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 73(5): e35-e66.
- Huang PL (2009) A comprehensive definition for metabolic syndrome. Dis Model Mech 2(5-6): 231-237.
- (2018) U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd Washington, DC: U.S. Department of Health and Human Services: 1-117.
- Metzl J (2017) The science of exercise. American College of Sports and Medicine.
- León Pedroza JI, González Tapia LA, Del Olmo Gil E, Castellanos Rodríguez D, Escobedo G, et al. (2015) Low-grade systemic inflammation and its relationship to the development of metabolic diseases: From molecular evidence to clinical application. Surgery and Surgeons 83(6): 543-551.
- Catrysse L, van Loo G (2017) Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κ Trends Cell Biol 27(6): 417-429.
- Gowdaiah PK, Mamatha T, Nirgude D, Hosamani P (2016) High sensitivity C-reactive protein in metabolic syndrome. Int J Adv Med 3(3): 607-610.
- Ahmadnezhad M, Arefhosseini SR, Parizadeh MR, Tavallaie S, Tayefi M, et al. (2018) Association between serum uric acid, high sensitive C-reactive protein and pro-oxidant-antioxidant balance in patients with metabolic syndrome. BioFactors 44(3): 263-271.
- Docherty S, Harley R, McAuley JJ, Crowe LA, Pedret C, et al. (2022) The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Science, Medicine and Rehabilitation 14(1): 1-14.
- Prescott JA, Mitchell JP, Cook SJ (2021) Inhibitory feedback control of NF-κB signalling in health and disease. Biochem J 478(13): 2619-2664.
- Liu HW, Chang SJ (2018) Moderate Exercise Suppresses NF-κB Signaling and Activates the SIRT1-AMPK-PGC1α Axis to Attenuate Muscle Loss in Diabetic db/db Mice. Front Physiol 9: 636.
- Carpio Rivera E, Moncada Jiménez J, Salazar Rojas W, Solera Herrera (2016) Acute Effects of Exercise on Blood Pressure: A Meta-Analytic Investigation. Arq Bras Cardiol 106(5): 422-433.
- Khan MS, Ali A, Khan MA, Al Abbasi FA (2020) Cytokinemia and catecholamines: A possible role in hypertension and atherosclerosis. Journal of Cardiovascular Pharmacology and Therapeutics 25(4): 323-332.
- Tanase DM, Gosav EM, Radu S, Ouatu A, Rezus C, et al. (2019) Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? International Journal of Hypertension 2019: 1-17.
- Wexler DJ, Hu FB, Manson JE, Rifai N, Meigs JB (2005) Mediating effects of inflammatory biomarkers on insulin resistance associated with obesity. Obes Res 13(10): 1772-1783.
- (2010) The Hyperglycemia-Induced Inflammatory Response in Adipocytes Involves Activation of the Toll-Like Receptor Pathway (d.). American Diabetes Association.
- Lainampetch J, Panprathip P, Phosat C, Noppanath Chumpathat, Pattaneeya Prangthip, et al. (2019) Association of tumor necrosis factor alpha, interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: a retrospective cohort study of rural Thais. J Diabetes Res 2019: 9051929.
- Richter EA, Hargreaves M (2013) Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol Rev 3(3): 993-1017.
- Röhling M, Herder C, Stemper T, Müssig K (2016) Influence of Acute and Chronic Exercise on Glucose Uptake. Journal of Diabetes Research 2016: 1-33.
- Gordon B, Chen S, Durstine JL (2014) The effects of exercise training on the traditional lipid and beyond. Curr Sports Med Rep 13(4): 253-259.
- Wang Y, Xu D (2017) Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis 16(1):132.
- Zuo Y, He Z, Chen Y, Lei Dai (2023) Dual role of ANGPTL4 in inflammation. Inflamm Res 72(6): 1303-1313.
- Park YM, Myers M, Vieira Potter VJ (2014) Adipose tissue inflammation and metabolic dysfunction: role of exercise. Mo Med 111(1): 65-72.
- Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, et al. (2013) The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One 8(2): e56415.
- Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9(2): 191-200.
- Ahmed B, Sultan R, Greene MW (2021) Adipose tissue and insulin resistance in obese. Biomed Pharmacother 137: 111315.
- Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, et al. (2017) Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr 27(3): 229-236.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.