Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Electrochemical Deposition of Co-Ni-Fe Films at 0.08 Mole/L Electrolyte Concentration
*Corresponding author: RD Tikhonov, State Research Centre of Russia Scientific-Manufacturing Complex “Technological Centre”, Russia.
Received: August 07, 2023; Published: August 15, 2023
DOI: 10.34297/AJBSR.2023.19.002640
Abstract
The electrochemical deposition of Co-Ni-Fe films revealed the nature of the phenomena leading to the difference in the relative content of the elements in the electrolyte and in the film. The dependence of hydrogen chloride electrolytes in the temperature range of 25-70 degrees Celsius and electrochemical deposition of Co-Ni-Fe films at 70 degrees Celsius were investigated. The deposition of Co-Ni-Fe films was carried out from chloride electrolyte with a component content ratio of 1:1:1 at an average concentration of 0.083mole/L of each component. The content of the component in the film in electrochemical deposition of three component solution CoCl2, NiCl2, FeCl2 with equal concentration of each component does not correspond to the composition of the electrolyte but approaches the composition of the electrolyte while reducing the concentration of each component at high current density.
Keywords: Сo-Ni-Fe films, Chloride electrolyte, Ion balance, Electrochemical deposition
Introduction
The Co-Ni-Fe magneto-soft triple alloy is used in microelectronics products and high-density magnetic memory. The Electrochemical coating of Co-Ni-Fe is used in many areas: to reduce corrosion and wear, to use in magnetic and electrical devices, and for electro catalytic materials. Compared to “dry” processes, electrochemical deposition gives a smoother coating and with fewer defects, and also allows you to increase the thickness of films without mechanical stresses if necessary.
In the work [1], the thin magnetic films Co65Ni12Fe23 are made of sulfate electrolyte and have a high magnetization saturation of 2.4- 2.59 T. When ammonium chloride is added to the electrolyte, the ratio of the component in Co-Ni-Fe films changes [2]: iron or nickel is enriched, cognitive properties are improved, сoercive force is reduced and magnetic permeability increases.
The low-stress high 2.2 T magnetic moments and сoercive force 60 Oe Fe70Co29Ni1 thin films were electrodeposited [3] from acidic chloride bath. The stresses in the Fe-Co-Ni thin films decreased with increasing the electrodeposition temperature from 20 to 70°C. From XRD analyses, it is suggested that the stress changes in the Fe-Co-Ni films may be a result of grain sizes. As the electrodeposition temperature was increased, the grain sizes increased. Ascorbic acid extended the life of solution baths and allows operation at higher pH=2.2 range while maintaining low-stress film deposition to prevent precipitate formation. The mathematical model, which describes the electrodeposition of the nanometer multi-layered magnetic-sensitive structures of Fe-Co-Ni-Cu/Cu, is designed to produce nanowires [4].
The electrochemical method of Co-Ni-Fe films from the sulfate- chloride electrolyte, containing, mole/l: NiSO4- 0.304, NiCl2- 0.084, CoSO4 - 0.1, FeSO4 - 0.036, H3BO3 - 20g/L, showed [5] that cobalt and iron are deposited with concentration 3 times more than in electrolyte, and nickel is twice less. Abnormality composition of triple alloy films on the composition of the electrolyte does not allow to obtaining compliance with the composition of films composition of the electrolyte. This phenomenon does not find an explanation in the literature. Despite the successes in the use of Co-Ni-Fe films, the nature of the phenomena that determine the chemical processes in the electrolyte and the composition of the films remain unclear and requires further research (Figure 1).
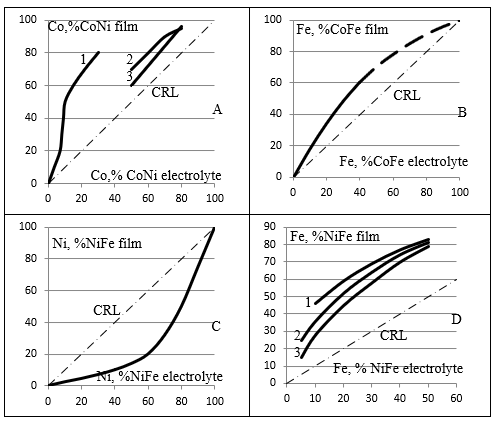
Figure 1: Dependence of the composition of the films of double alloys obtained by electrochemical deposition from the composition of the electrolyte according to the work [8] data A1, [9] - A2, 3 for Co-Ni; [10] - B for Co-Fe; [6,7] - C and D for Ni-Fe.
A number of potentials characterize the comparative activity of metals in oxidative-restorative reactions in water solutions. Electrochemical potentials, φ0, B: Fe2+ -0.441; Fe3+ -0,0425; Co2+ -0,28; Ni2+ -0,234. According to the electrochemical potential, nickel should be deposited well than iron and cobalt, and iron is worse than cobalt.
Experimental data on the dependence of the composition of films on the composition of electrolyte in electrochemical deposition of alloys Co-Ni, Co-Fe, Ni-Fe are presented in the works of [6,7] for Ni-Fe, [8,9] for Co-Ni, and Co-Fe for [10] built dependency on Figure 1. The CRL is marked with a line that defines the equality of the compositions of the electrolyte and the beleaguered film. For the first time, Fe deposition anomalies in relation to Ni in the concentration range of 5 to 50% of iron in the electrolyte for the deposition of the Ni-Fe alloy and the dependence of iron concentration in the film on current density were shown [6]. In the paper, Fe’s anomaly of deposition in relation to Ni is demonstrated [7] at all iron concentrations in the electrolyte for the deposition of the Ni-Fe alloy.
For electrochemical deposition of iron group metals, the abnormality of deposition is characteristic. Iron is deposited more intensely than cobalt and nickel. Cobalt is deposited more intensely than nickel. Assessing the deposition rate by electrochemical potential is important, but there are many factors in the processes that determine metal deposition.
Normal electrochemical deposition determines the equality of electrolyte compositions and precipitation of electrochemical equivalents. The concept of congruent electrochemical deposition determines the equality of the compositions of electrolyte and sediment.
The anomaly of deposition is also evident in the triple alloy Co- Ni-Fe though the composition of the films depends on the temperature, hydrogen pH, concentration and composition of the electrolyte. In this paper, to determine the effect of the factors determining the deviation of the composition of the Co-Ni-Fe films from the composition of the electrolyte, a study of the dependence of the hydrogen indicator on the temperature of chloride electrolytes with the same component concentration and electrochemical deposition of the films of Co-Ni-Fe at a temperature of 70 degrees Celsius.
Materials and Methods
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results, which provides [11-17] normal and congruent deposition of the alloy Ni-Fe and electrochemical deposition of the alloy Co-Ni-Fe [18-21].
Congruent Electrochemical Deposition of Ni-Fe from Chloride Electrolyte
The study of electrochemical deposition of Co-Ni-Fe triple alloy films was conducted using the experience of congruent electrochemical deposition of Ni-Fe films from the chloride electrolyte (Figure 2).
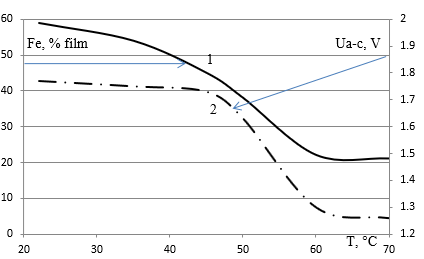
Figure 2: The dependence of Fe content in Ni-Fe films on the temperature of the sulfate is a chloride electrolyte with a ratio of Ni/Fe=3.4 and a content of 0.085/0.025 mole/l. 2. Change of voltage between the anode and the cell cathode for electrochemical deposition of the permalloy at a current density of 15 mA/cm2.
In electrochemical deposition with a bath temperature of more than 60 degrees Celsius, the composition of the Ni-Fe film corresponds to the composition of the electrolyte with Fe 20% content, as shown in Figure 2 of the curve 1. At room precipitation temperature, the concentration of iron in the films differs more than twice from the electrolyte. The tension between the electrodes (Figure 2 curve 2) decreases as the temperature rises and the current is constant. The decrease in voltage between the cathode and the anode as the temperature rises indicates an increase in the conductivity of the electrolyte due to the more complete dissociation of salts and the formation of two-charge ions instead of single-charge ions. It turns out that by choosing the temperature of the electrolyte of 70degrees Celsius, congruent electrochemical deposition of Ni-Fe is achieved.
In NiCl2 salt-based electrolytes, the FeCl2 deposition process occurs at room temperature in accordance with different iron ions in diluted solutions. Figure 3 shows the dependence of iron content in films from 34% to 13% of the concentration of salts in the electrolyte with the ratio of nickel and iron concentrations 4.26. Reducing the concentration of nickel and iron chlorides in the electrolyte leads to their complete ionization at room temperature. Charges of nickel ions Ni2+ and iron Fe2+ have the same value of valence 2. It turns out that the temperature of the electrolyte of 20 degrees Celsius is achieved congruent electrochemical deposition Ni-Fe by choosing a low concentration of electrolyte. Changing the balance of Ni-Fe ions when the temperature rises and when the electrolyte is diluted is further used in the analysis of electrochemistry deposition of the triple system Co-Ni-Fe (Figure 3).
The pH Ratio on the Temperature of the Solutions CoCl2, NiCl2, FeCl2
In the water solutions CoCl2, NiCl2, FeCl2 with a component concentration of 0.01 to 1mole/L measured the hydrogen pH with the testo 206 meter by Testo SE Co. KGaA, Germany. At a concentration of 1 mole/L at 25 degrees Celsius the dissolution [19] of NiCl2 gives a pH value of 5.8, CoCl2 gives a pH of 4.7, FeCl2 gives a pH of 2.7. The pH of the mixed electrolyte in the entire range of changes in concentrations of each component from 0.01 to 1mole/L triple electrolyte with equal concentrations of all three salts CoCl2, NiCl2, FeCl2 is determined by the actual hydrolysis of iron chloride. The pH ratio on the temperature of the solutions with concentrations of 0.083mole/L of each component is given in Figure 4. The temperature rise to 70 degrees Celsius increases hydrolysis (Figure 4).
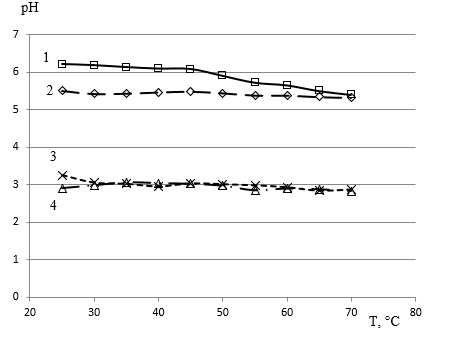
Figure 4: Electrolyte pH dependence for Co-Ni-Fe alloy deposition with concentrations of 0.083mole/L of each component 1 - Co; 2 - Ni; 4 - Fe separately and in a mixture of 3 - Co-Ni-Fe at a temperature of 25 to 70 degrees Celsius.
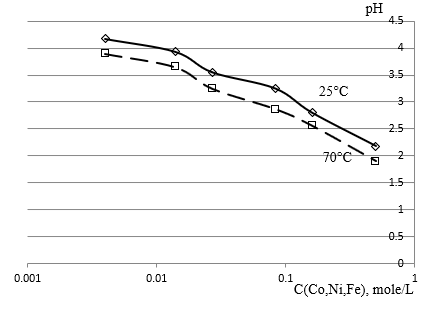
Figure 5: Electrolyte pH dependence for Co-Ni-Fe alloy deposition with concentrations of 0.004 - 0.5 mole/L of each component at 25 and 70 degrees Celsius.
The Figure 5 of the mixed electrolyte pH dependence for Co-Ni- Fe alloy deposition with concentrations of 0.004 to 0.5mole/L of each component shows that the increase in temperature from 25 to 70 degrees Celsius increases the hydrolysis of the mixed electrolyte throughout the studied range of concentrations almost evenly. An experimental study of the hydrogen pH of FeCl2, NiCl2 and CoCl2 salt solutions shows changes in salt hydrolysis in single and mixed solutions depending on temperature and concentration (Figure 5).
Electrochemical Deposition of Co-Ni-Fe films at 0.08 mole/L Electrolyte Concentration
Co-Ni-Fe films are equipped with the same 0.08mole/L boon component CoCl2 6H2O; FeCl2 4H2O; NiCl2 6H2O. In electrolyte added: boric acid H3BO3-20g/L, sodium hydrate C7H4NaNO3S-1. 5g/L, salt acid 3 mmole/L. Film from the specified electrolyte is deposited in an electrochemical plant with a galvanic bathroom volume of 2liters with graphite anode. Nickel ring-to-point to the perimeter of a metallized vertically positioned silicon electrode with a working area of 54 cm2. The electrolyte had a temperature of 70degrees Celsius due to heating by a submersible heater and mixed with a magnetic mixer. The process was carried out in a galvanostatic mode.
The composition of the films obtained by electrochemical deposition from the three component solution FeCl2, CoCl2, NiCl2 with the concentration of each of the component 0.006mole/L gives a [18] component content in the film close to the composition of the electrolyte. In three component solution CoCl2, NiCl2, FeCl2 with a concentration of each component of 0.48mole/L at the same cathode current density and temperature, as at low concentration the component content is far from the composition of the electrolyte. The effect of electric current density on the cathode in the range of 5÷20mA/cm2 on the composition of the films is investigated in electrochemical deposition from the electrolyte with a concentration of impurities 0.48mole/L. The content of metals in precipitation is measured by a Philips XL40 energy dispersive X-ray microanalyzer.
The relative content of the component Co, Ni, Fe in the film differs from the composition of the electrolyte and varies greatly depending on the density of the current. It is not possible to select the current density to obtain the composition of the film equal to the composition of the electrolyte (Figure 6).
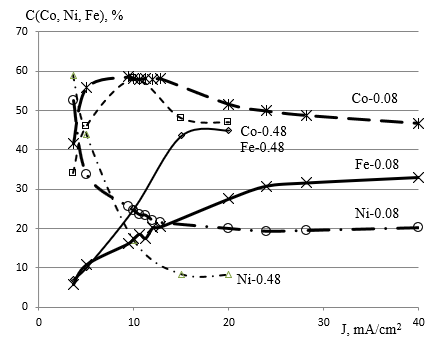
Figure 6: Dependence on current density content of Co-Ni-Fe films when deposited from three component solutions CoCl2, NiCl2, FeCl2 with concentration of each component Co, Ni, Fe equal 0.48 mole/L or 0.08 mole/L.
The studied relative content of the Co, Ni, Fe component in the film when deposited from the electrolyte composition with a concentration of 0.08mole/L of each component is shown in Figure 6. There is a low dependence on current density at a magnitude of 25 mA/cm2. In the Co-Ni-Fe film, the nickel content is 19 - 22%, iron is 28 -33%, and the cobalt content is 55-47%. With a current density of less than 25mA/cm2, the composition of the Co-Ni-Fe film changes dramatically - the nickel content increases from 19 to 57%, the iron content drops from 28 to 5%, and the cobalt content is 55-42%.
Depending on the current density of the Co-Ni-Fe films when deposited from three component solutions CoCl2, NiCl2, FeCl2 with the concentration of each component Co, Ni, Fe equal 0.48mole/L or 0.083mole/L have common patterns. Comparison of dependences on the current density of the composition of films derived from the three-component solution CoCl2, NiCl2, FeCl2 with the concentration of each component co, Ni, Fe equal 0.48mole/L or 0.08mole/L the following showed. The predominance of cobalt deposition persists at all electrolyte concentrations. Increased current density leads to a decrease in nickel content and an increase in iron content. Reducing the concentration of electrolyte to 0.083mole/L gives in the stabilization of the dependence of the composition of films on the current density of 28mA/cm2 approximation of the concentration of nickel and iron in the film to the composition of the component in the electrolyte. Cobalt is deposited with a concentration of 1.44 times more than in an electrolyte. Iron is deposited with a concentration of 0.97 times less than in an electrolyte. Nickel is deposited with a concentration of 0.54 times less than in an electrolyte.
In experiments on electrochemical deposition of Co-Ni-Fe films from chloride electrolyte with a relic ratio of 1:1:1 and content of the component of 0.48; 0,08; 0.006mole/L is a strong dependence of the composition of the film on the tension between the anode and the cathode and the concentration of salts in the electrolyte. The concentration of the electrolyte depends on the drop of voltage on the electrodes of the anode and cathode at a given current density. The rate at which Co-Ni-Fe films are deposited is determined by the density of current. Changes in composition at high voltages may be due to a change in the speed of the drift of ions in the electric field.
Discussion of Results
The experimental features of electrochemical deposition are described by a sequence of chemical and electrochemical reactions. An experimental study of hydrogen pH of CoCl2, NiCl2 and FeCl2 salt solutions at variable concentrations shows how hydrolysis response in solutions occurs [19].
The dissolution of CoCl2 6H2O cobalt chloride salts, NiCl2 6H2O nickel and FeCl2 4H2O iron, which crystallize in conjunction with water molecules, is accompanied by a hydrolysis reaction. FeCl2, NiCl2, CoCl2 are formed by weak bases FeOH2, NiOH2, CoOH2 and strong hydroid acid HCl. In hydrolysis of salts formed by weak base and strong acid, hydrolysis goes on cation and the solution acquires an acidic reaction.
First of all, the strongest restorers react to the anode - the substances that have the most negative potential. In the electrolysis of water solutions on the anode there is an electrochemical reaction and in accordance with the size of the current and the potentials of ionization are isolated anions and formed positive ions of cations of different concentrations for different atoms.
Under the influence of the electric field in the electrolyte there is a drift of positive ions of hydroxide metals from the anode to the cathode at a speed determined by the magnitude of the mobility of the ions and the tension of the electric field. Partial ions currents are formed. The cathode has an electrochemical reaction of metal discharge from hydroxide and the formation of water molecules.
The cycle of reactions of dissociated water ions with metal chlorides has closed. Experimentally confirmed [7] near the cathode formed an area with an elevated pH value. The current density increases and the composition of the electrolyte near the cathode deviates more from the uniform homogeneous, corresponding to the thermo-dynamic equilibrium.
Changing the potential of the electrode due to the change in the concentration of reagents near the electrodes during the passage of the current is called concentration polarization. Changes in the concentration of reactive substances cause a slowing down of the reagents or the diversion of reaction products. The difference in diffusion or ion mobility ratios of the electrolyte component determines the different dependence of the content of the component in the film on the current density, which creates a layer of concentration polarization.
Magnetization and Сoercive Force of Co-Ni-Fe Films
The magnetization and сoercive force of the Co-Ni-Fe films were determined by the hysteresis loop of the magnetic field flux on the magnetic properties analyzer. The composition of the films on the plates was determined using an energy-dispersive X-ray microanalyzer. The results of measurements of magnetic parameters: specific magnetization B/h, coercive force Hc and composition of films of triple alloy Co-Ni-Fe are presented in Figure 7. Magnetization of Co-Ni-Fe films of 130 nWb/μ at a Fe content of 13% to 23%. The сoercive force of Co-Ni-Fe films has a minimum value of 1.25 Oe with a Fe content of 16% (Figure 7).
Conclusion
The deposition of Co-Ni-Fe films provided the choice of chloride electrolyte with the ratio of CCo/CNi/CFe = 1/1/1, the development of electrolyte filtration technology and the deposition process at 70 degrees Celsius. An electrolyte with equal concentrations of CoCl2, NiCl2, FeCl2 equal to 0.08mole/L has optimal for the deposition of soft magnetic Co-Ni-Fe films. Co-Ni-Fe films in electrochemical deposition are reproduced with minimal mechanical stress and with good adhesion to the nickel sub layer. The study of the Co-Ni-Fe alloy electrochemical deposition mechanism of different concentrations does not provide congruent deposition of the three-component alloy. Based on the abnormal deposition of two component alloys, congruent electrochemical deposition of Ni-Fe and the temperature dependence of hydrogen salt solutions, it showed that it is necessary to continue the search for Co-Ni-Fe’s congruent electrochemical deposition modes while reducing the concentration of each component in the electrolyte at high current density.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- I Tabakovic, V Venkatasamy (2018) Preparation of metastable Co-Fe-Ni alloys with ultra-high magnetic saturation (Bs= 2.4 - 2.59 T) by reverse pulse electrodeposition. Journal of Magnetism and Magnetic Materials 452: 306-314.
- T Yanai, K Koda, J Kaji, H Aramaki, K Eguchi, et al. (2018) Electroplated Fe-Co-Ni films prepared in ammonium-chloride-based plating baths. Aip Advances 8(056127): 1-5.
- DY Park, BY Yoo, SKelcher, NV Myung (2006) Electrodeposition of low-stress high magnetic moment Fe-rich Fe-Co-Ni thin films. Electrochimica acta 51: 2523-2530.
- D Li, E Podlaha (2017) Template-Assisted Electrodeposition of Fe-Ni-Co Nanowires: Effects of Electrolyte pH and Sodium Lauryl Sulfate. Journal of The Electrochemical Society 164(13): D843-D851.
- Y Yang (2015) Preparation of Fe-Co-Ni ternary alloys with electrodeposition. Int J Electrochem Sci 10: 5164-5175.
- Korovin NV (1957) Cathode processes at electrodeposition of nickel and iron. Zhur. Neorg. Khim 2(9): 2259-2263.
- H Nakano, M Matsuno, S Oue, M Yano, Sh Kobayashi, et al. (2004) Mechanism of Anomalous Type Electrodeposition of Fe-Ni Alloys from Sulfate Solutions. The Japan Institute of Metals, Materials Transactions 45(11): 3130-3135.
- L Wang, Y Gao, Q Xue, H Liu, T Xu, et al. (2005) Microstructure and tribological properties of electrodeposited Ni - Co alloy deposits. Appl Surf Sci 242: 326 - 332.
- S Tebbakh, Y Messaoudi, A Azizi, N Fenineche, A Dinia, et al. (2015) The influence of0020saccharin on the electrodeposition and properties of Co - Ni alloy thin films. T I Met Finish, The International Journal of Surface Engineering and Coatings 93(4): 196-204.
- ВG Tóth, L Peter, L Pogány, A Revesz (2014) Preparation, Structure and Giant Magnetoresistance of Electrodeposited Fe-Co/Cu Multilayers. Journal of The Electrochemical Society 161(4): D154-D162.
- RD Tikhonov (2017) Normal Electrochemical Deposition of NiFe Films. Advances in Research 11(2): 1-10.
- RD Tikhonov (2018) The effect of ion charge on ferric chloride hydrolysis during electrochemical deposition of NiFe alloy. British Open Journal of Chemical Sciences 2(1): 1-14.
- RD Tikhonov (2018) Magnetic properties of permalloy films deposited electrochemically by the Tikhonov method. British Open Journal of Chemical Sciences 2(2): 1-10.
- RD Tikhonov (2019) Model of electrochemical deposition Ni-Fe: experiments and theory. British Open Journal of Chemical Sciences 3(1): 1-11.
- Robert Tikhonov (2019) Congruent electrochemical deposition of Ni-Fe alloy. Lambert Academic Publishing: 193.
- RD Tikhonov (2020) Electrochemical Deposition of Ni-Fe Alloy at a Temperature of 70°C. Russian journal of Electrochemistry 56(7): 611-614.
- RD Tikhonov (2020) Normal Electrochemical Deposition Ni-Fe: Experiments and Theory. European Journal of Engineering Research and Science 5(8): 828-834.
- RD Tikhonov (2021) Features of electrochemical deposition of films of the triple system of the Co-Ni-Fe. European Journal of Engineering Research and Science 6(2): 19-28.
- RD Tikhonov (2020) Mechanism of the Electrochemical Deposition of a Co-Ni-Fe Alloy. Chemical Science International Journal 29(7): 1-8.
- RD Tikhonov, AA Cheremisinov, MR Tikhonov (2021) Ion discharge in electrochemical deposition of Co-Ni-Fe films. Russian journal of Electrochemistry 57(12): 756-761.
- RD Tikhonov, SA Polomoshnov, VV Amelichev, AA Cheremisinov, AM Kovalev (2021) Co-Ni-Fe Triple System Films Formation by Electrochemical Deposition. Proc Univ Electronics 26(3-4): 246-254.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.