Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Changing Body Composition with A Transcutaneous Serum: Biometric and Lipid Panel Analysis
*Corresponding author: Diane Duncan, M.D., FACS, Plastic Surgical Associates, Fort Collins CO, 1701 East Prospect Road, USA.
Received: July 18, 2023; Published: August 03, 2023
DOI: 10.34297/AJBSR.2023.19.002627
Abstract
The link between sarcopenia and osteopenia is obvious, but the connection between lipid metabolism and sarcopenia is harder to understand. As muscle mass decreases with age, lower energy expenditure will translate into a lower basal metabolic rate. Extra calories are thus converted to fat. With age, fat depots in the subcutaneous location tend to diminish as fat accumulates intraabdominally as well as in cardiac, intramuscular, hepatic and skeletal muscle locations. Waistline measurement in adult women tends to increase by an average of 4cm every nine years. An increase in visceral fat is linked to many types of metabolic dysregulation. Given the rapid global increase in prevalence of metabolic syndrome, a targeted visceral fat solution is surely needed.
Most institutions continue to recommend diet and exercise as the cornerstones of treatment for increased visceral adipose tissue (VAT>100cm2). Given age related muscle loss and subsequent metabolic disturbance, that solution by itself does not always work. A prospective single site study was performed in order to evaluate a new transcutaneous topical serum for the purpose of enhancing visceral fat reduction and skeletal muscle mass preservation as a supplement to diet and exercise. Seventeen patients were enrolled; fifteen completed the study. A transcutaneous serum to be applied in the abdominal region was given to fifteen adult volunteers to see if clinical appearance and biometric indices would improve. Six men and nine women participated. Study subjects were instructed to continue their current diet and exercise program. Biometric indices were measured prior to study inception, and again at one month and two months. Standardized photographs were taken, and liver function studies and lipid panel values were evaluated at the same time points. Patients kept a daily journal in order to note any adverse events or side effects.
Results showed that reduction of visceral fat averaged 6.53cm2, with a range of +6.40 to -20.00cm2. Percent body fat was reduced by 1.27%, skeletal muscle mass increased by 0.95lb, and basal metabolic rate increased by an average of 14.50kcal. Liver function studies remained normal. Interestingly, lipid values improved slightly including triglyceride measurement, total cholesterol, and LDL. Patient weight remained within 5 pounds of the baseline.
Introduction
Sarcopenic obesity is difficult to exactly define, as different cultures have varying norms. Donini et al. defines sarcopenic obesity as the coexistence of obesity, characterized as >35 per cent body fat, and sarcopenia, determined by low skeletal muscle mass and function [1]. In the US, the prevalence of sarcopenic obesity is 18.1% in women over 60 and 42.9% in similarly aged men [2]. Basal metabolic rate declines by over 4% per decade after age 50 [3]. The rate of skeletal muscle loss varies between 3-8% every ten years after SMM peaks in early adulthood [4]. In patients older than 65, the rate of loss accelerates with a range of 6-15% prevalence of clinical sarcopenia [5]. The general deterioration of the musculoskeletal system leads to negative consequences, including a higher likelihood of falls, reduced functionality, increased frailty, and mortality. These outcomes are closely associated with a decrease in quality of life and an elevated risk of cardiometabolic conditions such as diabetes and hypertension [6].
Sarcopenic obesity is frequently observed among the elderly population, with both its risk and prevalence rising as individuals age [7]. Visceral fat area greater than 100cm2 in both women and men is the definition of visceral obesity [8]. A centralization of fat from the subcutaneous to visceral deposition peaks between the ages of 60 and 75 [9]. A downward metabolic spiral nicknamed the “metabaging cycle” [10] describes an aging process influenced by hyperlipidemia. Excess serum lipids generate an inflammatory cycle, including fat deposition intramuscularly, in increase in ROS (reactive oxygen species), and production of adipokines and inflammatory cytokines. “Inflammaging” then results, causing less fat to be stored, and a relative excess of circulating FFA (free fatty acids) [11]. Kob, et al., note that obesity can predispose progenitor cells to differentiate into adipose rather than muscle cells, when given a paracrine signal to do so from inflammatory cytokines [12].
Several studies have indicated that obesity can worsen sarcopenia, leading to increased fat infiltration into the muscles, diminished physical function, and a higher mortality risk [13,14]. There are several ways to assess sarcopenia with dual-energy X-ray absorptiometry DEXA and high-frequency bioelectrical impedance analysis (HF-BIA) being common modalities to measure body composition. HF-BIA, such as InBody, has been shown to be highly correlated with the DEXA for assessing skeletal muscle mass (standard coefficient beta (β)≥0.95) and percent body fat (β ≥0.94, R2 ≥0.89) [15]. Thus, the InBody 770 was deemed an appropriate measurer of body biometrics for this study.
Resistance training is recognized as an effective method for mitigating the symptoms of sarcopenia and osteoporosis [16]. Nevertheless, the dropout rate from such training programs among older adults can reach as high as 33% [17], with increased age and poorer physical performance linked to a greater likelihood of discontinuation [18,19]. Consequently, there is a necessity for alternative approaches to enhance body composition and mitigate muscle and bone loss, as well as total body fat reduction.
Methods
Eligible patients (21-80 yrs, BMI< 33kg/m2) were included in the study after reviewing inclusion and exclusion criteria. Patients accepted into the study expressed a desire to improve their body composition, with willingness to apply a topical transcutaneous serum twice a day, in combination with a healthy diet and exercise program. Exclusion criteria were cardiac disorders requiring medication or treatment, immunosuppressive diseases, poorly controlled endocrine disorders, recent abdominal surgeries, pregnancy, breast feeding, and the use of weight loss medications or testosterone. Fifteen eligible patients (9 women, 6 men, 30-80 years old, BMI 21 to 33kg/m2) were enrolled in the study. All participants received treatment instructions and provided written informed consent. They were instructed to maintain their usual diet and physical activity regimen.
The treatment protocol included application of three pumps of the serum to the abdominal skin twice daily, once in the morning and again at night. Patients were strictly instructed to maintain their regular routine diet and exercise program, and to avoid weight gain or loss of over 5 lb from baseline for the study duration. Evaluation parameters included 2D and 3D photographs that were taken in a standardized studio setting before treatment, and again at one month a 2 months following treatment inception. Biometric index measurements were taken at the same time points using the In Body 770, including weight (lb), percent body fat (PBF, %), skeletal muscle mass (SMM, lb), visceral fat area (VAT, cm2), and basal metabolic rate (BMR, kcal). Fasting liver enzyme panel and lipid panels were drawn from each study subject prior to the start of the study, and again at one month and 2 months post inception.
Primary Outcomes
The primary evaluation method assessed body biometrics including weight (lb), percent body fat (PBF, %), skeletal muscle mass (SMM, lb), visceral adipose tissue (VAT, cm2), and (BMR, kcal). The InBody 770 (Cerritos, CA, USA) was used to measure the changes of these variables, and standardized photographs were taken at baseline, at 1-month, and 2-month follow-up visits. Additionally, as a secondary primary evaluation, blind reviewers assessed patient aesthetic improvement including overall abdominal contour, degree of abdominal protuberance, periumbilical skin laxity, and apparent muscle tone using the 5-point Likert scale. The statistical significance was tested by RStudio and MS Excel utilizing the paired sample t-test with the significance level, α, set at 5% and the null hypothesis set to 0.
Secondary Outcomes
The secondary outcomes focused on the complete metabolic panel blood values, specifically the lipid profile and liver metabolism values. Parameters such as triglycerides, cholesterol, HDL, LDL, alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase were assessed through blood tests. The liver function tests were conducted to ensure the safety of the topical serum regarding its impact on liver function. And lipid metabolism. Measurements were taken at baseline, 1-month, and 2-month follow- up visits.
Results
All fifteen patients completed the treatment regime and attended follow-up appointments. No adverse events or significant expected sequelae were reported. Participants did not experience any discomfort or difficulties during the at-home application of the serum and were able to resume their daily activities after treatment. The weight of the subjects did not significantly change throughout the study, as their weight remained within a 5lb range of baseline (p-value >0.05).
Primary Outcomes
Biometric analysis showed that at baseline, mean weight measured 162.96lb. The average per cent body fat was 29.22%. Mean skeletal muscle mass (SMM) measured 64.42lb. Mean visceral adipose tissue (VAT) value was 110.03cm2, with a normal value of <100cm2. Basal metabolic rate (BMR) averaged 1519.70kcal. At the conclusion of the study, the average PBF was lowered to 27.95% (p-value <0.05), a decrease of 1.27%, which translates to a 4.2% lowering of this biometric index. SMM increased to 65.37lb (p-value <0.05), an increase of 0.95 pounds, without any change in diet or exercise regimes.
VAT decreased to 103.51cm (p-value <0.05), a 5.9% improvement. There was an increase in the average BMR by 14.42kcal (p-value <0.05). Average weight at baseline was 162.96lbs, and the mean weight decreased slightly to 162.14lb at the conclusion of the study with a change of -0.82lb (p-value >0.05). The average changes in the body biometrics are exhibited in Figure 1. Overall, the blinded reviewers evaluating the body morphology improvement in the patients observed positive scores for improvement across all categories. No ratings were given by the reviewers to indicate any worsening of appearance. Average of all patient morphology evaluations are shown in Figure 2.

Figure 1: Average changes in body biometrics across patient population at 2 months. PBF = percent body fat (%); SMM = skeletal muscle mass (lb); VAT = visceral adipose tissue (cm2), BMR = basal metabolic rate (kcal).
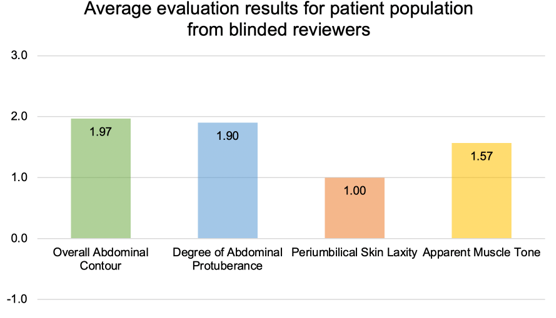
Figure 2: Overall body morphology change evaluation 2 months following twice daily application of topical body composition serum, as graded by 3 blinded evaluators using a 5-point Likert scale.
Clinical Examples
Figure 3 shows a 39-year-old patient following childbirth and four pregnancies. After twice daily use of the transcutaneous serum, she noted improvement in abdominal contour. She maintained a healthy diet and exercised three times a week.
Figure 4 depicts a 41 year old male with central abdominal protuberance. Two months post-treatment, the epigastric and periumbilical region showed significant improvement in both abdominal protuberance and apparent muscle tone. Figure 5 shows his biometric measurement index changes. His PBF decreased by 2.80 percent, and he had a loss of VAT by 13.00cm2 (-16.31%). His SMM improved by 1.80lb (+2.11%) and he had a 27.00kcal improvement in BMR as shown in Figure 5. The patient lost 3.00lb after 2 months of topical serum use. The patient’s photos (Figure 4) were evaluated by 3 blinded reviewers that assessed the patient’s aesthetic improvement as seen in Figure 6.

Figure 3: 39-year-old before (left) and 2 months post twice daily use of a topical serum for body composition in addition to her routine diet and exercise program (right).

Figure 4: 41-year-old male before (left) and 2 months after twice daily application of the topical serum in the abdominal region.

Figure 5: Changes in biometrics for a 41-year-old male at one and two months since start of twice daily application of the topical serum. PBF = percent body fat (%); SMM = skeletal muscle mass (lb); VAT = visceral adipose tissue (cm2), BMR = basal metabolic rate (kcal).

Figure 6: Evaluation result of blind reviewers assessing study subject 010’s body morphology changes at 2 months using the 5-point Likert scale.
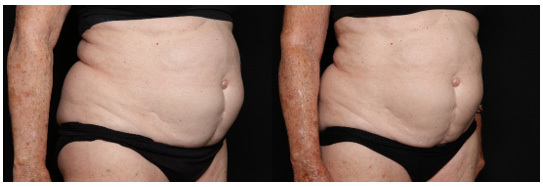
Figure 7: 78 year old female before, left, and two months following daily topical application of body composition enhancing serum (right).
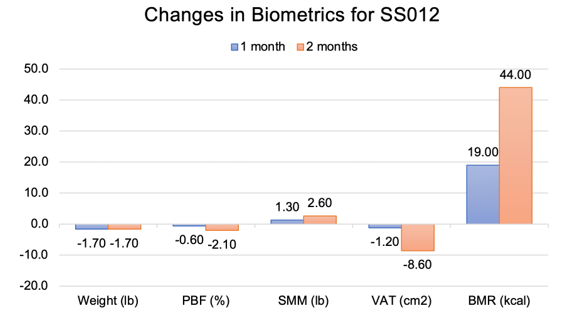
Figure 8: Changes in biometrics for a 78-year-old female at one and two months since start of twice daily application of the topical serum. PBF = percent body fat (%); SMM = skeletal muscle mass (lb); VAT = visceral adipose tissue (cm2), BMR = basal metabolic rate (kcal).
Figure 7 shows a 78 year old female patient before treatment, and two months following twice daily application of topical serum. This patient body morphology type and age group are clinically difficult to improve. Her body biometrics showed improvement after 2 months with a decrease in PBF by 2.10%, reduction in VAT by 8.60cm2 (-6.88%), an increase in SMM by 2.60lb (+3.49%), and a 44.00 kcal improvement in BMR as shown in Figure 8. Patient weight stayed within 2.0lb (p-value >0.05) of baseline. The patient’s photos were evaluated by 3 blinded reviewers that assessed the patient’s aesthetic improvement as seen in Figure 9.
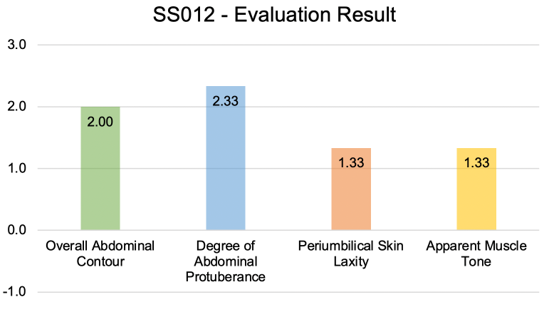
Figure 9: Morphology evaluation results for SS 012 by blinded reviewers 2 months following use of the topical transcutaneous serum.
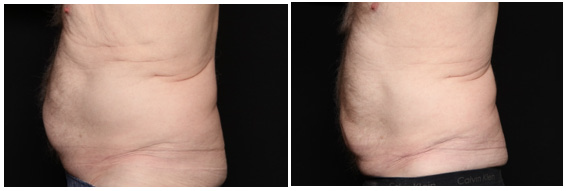
Figure 10: 80 year old man before, left, and 30 days after using the body composition enhancing serum twice daily. He noted significant improvement in central abdominal protrusion.
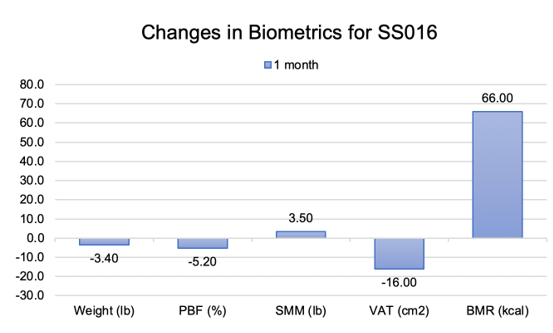
Figure 11: Biometrics for an 80 year old man following 30 days of daily application of transabdominal topical serum. PBF = percent body fat (%); SMM = skeletal muscle mass (lb); VAT = visceral adipose tissue (cm2), BMR = basal metabolic rate (kcal).
An 80 year old male patient’s body biometrics showed improvement after 1 month of treatment. He had significant reduction of visceral fat, as seen in Figure 10. Biometric indices showed improvement with a decrease in PBF of 5.20%, reduction in VAT by 16.00cm2 (-16.04%), an increase in SMM by 3.40lb (+4.77%), and a 66.00kcal improvement (-3.95%) in BMR as shown in Figure 11. Patient weight stayed within the required 5.0 lb weight change limit required by the study. (p-value >0.05) of baseline.
Secondary Outcomes
The majority of patients showed a reduction in triglycerides while the other patients remained within their pre-treatment normal limits. There was an average decrease in triglycerides of 28.64mg/dL across the patient population (p-value <0.05). There was also a decrease in average cholesterol and LDL across the patient population by 19.27mg/dL (p-value <0.05) and 14.00mg/dL (p-value <0.05), respectively. HDL values increased slightly but stayed within the normal range of 35.0 to 65.0mg/dL for men, 35.0 to 80.0mg/dL for women. Results of the lipid metabolism evaluation are portrayed in Figure 12. Liver function tests overall changed very minimally (p-value >0.05) and stayed within the normal limits as seen in Figure 13.

Figure 12: Average changes in lipid metabolism across patient population from start to end of treatment.

Figure 13: Changes in liver function studies across the patient population over the two-month treatment period. Average changes of liver function tests including alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) along with their reference ranges (19). Error bars depict 10-point deviation.
Adverse Events and Expected Sequelae
There were no adverse events. No patient experienced nausea, diarrhea, abdominal cramping, or pain. One patient reported transient tingling upon application. There were no instances of skin rash, erythema, itching, or hives. No patient reported an allergic reaction.
Limitations
The study cohort was small, with an n of fifteen. A larger number of subjects should be treated in order to verify consistent results. Though the InBody device used for measurement of biometric indices correlates well with dual X-ray absorptiometry, DEXA is considered the gold standard for these calculations. Evaluation of the change in physical manifestations of body composition were subjective, though reviewers were blinded and photographs were taken in a studio with standardized lighting and positioning. Two patients showed improvement during the first 30 days, only to lose biometric and morphologic improvements during the second 30 days due to admitted dietary indiscretions. One patient noted limited improvement late in the study due to travel and limited exercise and diet options.
Discussion
Many aging patients attribute their aging body morphology to genetics, claiming that they inherited these characteristics from their parents. Another common belief is that thickening waistlines and protuberant midsections are an unavoidable consequence of getting older. Many aspects of this type of thinking are valid. Before 1990, inherited patterns of aging were felt to be unalterable [20]. However, only 25-40% of a human’s aging pattern is genetic; the remainder is epigenetic and genomic [21,22]. Thus, changing the way humans age has become increasingly possible [23]. The general population loses 0.5 to 1% of muscle mass per year after the age of 50 [24] and undergoes an increase in per cent body fat until late middle age [25]. Resulting weakness and frailty can decrease the quality of life while shortening its span [26].
In 2004 the term “sarcopenic obesity” was introduced; Ruebenoff noted that obese older individuals had less strength per unit of muscle mass than their thinner counterparts [27]. An explanation for the metabolic consequences of sarcopenia is that as muscle atrophies, there is a lesser amount of target tissue (SMM) that responds to the effects of circulating insulin, thus stimulating insulin resistance [28]. Another way of looking at this is that as muscle mass decreases, less energy is expended, which lowers basal metabolic rate while promoting fat accumulation and subsequent obesity [29].
While targeting treatments for overall fat loss and gain in skeletal muscle mass is a logical solution, specifically targeting visceral fat may be even more effective for reducing the risk for both metabolic syndrome and sarcopenic obesity. With age, less fat is stored as subcutaneous fat, while an increasing percentage of extra calories are stored centrally, in the intra-abdominal location [30]. The toxic effects of increased visceral fat have several pathways. In general, insulin drives hepatic glucose production, which is directly affected by the concentration of free fatty acids (FFA) delivered to the liver from the visceral fat depot through the portal circulatory system [31]. Sterol regulatory element-binding transcriptional protein factor- 1 (SREBP-1) is a marker for increased visceral fat, as noted in a study comparing dogs fed a normal fat diet (35% kcal derived from fat) vs. a cohort fed a high fat diet (42% fat) [32]. Visceral to subcutaneous fat ratios increased in this study in the higher fat fed dogs as measured by peroxisome proliferator activated receptor gamma (PPARG) and hormone sensitive lipase (HSL), verifying a selective increase in visceral fat deposition in the test subject cohort [33].
The topical serum (Viscetrim, Palm Harbor, Florida) is comprised of a specialized blend of highly concentrated and specific botanical extracts, all focused on non-drug enhancement on lipid and muscle metabolism. The composition of the topical serum is plant based. No manufactured pharmaceuticals are included. The delivery system is based on reversible electroporation, in which electrostatic repulsion is used to create short-lived but high voltage pulses in order to open ionic channels on the skin. This allows active compounds to be absorbed. There is a cascade of physiologic triggers that promotes enhancement of lipid metabolism when the serum is used in conjunction with a healthy diet and exercise program. SREBP-1, a transcription factor which regulates the conversion of glucose into fatty acids, is inhibited by a polysaccharide included in the serum [34]. Inhibiting this protein therefore limits lipogenesis and helps to prevent long-term energy storage in the form of fat deposition. Another target is stimulation of AMPK (adenosine monophosphate activated protein kinase), an important regulator of cellular metabolism [35,36].
AMPK, which declines with advancing age, is found in every cell in the body. It is essential for energy production and utilization of fat for fuel, especially visceral fat. Botanically based ursolic acid is used to reduce inflammation, as visceral fat releases several proinflammatory cytokines [37]. Other beneficial effects include an increase in expression of AMPK as well as irisin. Irisin is a powerful cytokine generated through exercise that improves insulin sensitivity while increasing energy expenditure and reducing body weight [38]. Ursolic acid increases the body’s levels of uncoupling proteins, both UCP-1 and UCP-3. These proteins are responsible for generating thermogenesis in a non-shivering manner within brown adipose tissue. Overexpression of these uncoupling proteins can also transform WAT (white adipose tissue) into beige adipose tissue, which increases utilization of fat tissue for heat and energy generation [39].
The gold standard for biometric analysis is the dual-energy X-ray absorptiometry (DEXA) [40] HF-BIA, such as InBody, has been shown to be highly correlated with the DEXA for assessing skeletal muscle mass (standard coefficient beta (β) ≥0.95) and percent body fat (β ≥0.94, R2 ≥0.89) [41]. Other biometric indices available through this device include weight, BMI, visceral adipose area in cm2, and calculated basal metabolic rate. Basal metabolic rate (BMR) is the amount of energy expended during rest in a neutral environment over a 24-hour period [42]. The InBody 770 utilizes John J. Cunningham’s equation which uses lean body mass (LBM, kg) to estimate BMR [43]
BMR (kcal) = 21.6 x LBM + 370
The most influential factor in determining BMR among body composition measures is lean body mass; body fat mass, levels of physical activity, and nutrition are also significant determinants [44]. As SMM is a component of LBM, it can be expected that an increase in BMR is correlated with increased SMM and decreased PBF. The transcutaneous serum tested showed improvement in patient SMM and BMR in conjunction with reduced PBF and VAT following treatment.
While liposomes and lipid particles are routinely used in skin creams [45], the use of topical creams for the purpose of altering lipid metabolism is rare. Testosterone formulations, topically applied in female patients infected with HIV, have been shown to affect body composition and metabolism to some extent [46]. Estrogen, topically applied in one study, worsened insulin resistance [47]. Transdermal delivery systems can include microemulsions, transdermal patches, nanoparticles, transferosomal gels, and procedures such as iontophoresis and electroporation [48]. The majority of the patients in this study who used the transcutaneous emulsion (Viscetrim) saw visible improvement in body morphology as measured by both independent reviewers and by objective biometric indices.
The moderate viscosity serum is well absorbed and uses reversible electroporation in order to enhance absorption. To date no adverse events or significant expected sequelae have been noted. However, one difficulty with the transcutaneous system is the human tendency to discontinue use of any supplement unless the need is dire. Another human trait is that of abandoning dietary discipline if a mitigating substance is at hand. Notably, a positive effect was seen in patients who did not adhere to a strict diet and exercise program, but merely continued their current regime. In this study, patients who were already disciplined and continued to follow or slightly improve their diet and exercise routine fared better than their counterparts that relaxed that vigilance.
Ethical Statement
This study was performed following the general principles of medical ethics in clinical research derived from the Declaration of Helsinki (June 1964) and its successive amendments. Investigators followed the international recommendations relating to Good Clinical Practices for conducting clinical trials for drugs and supplements ICH TOPIC E6 (R2) of November 2016 (CPMP/ICH/135/95).
Conclusion
An inevitable decline in muscle mass accelerates sarcopenic obesity or “metabaging”. Elderly adults face the twin specters of frailty and dyslipidemia. As visceral fat increases and waistlines expand, insulin resistance grows and inflammatory cytokines cause intramuscular fat deposition, which further negatively influences muscle function. Diet and exercise can improve the situation but will not reverse the process. A transcutaneous supplement applied to the abdomen twice daily can help to improve body morphology as well as biometric indices such as skeletal muscle mass and basal metabolic rate, while reducing per cent body fat and visceral fat area. The supplement is not intended to influence weight loss. Slight improvement in lipid metabolic markers may be seen. Best success is achieved when combined with a healthy and consistent diet and exercise program.
References
- Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, et al. (2022) Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts 15(3): 321-335.
- Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, et al. (2017) Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord 16: 21.
- Zoico E, Di Francesco V, Mazzali G, Vettor R, Fantin F, et al. (2004) Adipocytokines, fat distribution, and insulin resistance in elderly men and women. J Gerontol A Biol Sci Med Sci 59: M935-939.
- SMM loss 3-8%
- Vincent HK, Raiser SN, Vincent KR (2012) The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev 11(3): 361-73.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022505s012s013lbl.pdf.
- Johnson Stoklossa CA, Sharma AM, Forhan M, Siervo M, Padwal RS, et al. (2017) Prevalence of Sarcopenic Obesity in Adults with Class II/III Obesity Using Different Diagnostic Criteria 7307618.
- Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, et al. (2010) Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 33(7): 1652-1654.
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, et al. (2008) Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 11(6): 693-700.
- Li CW, Yu K, Shyh Chang N, Jiang Z, Liu T, et al. (2022) Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle 13(2): 781-794.
- Lackey DE, Olefsky JM (2016) Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 12(1): 15-28. 58.
- Kob R, Bollheimer LC, Bertsch T, Fellner C, Djukic M, et al. (2015) Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology 16(1): 15-29.
- Kalinkovich A, Livshits G (2017) Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing research reviews 35:200-221.
- Barbat-Artigas S, Pion CH, Leduc-Gaudet JP, Rolland Y, Aubertin-Leheudre M, et al. (2014) Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J Am Med Dir Assoc 15(4): 303.e313-320.
- Yi Y, Baek JY, Lee E, Jung HW Jang, I Y J, et al. (2022) A Comparative Study of HighFrequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Body Composition Life 12(7): 994.
- Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ, et al. (2010) Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol 109(3): 517-525.
- Katz B, Duncan D (2021) Lifting and Toning of Arms and Calves Using High-Intensity Focused Electromagnetic Field (HIFEM) Procedure Documented by Ultrasound Assessment. J Drugs Dermatol 20(7): 755- 759.
- Rosado H, Bravo J, Raimundo A, Carvalho J, Marmeleira J, et al. (2021) Effects of two 24-week multimodal exercise programs on reaction time, mobility, and dual-task performance in community-dwelling older adults at risk of falling: a randomized controlled trial. BMC Public Health 21(Suppl 2): 408.
- Lala V, Zubair M, Minter DA (2023) Liver Function Tests, National Library of Medicine.
- Johnson TE (2002) A personal retrospective on the genetics of aging. Biogerontology 3(1-2): 7-12.
- Passarino G, De Rango F, Montesanto A (2016) Human longevity: Genetics or Lifestyle? It takes two to tango. Immun Ageing 13: 12.
- Bin-Jumah MN, Nadeem MS, Gilani SJ, Al-Abbasi FA, Ullah I, et al. (2022) Genes and Longevity of Lifespan. Int J Mol Sci 23(3): 1499.
- Yang JH, Petty CA, Dixon-Mc Dougall T, Lopez MV, Tyshkovskiy A, et al. (2023) Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY) 15(13): 5966-5989.
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, et al. (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10): 1059-64.
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, et al. (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3: 260.
- Newman AB, Lee JS, Visser M (2005) Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 82(4): 872-878.
- Roubenoff R (2004) Sarcopenic obesity: the confluence of two epidemics. Obes Res 12(6): 887-888.
- Reaven GR (1988) Role of Insulin Resistance in Human Disease. Diabetes 37(12): 1595-1607.
- Villareal D, Banks M, Sienerc C, Sinacore D, Klein S, et al. (2004) Physical frailty and body composition in obese elderly men and women. Obes Res 12(6): 912-919.
- Tchernof A, Després JP (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93(1): 359-404.
- Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, et al. (2019) Exercise-Induced Changes in Visceral Adipose Tissue Mass Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab 29(4): 844-855.e3.
- Castro AV, Woolcott OO, Iyer MS, Kabir M, Ionut V, et al. (2015) Increase in visceral fat per se does not induce insulin resistance in the canine model. Obesity (Silver Spring) 23(1): 105-111.
- Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, et al. (2021) PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int 41(11): 2659-2670.
- Chen M, Xu J, Wang Y, Wang Z, Guo L, et al. (2020) polysaccharide can regulate lipid metabolism in type 2 diabetic rats through the SREBP-1/SCD-1 axis. Carbohydr Res.
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, et al. (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4): 376-388.
- Jeon SM (2016) Regulation and function of AMPK in physiology and diseases. Exp Mol Med 48(7): e245.
- Katashima CK, Silva VR, Gomes TL, Pichard C, Pimentel GD, et al. (2017) Ursolic acid and mechanisms of actions on adipose and muscle tissue: a systematic review. Obes Rev 18(6): 700-711.
- Cheng CF, Ku HC, Lin H (2018) PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int J Mol Sci 19(11): 3447.
- Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA (2017) Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig 31(1).
- Pineau JC, Frey A (2016) Comparison of skinfold thickness models with DEXA: impact of visceral adipose tissue. J Sports Med Phys Fitness 56(5): 541-545.
- Jensky-Squires NE, Dieli-Conwright CM, Rossuello A, Erceg DN, Mc Cauley S, et al. (2008) Validity and reliability of body composition analysers in children and adults. Br J Nutr 100(4): 859-865.
- Anthanont P, Jensen MD (2016) Does basal metabolic rate predict weight gain? Am J Clin Nutr 104(4): 959-963.
- Cunningham JJ (1991) Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr 54(6):963-969.
- Konarzewski M, Książek A (2013) Determinants of intra-specific variation in basal metabolic rate. J Comp Physiol B 183(1): 27-41.
- lsayed MM, Abdallah OY, Naggar VF, Khalafallah NM (2007) Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm 332(1-2): 1-16.
- Herbst KL, Calof OM, Hsia SH, Sinha-Hikim I, Woodhouse LJ, et al. (2006) Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus-infected women with mild to moderate weight loss. Fertil Steril 85(6): 1794-1802.
- Chu MC, Cosper P, Nakhuda GS, Lobo RA (2006) A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertil Steril 86(6): 1669-1675.
- Rao R, Mahant S, Chhabra L, Nanda S (2014) Transdermal innovations in diabetes management. Cur Diabetes Rev 10(6): 343-359.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.