Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Daily Response of Vital Signs to COVID-19 Infection: A Case Study of an Unvaccinated 70-Year-Old Male with Type II Diabetes Treated with Monoclonal Antibodies and Selected Supplements
*Corresponding author: Walter R Schumm, Adjunct Instructor, Math Department, Highland Community College, Wamego, Kansas, 66547 USA; Emeritus professor of Applied Family Science, Kansas State University, Manhattan, 66506-1403 USA.
Received: July 08, 2023; Published: July 12, 2023
DOI: 10.34297/AJBSR.2023.19.002602
Abstract
In much of the current scientific literature on COVID-19, potential treatments are studied singly or in combination with two or three other items. Treatment options for unvaccinated individuals at high risk for COVID-related complications and mortality have improved but may still be limited for remote locations. A case study of an unvaccinated older male with co-morbidities at higher risk for COVID-19 related mortality is used to illustrate possible solutions to some of the issues. His vital signs, including O2 saturation, breathing rate, heart rate variability, night skin temperature, and resting heart rate, were monitored for 30 days before and after COVID-19 infection, providing more individual details than available in most studies to date. Supplements may have limited his vulnerability to COVID-19 while monoclonal antibodies led to rapid improvement in multiple vital signs. Tests for COVID-19 may not detect the earliest stages of COVID infection as fever levels increased before the patient tested positive for COVID-19.
Keywords: COVID-19, Monoclonal antibodies, Vitamin D, Quercetin, Resveratrol, Zinc, Combination therapies, Vaccine resistance
Statement of the Problem
Although its exact origins remain unclear, the COVID-19 virus appears to have originated in the city of Wuhan, China in the late fall of 2019. By January 2020 it had reached the United States and many other nations. Since then, millions of infected persons have perished (over one million in the USA) while some survivors have experienced long-term COVID symptoms, up to at least two years [1,2]. Adverse psychological outcomes have been common [3]. Due to vaccine hesitancy or medical restrictions, some potential victims of COVID-19 may not have been vaccinated. Vaccine hesitancy is not without legitimate concern; the Open VAERS website [https:// www.openvaers.com] lists over 1.5 million adverse event reports as of June 2023, including over 35,000 deaths, over 200,000 hospitalizations, and nearly 67,000 permanent disabilities. Young men may be at higher risk for myocarditis after COVID vaccination [4]. There may be other unintended consequences of vaccination [5]. Some have proposed that vaccination may result in autoimmune disease, cancer growth, and autoimmune myocarditis, as well as reduced immunity to COVID [6]. Natural immunity from mild COVID infection may be more protective against severe reinfection than vaccination [7,8]. Vaccine immunity may wane over time, especially against new variants [9]; some have claimed that “all-cause mortality” may be higher among the vaccinated than those not [10]. In some data, vaccination rates have not correlated with infection rates [11]. Data seem to support less need for vaccination of children against COVID [12]. Five trillion dollars were spent to fight COVID in the USA, including vaccine development [13], but the effectiveness of societal lockdowns remains questionable [14]. Older individuals, including men, African Americans, persons with high BMI or other comorbidities, including diabetes, heart problems, or cancer appear to be at much higher mortality risk if they become infected with COVID-19. A final basis for vaccine hesitancy is the alarming rate of retractions of scientific articles on COVID vaccines and other treatments [15]. However, the question remains - how to treat unvaccinated individuals at high mortality risk from COVID-19? Furthermore, how could they be treated at lower cost? Not all nations may be able to afford higher cost treatments for all their vulnerable citizens.
Literature Review
Several reports have highlighted the potential role of nutrition in preventing COVID infection or reducing its severity [16-20]. Ashok, et al., [16] have recommended specific fruits and vegetables for consumption to obtain nutrients for improving immune response. Ali [21] has stated that “preventive health measures that can reduce the risk of infection, progression and severity of COVID-19 are desperately needed”. Improved nutrition might enhance vaccine effectiveness in older persons [17]. Our focus will be on over-the-counter supplements or medicines due to their relatively low cost and greater availability than for prescription medications. Even if some nutritional supplements might assist a human body’s resistance to colds or flu, it might not be certain that those supplements would be helpful for dealing with the novel COVID-19 virus. Ideally, a supplement would reduce one’s chances of being infected with the COVID-19 virus (presumably by enhancing the body’s own immune system prior to infection), reduce the ability of that virus to replicate and establish itself inside the human body, limit its damage to the human body’s cells and organs, limit the body’s inflammatory overreaction to the virus, preventing the cytokine storm that often leads to fatal cell and organ damage, especially to the heart and lungs, and lastly, minimize the damage, if it occurs, to the heart, lungs, and other critical organs. Of course, not all substances will help with all those ideal goals, so each substance must be evaluated for the conditions under which it would be most helpful, if at all. Evidence for a substance might range from mere theoretical speculation to results from double-blind randomized trials with low bias and high quality. It is also possible that a deficiency of a substance might be a risk with respect to different stages of COVID-19 infection, but that more of the same substance above deficiency levels might not provide greater benefits against COVID-19. The value of substances may also vary with a person’s comorbidities. Perhaps some substances might work to offset the disadvantages of diabetes (or other co-morbidities) as well as dealing with COVID-19. Furthermore, we must remain alert to potential interactions between substances with each other or with prescription drugs.
Covid Symptoms
All of the authors of this paper have witnessed the severe symptoms of COVID-19 infection for themselves or for friends or family members, including mortality. In many cases, victims have been unable to work, even remotely, due to the extreme fatigue experienced (up to two weeks), as well as high fevers and other symptoms. Severe symptoms have lasted up to two weeks for some while long-term symptoms have remained for up to several months. Some of our friends have died from or with COVID-19. In summary, COVID-19 infection is not to be taken lightly. However, many victims have not had access to day-by-day readings of their vital signs.
Methodology
A 70-year-old male with type 2 diabetes and type O blood [22,23] had avoided COVID-19 infection until October 2021 and served as the patient and research participant for this study (and is senior author). Older age [24] and a type 2 diabetes diagnosis placed him at higher risk from COVID infection [25]. The patient did not suffer from obesity (BMI 22) obesity being related to a poor immune response to infections [20] nor from hypertension or cardiovascular disease. He had undergone prostate surgery for cancer in 2015. However, on 2 October 2021 his wife fell and broke her femur, necessitating surgery and a week of hospitalization, followed by seven weeks of care at a rehabilitation center in the midwestern USA. A terminal case of COVID occurred at the rehabilitation center in a room immediately adjacent to his wife’s, and his wife became infected. The patient’s wife first tested positive for COVID-19 on October 14; the patient tested positive for COVID-19 on October 19 and 21 after testing negative on October 16 and 18. After his wife tested positive, the patient was only allowed to visit her if wearing a disposable gown and a face mask; the patient also had to depart the rehabilitation center via a back door rather than through the front entrance. The patient’s positive test results were reported to his county’s health department who placed him under a 10-day quarantine, which prevented him from visiting his wife at the rehabilitation center, other than leaving items at the front desk to be taken to her. His wife never felt any symptoms from COVID-19; the patient only felt like he had a mild cold - without a positive test for COVID-19, he would not have sought treatment. However, in addition to more frequent COVID-19 testing (freely available at several nearby locations) the patient responded to the risk with a regime of supplements thought to help him avoid infection or at least reduce any impact of infection, while knowing that his physician had told him that if he did become infected, he could apply for treatment with monoclonal antibodies due to his high risk of mortality due to his age and type 2 diabetes. In essence, plan A was a combination of increased testing for infection and increased nutritional supplements while plan B was the antibody treatment. He did become infected and elected to take the monoclonal antibody treatment as soon as it was available.
Smart watches can be used to collect data from patients at risk for COVID Banks, et al., (2021) [26]. Data from the patient’s smart watch were recorded from October 13 to November 15, as presented in Table 1. Data were analyzed using SPSS [27]. The patient was teaching a basic statistics class at Highland Community College that fall, and this became the second research project in which the class was involved, the first previously published in this journal [28] (Table 1).
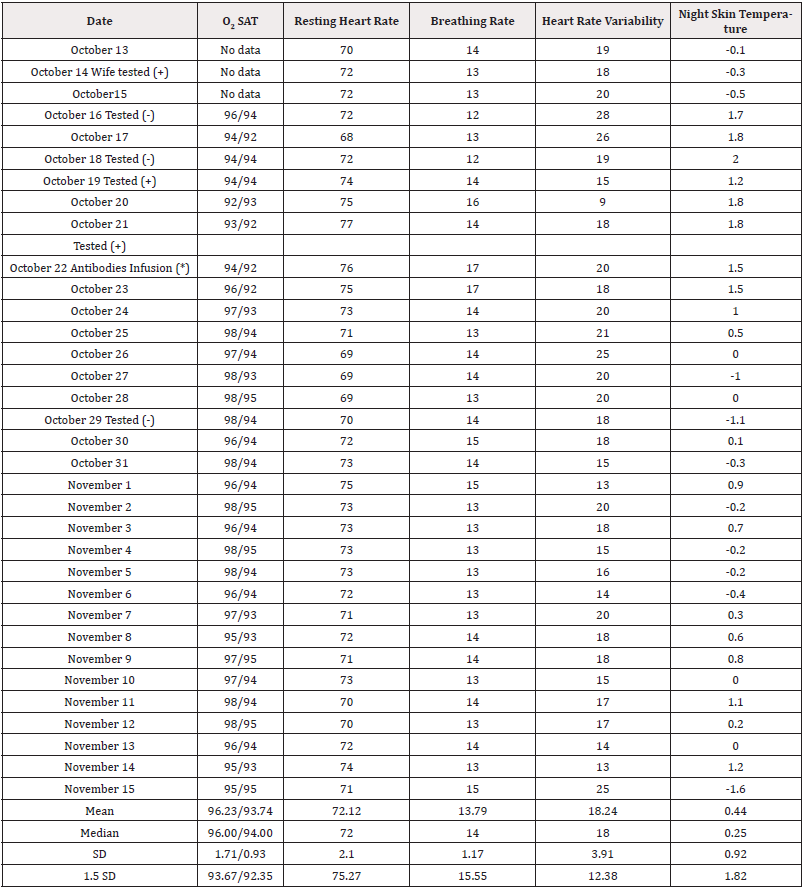
Table 1: Case Study Health Indicators Over Time During a Confirmed COVID-19 Infection.
Note*: (*) Antibodies infused were casirivimab and imdevimab, 1200 units each, manufactured by Regeneron Pharmaceuticals. See: U.S. Food and Drug Administration. Letter of Authorization for Monoclonal Antibodies Casirivimab and Imdevimab. November 21, 2020. Washington, DC.
During this period (and before to a lesser extent) the patient was taking 2000 to 6000 IU of vitamin D [17,18,20,21,29-36], 1000- 3000 IU of Vitamin C [17,36-40], 100-200mg of zinc tablets [41], four resveratrol tablets [42-44] daily that each included 200 mg red wine extract (30% polyphenols), 200mg red wine powder, 200mg Japanese knotweed extract, and 100 mg grape seed extract [45]; 2000mg, vitamin C [17,36-40]; 45mcg of vitamin K2 [46,47]; multivitamins that included a total of 2400mcg of vitamin A [17,20,48- 51], 150mg of vitamin C, 800 IU of vitamin D, 27mg of vitamin E [17,36,52], 10mg of vitamin B-6 [17,53], 800mcg of folic acid [53], 30mcg of vitamin B-12 [35,53], 1200mcg of biotin [54,55], 20mg of pantothenic acid [55], 12mg of choline, 300mcg of iodine, 10mg of zinc [17,54,55], 220mcg of selenium [17,37,56-58], and 80mcg of inositol [59]; 1000mg of magnesium [35,60], 2grams of beet root, 600mg of alpha lipoic acid [61-63], 200mg grapeseed extract [45] with 800mg of grapefruit powder, 1500mg quercetin [64-69], and 60-200mg of Coenzyme Q-10 [70-73]. The patient was taking 1500 units of metformin for diabetes as well as 25 units of insulin per day; the patient took 325mg of aspirin [74-81] two or three times during the first week to relieve general discomfort.
Hypotheses
The general research question was how the dependent variables would vary over time from before COVID infection, during infection, and post infection. The general hypothesis was that levels would become worse during infection and recover, often leading to a quadratic effect. Small declines in health outcomes before and during COVID infection might suggest that there was an early onset of COVID infection before detection. Larger declines in health outcomes would suggest that COVID infection had a more substantial impact during detectable infection. Improvements from infection to post infection health outcomes would indicate the degree of recovery. Changes from pre infection to post infection would reflect if recovery was partial or more complete.
Methods
Groups. Results for October13 to 18 (3-6 cases) were classified as a pre-COVID baseline. Results for October 19 to 24 (6 cases) were classified as during-COVID. Results between October 25 and November 15 (22 cases) were classified as post-COVID treatment. Because patients may become contagious 2-3 days before onset of symptoms [82], it is possible the patient was contagious as early as October 18.
Measures. Oxygen saturation levels were measured on a oximeter at the patient’s home and as measured by the patient’s smart watch, as averaged over each day. Resting heart rate was measured by the smartwatch as averaged over each day. Breathing rate, heart rate variability, and night skin temperature (above/below 98.6 degrees) were also measured by the patient’s smartwatch, averaged over the day. Complete data are reported in Table 1 and correlations among the vital signs are presented in Table 2, showing interrelationships among the different vital signs (Table 2).
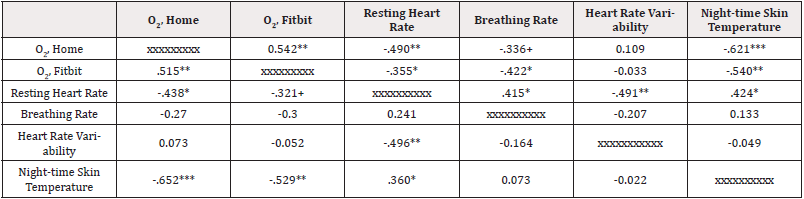
Table 2: Vital Signs Correlations.
Note*: Correlations above the diagonal are Pearson zero-order correlations; below the line are Spearman rho correlations.
Analysis. Levels of the dependent measures were compared across three groups using one way analysis of variance, a more conservative approach than using repeated measures. Post hoc tests were conducted with LSD comparisons. Tests for quadratic trends were conducted as part of the analysis of variance. Cohen’s d and Hedge’s g were calculated as noted in Table 3.
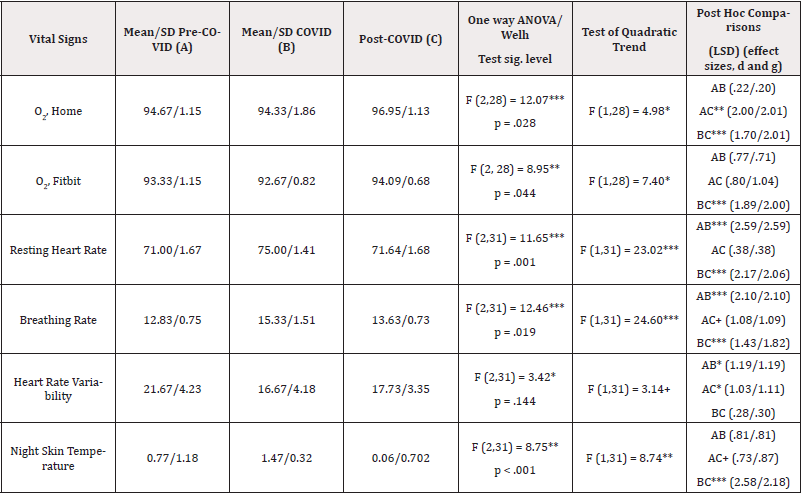
Table 3: Analysis of Variance of Vital Signs Comparing Across Three Times with Test for Quadratic Trends and Post Hoc Comparisons with Effect Sizes.
Note*: Effect sizes were Cohen’s d and Hedge’s g, the latter more useful when group sizes are not equal. Effect sizes were calculated from the website https://www.socscistatistics.com/effectsize/default3.aspx.
Results
Overall tests. One-way analyses of variance yielded statistically significant results for all six health outcome variables while the Welch test yielded five significant results. Five of the quadratic trends were statistically significant, while a sixth was not significant (p<.10). From Table 1, it can be seen that all of the six measured health outcomes improved at least somewhat within three days of medical treatment (infusion of monoclonal antibodies). Early shifts in health outcomes (pre-COVID to during COVID infection). The largest deteriorations for health outcomes occurred for resting heart rate and breathing rate with smaller changes for both measures of oxygen saturation, heart rate variability, and night skin temperature. The changes were small enough for night skin temperature yet lower post infection to suggest that an increased night skin temperature might have been a leading indicator of an oncoming COVID infection. Recovery from COVID (during COVID to after COVID infection). Except for heart rate variability, all health outcomes featured very substantial (effect sizes of 1.43 to 2.58) and statistically significant (p<.001) improvements after monoclonal antibodies treatment of the patient. Heart rate variability improved but only slightly, possibly a factor in long COVID (slower heart tissue recovery) and remained significantly (p<.05) well below (effect size of at least 1.03) initial levels even after treatment. While both measures of O2 levels improved upon pre-COVID levels as well as COVID levels, resting heart rate, breathing rate, and night skin temperature did not return completely to pre-COVID levels, even after treatment (Table 3).
There are many potentially useful drugs, supplements, or treatments that were not used by the patient that might have been useful or were used but without apparent effect: nicotine, ivermectin, remdesivir, chloroquine, nasal disinfection, green and black teas, catechins, prednisone, kampo, rosemary, molnupiravir, ranolazine, Paxlovid, antihistamines, trimetazidine, thiamine, riboflavin, L-carnitine tartrate, niacin, pantothenic acid, iodine, nitric oxide, Glutathione (GSH), myo-inositol, iron, probiotics; papaya, chamomile, bitter orange, hawthorn, echinacea, golden poppy, Siberian ginseng, tea tree oil, lemon balm, holy basil, panax ginseng, purple passionflower, dexamethasone, Asian knotweed, golden root, sage, elderberry, cat’s claw, valeric acid [19]; inhaled corticosteroids, nitazoxanide, mometasone, ciclesonide, doxycycline, albendazole, theophylline, fluvoxamine, Pepcid, albendazole, copper, turmeric/ curcumin, various antibiotics, tocilizumab, N-Acetylcysteine (NAC), pantoprazole, Eliquis, beet root, iodine, choline, metformin, insulin, hyperbaric oxygen, favipiravir, colchicine, anticoagulant therapy, clopidogrel, budesonide, hydrogen peroxide nasal rinse, povidone- iodine nasal rinse, ozone blood therapy, omega-3 fatty acids (docosahexaenoic acid), and melatonin, among others. The possible effects for these items with respect to COVID can be found using Google Scholar or medical indices.
Discussion
The origins of COVID remain controversial. One of our neighbors contracted a virus-like illness in late November 2019 after visiting with a Chinese student who had just flown into our hometown from Wuhan, China. Was it an early case of COVID - or were there other infectious agents circulating in Wuhan at the time? Many facilities checked temperatures of persons entering as a way to detect infections, but the results here suggest that higher body temperatures may indeed be a leading indicator of a COVID infection not yet detected officially but if a pre-COVID body temperature is lower than average, a rise in temperature may not show up as abnormal, allowing COVID infected persons to enter facilities with undetected infection. A variety of nutritional supplements may reduce the severity of infection by promoting the human immune system and by reducing inflammation, but more research needs to be done to determine which supplements are best, at which times before or during a COVID infection. Monoclonal antibody treatments appear effective and may be enhanced for those on effective supplements, even for high-risk individuals. In the future, physicians and medical researchers might be able to assess a patient’s condition more clearly and rapidly if they were provided access to personal vital signs detection devices, as can be found on many current smart watches. Those with cancer may need particular attention [83-85].
Acknowledgement
None.
Conflict of Interest
None.
References
- Wahlgren C, Forsberg G, Divanoglou A, Balkhed AO, Niward K, et al. (2023) Two-year follow-up of patients with post-COVID-19 condition in Sweden: a prospective cohort study. The Lancet Regional Health Europe 28: 100595.
- Sonnenschein L, Etyang T, Manga S, Wong E (2023) An initial evaluation of the effectiveness of a dietary supplement regimen on the effects of post-COVID infection. Am J Biomed Science & Research 18(6): 580-592.
- Liu Y, Xie YN, Li WG, He X, He GG, et al. (2022) A machine learning-based risk prediction model for post-traumatic stress disorder during the COVID-19 pandemic. Medicina 58(12): 1704.
- Cho JY, Kim KH, Lee N, Cho SH, Kim SY, et al. (2023) COVID-19 vaccination-related myocarditis: a Korean nationwide study. European Heart Journal 44(24): 2234-2243.
- Seneff S, Nigh G (2021) Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19 2(1): 38-79.
- Uversky VN, Redwan EM, Makis W, Rubio Casillas A (2023) IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 spike protein. Vaccines 11(5): 991.
- Forbes S (2022) Biden’s vaccine diktat is wrong. Forbes Magazine 204(6): 19.
- Schlubach J (2022) Covid-19: Vaccine effectiveness and risks - Where do we stand? Am J Biomedical Sci & Res 15(4): 377-379.
- Wang L, Wang QQ, Davis PB, Volkow ND, Xu R, et al. (2022) Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry 21(1): 124-132.
- Classen JB (2021) US COVID-19 vaccines proven to cause more harm than good based on pivotal clinical trial data analyzed using the proper scientific endpoint, “All cause severe morbidity”. Trends in Internal Medicine 1(1): 1-6.
- Subramanian SV, Kumar A (2021) Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. European Journal of Epidemiology 36(12): 1237-1240.
- Kostoff RN, Calina D, Kanduc D, Briggs MB, Vlachoyiannopoulos P, et al. (2021) Why are we vaccinating children against COVID-19? Toxicology Reports 8: 1665-1684.
- Moore S (2022) The greatest government failure in American history. Washington Examiner 28(5): 42.
- Atlas SW (2021) Science, politics, and COVID: Will truth prevail? Imprimis 50(2): 3-7.
- Yeo The NSL, Tang BL (2021) An alarming retraction rate for scientific publicatiosn on Coronavirus Disease 2019 (COVID-19). Accountability in Research 28(1): 47-53.
- Ashok AD, Ravivarman J, Kayalvizhi K (2020) Nutraceutical value of salad vegetables to combat COVID 19. Journal of Pharmacognosy and Phytochemistry 9(3): 2144-2148.
- Rayman MP, Calder PC (2021) Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. British Journal of Nutrition 126(12): 1919-1920.
- Muscogiuri G, Barrea L, Savastano S, Colao A (2020) Nutritional recommendations for CoVID-19 quarantine. European Journal of Clinical Nutrition 74: 850-851.
- Bertuccioli A, Cardinali M, Di Pierro F, Magi S, Zonzini G, et al. (2022) A practical perspective on the use of botanicals during the COVID-19 pandemic: From proven to potential interactions. Journal of Medicinal Food 25(1): 1-11.
- Calder PC (2020) Nutrition, immunity, and COVID-19. BMJ Nutrition Prevention & Health 3(1): 74-92.
- Ali N (2020) Role of vitamin D in preventing COVID-19 infection, progression, and severity. Journal of Infection and Public Health 13(10): 1373-1380.
- Zietz M, Zucker J, Tatonetti NP (2020) Associations between blood type and COVID-19 infection, intubation, and death. Nature Communications 11(1): 5761.
- Latz CA, DeCarlo C, Boitano L, Png CYM, Patell R, et al. (2020) Blood type and outcomes in patients with COVID-19. Annals of Hematology 99(9): 2113-2118.
- Bhattacharya J (2020) A sensible and compassionate anti-COVID strategy. Imprimis 49(10): 1-6.
- Yavari M, Afshar MM, Jabalameli F, Kharkani PS, Jafari M, et al. (2021) Diabetes, heart disease, fatty liver, blood groups, and COVID-19 disease: A brief review of evidence. Am J Biomed Sci & Res 13(2): 191-199.
- Banks MA (2021) In the wake of COVID-19, decentralized clinical trials move to center stage. PNAS 118(47): e2119097118: 1-4.
- Aldrich JO (2018) Using IBM SPSS statistics: an integrative hands-on approach. Sage.
- Schumm WR, Brady CA, Cerny J, Francis M, Mann M, et al. (2021) The importance of catastrophe theory and nonlinear modeling applied to the collapse of Afghanistan in the late summer of 2021. Am J Biomed Sci & Res 15(2): 128-138.
- Mercola J, Grant WB, Wagner CL (2020) Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 12(11): 3361.
- Danner OK, Danner ED, Matthews LR (2020) Optimizing vitamin D status improves outcomes in critical Ill and injured patients. Am J Biomed Sci & Res 8(2): 114-116.
- Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, et al. (2020) Early nutritional interventions with zinc, selenium, and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients 12(8): 2358.
- Ahmadi A, Heydari K, Mohammadi N, Hoseinnejad F (2022) Evaluation of the relationship between vitamin D deficiency and the epidemiology of COVID-19. Am J Biomed Sci & Res 15(4): 387-388.
- Petrelli F, Oldani S, Borgonovo K, Cabiddu M, Dognini G, et al. (2023) Vitamin D3 and COVID-19 outcomes: An umbrella review of systematic reviews and meta-analyses. Antioxidants 12(2): 247.
- Bilezikian JP, Binkley N, De Luca HF, Fassio A, Formenti AM, et al. (2023) Consensus and controversial aspects of vitamin D and COVID-19. The Journal of Clinical Endocrinology & Metabolism 108(5): 1034-1042.
- Tang CF, Ding H, Jiao RQ, Wu XX, Kong LD, et al. (2020) Possibility of magnesium supplementation for supportive treatment in patients in COVID-19. European Journal of Pharmacology 886: 173546.
- Darbar S, Saha S, Agarwal S (2021) Immunomodulatory role of vitamin C, D, and E to fight against COVID-19 infection through boosting immunity: A review. Parana Journal of Science and Education 7(1): 10-18.
- Bae M, Kim H (2020) The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 25: 5346.
- Feyaerts AF, Luyten W (2020) Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition 79: 110948.
- Moore A, Khanna D (2023) The role of vitamin C in human immunity and its treatment potential against COVID-19: A review article. Cureus 15(1): e33740.
- Abobaker A, Alzwi A, Alraied AHA (2020) Overview of the possible role of vitamin C in management of COVID-19. Pharmacological Reports 72(6): 1517-1528.
- Elalfy H, Besheer T, El Mesery A, El Gilany AH, Soliman MA A, et al. (2020) Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol 93(5): 3176-3183.
- Liao MT, Wu CC, Wu SV, Lee MC, Hu WC, et al. (2021) Resveratrol as an adjunctive therapy for excessive oxidative stress in aging COVID-19 patients. Antioxidants 10(9): 1440.
- Domi E, Hoxha M, Kolovani E, Tricarico D, Zappacosta B, et al. (2022) The importance of nutraceuticals in COVID-19: What’s the role of resveratrol? Molecules 27(8): 2376.
- Giordo R, Zinellu A, Eid AH, Pintus G (2021) Therapeutic potential of resveratrol in COVID-19 associated hemostatic disorders. Molecules 26(4): 856.
- Saaty AH (2022) Grapefruit seed extracts’ antibacterial and antiviral activity: Anti-severe acute respiratory syndrome Coronavirus 2 impact. Arch Pharm Pract 13(1): 68-73.
- Ali AM, Kunugi H, Abdelmageed HA, Mandour AS, Ahmed ME, et al. (2021) Vitamin K in COVID-19 – Potential anti-COVID-19 properties of fermented milk fortified with bee honey as a natural source of vitamin K and probiotics. Fermentation 7: 202.
- Anastasi E, Ialongo C, Labriola R, Ferraguti G, Lucarelli M, et al. (2020) Vitamin K deficiency and covid-19. Scandinavian Journal of Clinical and Laboratory Investigation 80(7): 525-527.
- Li R, Wu K, Li Y, Liang X, Tse WKF, Yang L, Lai KP (2020) Revealing the targets of mechanisms of vitamin A in the treatment of COVID-19. Aging (Albany NY) 12(15): 157784-15796.
- Stephensen CB, Lietz G (2021) Vitamin A in resistance to and recovery from infection: Relevance to SARS-CoV2. British Journal of Nutrition 126(11): 1663-1672.
- Alzaben AS (2020) The potential influence of vitamin A, C, and D and zinc supplements on the severity of COVID-19 symptoms and clinical outcomes: An updated review of literature. Current Research in Nutrition and Food Science 8(3): 703-714.
- Al Sumiadai MM, Ghazzay H, Al Dulaimy WZS (2020) Therapeutic effect of vitamin A on severe COVID-19. EurAsian Journal of Biosciences 14: 7347-7350.
- Tavakol S, Seifalian AM (2022) Vitamin E at a high dose as an anti-ferroptosis drug and not just a supplement for COVID-19 treatment. Biotechnol Appl Biochem 69(3): 1058-1060.
- Shakoor H, Feehan J, Mikkelsen K, Al Dhaheri AS, Ali HI, et al. Be well: A potential role for vitamin B in COVID-19. Maturitas 144: 108-111.
- Rahman MT, Idid SZ (2021) Can Zn be a critical element in COVID-19 treatment? Biological Trace Element Research 199(2): 550-558.
- Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, et al. (2020) Zinc and respiratory tract infections: Perspectives for COVID-19 (review). International Journal of Molecular Medicine 46(1): 17-26.
- Kieliszek M, Lipinski B (2020) Selenium supplementation in the prevention of coronavirus infections (COVID-19). Medical Hypotheses 143: 109878.
- Khatiwada S, Subedi A (2021) A mechanistic link between selenium and Coronavirus Disease 2019 (COVID-19). Current Nutrition Reports 10(2): 125-136.
- Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, et al. (2020) Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 12(7): 2098.
- Bermano G, Meplan C, Mercer DK, Hesketh JE (2021) Selenium and viral infection: Are there lessons for COVID-19? British Journal of Nutrition 125(6): 618-627.
- Bizzarri M, Lagana AS, Aragona D, Unfer V (2020) Inositol and pulmonary function: Could myo-inositol treatment downregulate inflammation and cytokine release syndrome in SARS-CoV-2? European Review for Medical and Pharmacological Sciences 24(6): 3426-3432.
- Guerrero Romero F, Micke O, Simental Mendia LE, Rodriguez Moran M, Vormann J, et al. (2023) Importance of magnesium status in COVID-19. Biology 12(5): 735.
- Cure E, Cure MC (2020) Alpha-Lipoic acid may protect patients with diabetes against COVID-19 infection. Medical Hypotheses 143: 110185.
- Rochette L, Ghibu S (2021) Mechanics insights of alpha-lipoic acid against cardiovascular disease during COVID-19 infection. International Journal of Molecular Sciences 22(15): 7979.
- Dragomanova S, Miteva S, Nicoletti F, Mangano K, Fagone P, et al. (2021) Therapeutic potential of alpha-lipoic acid in viral infections, including COVID-19. Antioxidants 10(8): 1294.
- Cheema HA, Sohail A, Fatima A, Shahid A, Shahzil M, et al. (2023) Quercetin for the treatment of COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol 33(2): e2427.
- Imran M, Thabet HK, Alaqel SI, Alzahrani AR, Abida A, et al. (2022) The therapeutic and prophylactic potential of quercetin against COVID-19: An outlook on the clinical studies, inventive compositions, and patent literature. Antioxidants 11(5): 876.
- Boretti A (2021) Quercetin supplementation and COVID-19. Natural Product Communications 16(9): 1-3.
- Derosa G, Maffioli P, D Angelo A, Di Pierro F (2020) A role for quercetin in coronavirus disease 2019 (COVID-19). Phytotherapy Research 35(3): 1230-1236.
- Di Pierro F, Iqtadar S, Khan A, Mumtaz SU, Chaudhry MM, et al. (2021) Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled, and open-label clinical trial. International Journal of General Medicine 14: 2807-2816.
- Bastaminejad S, Bakhtiyari S (2021) Quercetin and its relative therapeutic potential against COVID-19: A retrospective review and prospective overview. Current Molecular Medicine 21(5): 385-391.
- Fakhrolmobasheri M, Hosseini MS, Shahrokh SG, Mohammadi Z, Kahlani MJ, et al. (2023) Coenzyme Q10 and its therapeutic potencies against COVID-19 and other similar infections: A molecular review. Advanced Pharmaceutical Bulletin 13(2): 233-243.
- Medvegy M, Simonyi G (2021) Supplementary therapeutic possibilities to alleviate myocardial damage due to microvascular dysfunction in coronavirus disease 2019 (COVID-19). Cardiol Ther 10(1): 1-7.
- Polymeropoulos VM (2020) A potential role of Coenzyme Q10 deficiency in severe SARS-CoV2 infection. OBM Integrative and Complementary Medicine 5(4): 1-9.
- Know CS, Ramachandram DS, Hasan SS (2023) Coenzyme Q10 therapy in patients with post COVID-19 condition. The Lancet Regional Health – Europe 25: 100567.
- Diaz T, Trachtenberg BH, Abraham SJK, KosagiSharaf R, Durant Archibold AA, et al. (2020) Aspirin bioactivity for prevention of cardiovasculalr injury in COVID-19. Frontiers in Cardiovascular Medicine 7: 562708.
- Merzon E, Green I, Vinker S, Golan Cohen A, Gorohovski A, et al. The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection. The FEBS Journal 288(17): 5179-5189.
- Osborne TF, Veigulis ZP, Areola DM, Mahajan SM, Eliane Röösli, et al. (2021) Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration. PLoS One 16(2): e0246825.
- Meizlish ML, Goshua G, Liu Y, Fine R, Amin K, et al. (2021) Intermediate-dose anticoagulations, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis. Am J Hematol 96(4): 471-479.
- Mohamed Hussein AAR, Aly KME, Ibrahim MAA (2020) Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy? Medical Hypotheses 144: 109975.
- Yuan S, Chen P, Li H, Chen C, Wang F, et al. (2021) Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. Journal of Cellular and Molecular Medicine 25(2): 1263-1273.
- Wijaya I, Andhika R, Huang I, Purwiga A, Budiman KY, et al. (2021) The effects of aspirin on the outcome of COVID-19: A systematic review and meta-analysis. Clinical Epidemiology and Global Health 12: 100883.
- Choi Y, Yoo JS, Song EH, Kim BY, Park SA, et al. (2021) The importance of early treatment of COVID-19. Am J Biomed Sci & Res 14(5): 401-402.
- Ianniello N (2021) COVID-19 and the cancer patient. Am J Biomed Sci & Res 13(5): 571-572.
- Mian A, Khan S (2020) Coronavirus: The spread of misinformation. BMC Medicine 18(1): 89.
- Springer S, Ozdemir V (2022) Disinformation as COVID-19’s twin pandemic: False equivalences, entrenched epistemologies, and causes-of-causes. OMICS: A Journal of Integrative Biology 26(2): 82-87.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.