Short Communication 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Epidemiological, Clinical Characteristics and Therapeutic Results for Stage II Colon Cancer: Experience of the Medical Oncology Department of Fez
*Corresponding author: Youssef Elhaitmy, Department of Medical Oncology, University Hospital Center Hassan II, City of Fez, Morocco.
Received: May 15, 2023; Published: June 02, 2023
DOI: 10.34297/AJBSR.2023.19.002548
Abstract
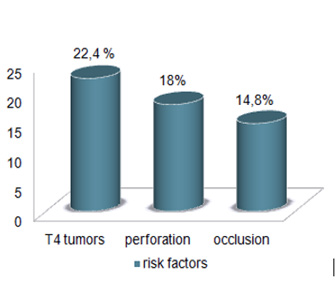
Adjuvant chemotherapy is the standard of care in patients with stage II colorectal cancer (CRC) with high-risk features. The objective of this study is to report survival benefits associated with adjuvant chemotherapy among patients with stage II colon cancer having one or more high risk features [T4 tumors, less than 12 lymph nodes examined (<12LN), positive margins, high-grade tumor, perineural invasion ,and lymphovascular invasion).This is a retrospective study which included 144 patients with stage II CRC treated at the medical oncology department of Fez over a period from December 2009 to November 2020. The Kaplan Meier method was used to estimate the median survival. 65% of patients (n= 93) received postoperative chemotherapy with a female predominance (44 %males 56%females). Microsatellite instability (MSI) was observed in 25 % of patients versus microsatellite stability (Mss) in 38 % of patients. Among the identified risk factors, occlusion, perforations and T4 tumors were observed in 14.8%, 18% and 22.4% of patients respectively. Median overall survival for MSI patients was higher than MSS patients (36 months versus 29 months). The majority of patients with MSS status and risk factors received chemotherapy with a median overall survival of 29 months. Chemotherapy was well tolerated on the hematological and digestive level. The acute toxicities observed were mostly grade 1 or 2. Our study showed an increased overall survival in patients with MSI. However, the indication for adjuvant chemotherapy must take into account the benefit/risk ratio for each patient.
Keywords: Stage II colon cancer; Epidemiology; MSI; Survival
Introduction
Colorectal cancer (CRC) is the 3rd most common cancer in men after prostate and lung cancer. It is the second most common cancer in women after breast cancer [1–3]. However, this cancer is usually developed on preexisting lesion, which makes this cancer accessible to a prevention strategy. The study of colorectal cancer carcinogenesis has showed an accumulation of successive mutations, on both tumor suppressor genes and oncogenes [4]. There are two main pathways of carcinogenesis in the CRC: the chromosomal instability pathway borrowed by 85% of sporadic colorectal cancers and all cancers developed in the context of familial adenomatous polyposis (FAP); and the microsatellites instability pathway, present mainly in colorectal cancers of hereditary non-polyposis co lorectal cancer (HNPCC) and in 15% of sporadic CRC. This pathway is caused by instability of repeated sequences caused by deficient DNA repair. Genes that are involved are the mismatch repair (MMR) [4–7]. Adjuvant treatment of patients with stage II colon cancer is an area of controversy in medical oncology. The aim of adjuvant chemotherapy is to eradicate micro metastatic disease present at the time of surgery and prevent the development of distant metastatic disease. National and international guidelines for the adjuvant treatment of stage II colon cancer depends on the presence or absence of high-risk features (poorly differentiated histology, presence of lymphovascular invasion, presence of perineural invasion, report of < 12 lymph nodes, bowel obstruction, localized perforation, or positive margins).
Methods
This is a retrospective study of 144 patients diagnosed with stage II colon cancer and treated at the medical oncology department of Fez over a period from December 2009 to November 2020. The Kaplan Meier method was used to estimate the median survival.
Results
65% of patients (n= 93) received adjuvant chemotherapy and included 44 %males 56%females. Mss status was reported in 38 % (n=56) of patients and MSI detected in 25 % (n=36). Regarding the reported risk factors, 14.8% of patients had an occlusion and 18% had perforations. T4 tumors observed in 22.4% of patients (figure 1). Median overall survival for MSI patients was 36 months versus 29 months for MSS patients. 88% of patients with MSS status and risk factors have benefited from chemotherapy with a median survival of 29 months. The patient’s tolerance profile was manageable on the hematological and digestive level. Acute toxicities observed were mainly grade 1 or 2 and consisted in vomiting and diarrhea in 25% of cases, peripheral neuropathy in 21.5% of cases and neutropenia in 8.5% of cases. (Table1)
Discussion
Stage II colon cancers form a very heterogeneous group from an anatomopathological point of view with a very heterogeneous prognosis associated with 5-year overall survival rates of 87.5% in stage IIa and 58.4% in stage IIc [8]. The results reported on the effect of adjuvant chemotherapy in the treatment of stage II colon cancers are also very heterogeneous and few specific trials have been conducted to try to answer the question. These are most often results from subgroup analyzes within trials that included stages II and III and from meta-analyses. Analysis using interaction tests of the data from the NSABP C01-4 trials showed that adjuvant chemotherapy benefited stage II patients included with a relative reduction in mortality identical to that of stage III. Overall, there is a trend in favor of a benefit of adjuvant chemotherapy for stage II cancers, but a priori only concerns a subgroup of patients. The demonstration of the potential interest of adjuvant chemotherapy for stage II cancers has been reported through the results of the QUASAR study [9–11]. This study (n = 3239 patients with colon or rectal cancer) compared adjuvant chemotherapy with 5-fluorouracil (5-FU) + folinic acid ± levamisole to one arm without adjuvant chemotherapy in patients with colorectal cancers predominantly of stage II (91%). In subgroup analysis, for stage II colon cancers, the relative risk of recurrence at 2 years was reduced by 29% with a hazard ratio (HR) of 0.71 (95% CI: 0.54 - 0, 92; p = 0.01) with a non-significant trend towards improvement in overall survival an HR of 0.83 (95% CI: 0.65 - 1.07; NS). The MOSAIC study, which included stage II and III patients, compared adjuvant chemotherapy with FOLFOX4 to LV5FU2 . In the subgroup of all stage II patients, there is no 10-year survival benefit in favor of FOLFOX4 (78.4% versus 79.8%; HR: 1.00; CI 95 %, 0.74-1.35; p=0.98). For the high-risk stage II subgroup (T4, perforated tumor, or number of nodes examined < 10), treatment with FOLFOX4 resulted in a statistically nonsignificant improvement in disease-free survival compared to LV5FU2 (RR: 0, 79; 95% CI: 0.55 - 1.13; NS) and overall survival at 10 years (RR: 0.89; 95% CI: 0.60 - 1.32; NS) [12–14].
Routine determination of MSI or dMMR status should be recommended to discuss the indication of adjuvant chemotherapy for a patient operated on for stage II colon cancer with poor prognostic factors [15]. In our study MSI observed in 25 % (n=36) of patients versus Mss in38 % (n=56) of patients. The good prognosis of patients operated on for stage II colon cancer with a molecular MSI and/or immunohistochemical dMMR phenotype (generally of faster evaluation than in molecular biology) is an argument for not offering adjuvant chemotherapy. This element can only appear in the discussion if the MSI status is available within a time frame compatible with the establishment of adjuvant chemotherapy (ideally within 4 weeks after the date of surgery). the indication of adjuvant chemotherapy for patients with stage II cancer should be discussed on a case-by-case basis with an assessment of the risk- benefit ratio of this adjuvant chemotherapy and with knowledge of the MSI status of the cancer. [16] The potential gain of adjuvant chemotherapy in stage II is to be weighed according to the toxicity of the chemotherapy and the risk-benefit ratio. The benefit in survival being moderate (between 2 to 5% in absolute value according to the risk of recurrence with fluoropyrimidines alone), it should encourage practitioners to separate stage II tumors according to their “relative” risk of recurrence:
1. risk “ relative” low or moderate recurrence: - MSS tumor with the following good prognostic factors: T3, analysis of more than 12 lymph nodes [17], absence of venous, perineural and/ or lymphatic emboli, well or moderately differentiated tumor, and absence tumor perforation - MSI tumor
2. high “relative” risk of recurrence (MSS tumors with one or more of the following poor prognostic factors): T4, analysis of less than 12 lymph nodes, presence of venous, perineural and/or lymphatic emboli, poorly differentiated tumour, tumor perforation and for some revealing occlusion and presence of isolated neoplastic cells in an N0 lymph node (CTI).
For some experts, the presence of venous, perineural and/or lymphatic emboli cannot be considered when it is a single risk factor as a high risk. The same is true of tumoral occlusion, the definition of which is often very vague and heterogeneous in the publications (the occlusive nature of the tumor in endoscopy is not a factor of poor prognosis, unlike the clinical and radiological occlusive syndrome treated by stoma or emergency colectomy). The poorly differentiated nature of the tumor is associated with a high risk only in the case of an MSS tumor (MSI tumors are very often poorly differentiated and have a good prognosis). The presence of isolated neoplastic cells in a ganglion - T3N0(CTI) - is a rare situation that is considered by some to be a high-risk factor. It should be noted that the data in the literature on these anatomopathological risk factors for recurrence for stage II come from a posteriori analyzes by subgroups and that the benefit of adjuvant chemotherapy as a function of these factors has not been demonstrated. It is likely that in the near future, a more precise molecular classification of colon cancers will make it possible to stratify the therapeutic management of patients with localized colon cancer with perhaps different treatments for mutated BRAF, MSI, HER2 amplified [18].
Another approach is the monitoring of residual disease using circulating DNA of tumor origin (ctDNA) in blood. [19,20] The detection of blood ctDNA postoperatively in patients treated for localized colon cancer seems very promising as a biomarker associated with disease-free survival and overall survival and for monitoring adjuvant chemotherapy. It will probably be an important future factor in the strategy of adjuvant treatment of localized colon cancer and prospective studies are currently underway to validate it, such as the CIRCULATE-PRODIGE 70 study in stage II [21,22].
Conclusion
Stage II colon cancers form a very heterogeneous group from an anatomopathological point of view with a very heterogeneous prognosis in terms of overall survival. Our study showed a higher overall survival in patients with MSI. However, the indication of adjuvant chemotherapy for patients with stage II cancer is to be discussed on a case-by-case basis with an assessment of the risk-benefit ratio of this adjuvant chemotherapy and with knowledge of the MMR/MSI status of the cancer.
Acknowledgement
None.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pan SY, Morrison H (2011) Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 3(3): 33‑
- Nishida H, Urano S (2011) Effectiveness of repeated screening using the fecal occult blood test and its impact on reducing false-negative cancer cases. European Journal of Cancer Prevention 20(3): 184‑18
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, et al. (2004) Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 126(7): 1674‑16
- Worthley DL, Whitehall VL, Spring KJ, Leggett BA (2007) Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol 13(28): 3784‑37
- Grady WM (2004) Genomic instability and colon cancer. Cancer Metastasis Rev 23(1‑2): 11‑
- Worthley DL, Leggett BA (2010) Colorectal Cancer: Molecular Features and Clinical Opportunities. Clin Biochem Rev 31(2): 31‑3
- Smith G, Carey FA, Beattie J, Wilkie MJV, Lightfoot TJ, et al. (2002) Mutations in APC, Kirsten-ras, and p53-alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A 99(14):9433‑940
- Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, et al. (2018) Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients with Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA 319(20): 2095‑2
- Gray RG, Quirke P, Handley K, Lopatin M, Magill L, et al. (2011) Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 29(35): 4611‑460
- QUASAR Collaborative Group (2000) Comparison of fluorouracil with additional levamisole, higher dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. Lancet 355(9215): 1588‑15
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, et al. (2007) Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370(9604): 2020‑202
- Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, et al. (2012) Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 30(27): 3353‑33
- André T, Boni C, Navarro M, Tabernero J, Hickish T, et al. (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19): 3109‑30
- André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, et al. (2015) Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 33(35): 4176‑41
- Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138(6): 2073-2087.
- Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L (2004) Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol 22(16): 3395‑3
- Choi HK, Law WL, Poon JTC (2010) The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer 10: 267.
- Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, et al. (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 28(3): 466‑4
- Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, et al. (2019) Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients with Stages I to III Colorectal Cancer. JAMA Oncol 5(8): 1124‑11
- Lecomte T, Berger A, Zinzindohoué F, Micard S, Landi B, et al. (2002) Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer 100(5): 542‑54
- Taïeb J, Benhaim L, Laurent Puig P, Le Malicot K, Emile JF, et al. (2020) Decision for adjuvant treatment in stage II colon cancer based on circulating tumor DNA:The CIRCULATE-PRODIGE 70 trial. Dig Liver Dis 52(7): 730‑73
- Fan G, Zhang K, Yang X, Ding J, Wang Z, et al. (2017) Prognostic value of circulating tumor DNA in patients with colon cancer: Systematic review. PLoS One 12(2): e0171991.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.