Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Genetic Disorder and Cancer
*Corresponding author: Parimal Das, Banaras Hindu University, India.
Received: July 10, 2023; Published: August 11, 2023
DOI: 10.34297/AJBSR.2023.19.002635
Abstract
The human body is composed of different cell types and tissues and cancers can arise from any of these cells. Cancer, the last stage of tumour growth is driven by genetic and epigenetic alterations that allow cells to proliferate indefinitely and escape mechanisms that normally control their survival and migration and cellular defense barriers. Oncogenes and tumour suppressors are the class of the genes which are responsible for the initiation and progression of tumour. Cancer is of three types of Carcinoma, Sarcoma and Leukemia depending upon the cell of origin and caused by different cancer causing agents known as carcinogen. Cancer is a genetic disease, and a spectrum of DNA variations is responsible or associated with particular type of cancer. Many of these changes map to signalling pathways that control cell proliferation and death leading to distortions of wider signalling networks. These mutations cremoveng cellular proto-oncogenes to oncogenes can cause hyper activation of these signalling, whereas inactivation of tumour suppressor genes removes negative regulators of signalling pathways. These information at molecular level gives insight for the cancer therapy.
Introduction
Cancer is dreadful disease causing a serious burden on human society affecting the health and lifespan. According to GLOBOCAN project, in (2012), 8.2 million people die because of cancer [1]. The fundamental abnormality in the development of cancer is the unregulated cell division and proliferation of cells. Fundamentally cancer is a genetic disease mainly caused by mutations in DNA repair genes in somatic cells leading to accumulation of genetic and epigenetic lesions. Many genes are known that are repeatedly altered in human cancer, such genes are termed as cancer-critical genes, meaning genes whose mutation contributes to the causation of cancer. These changes are inherited, spontaneous and external agents such as radiation or chemical induced. Cancer, the last stage of tumour growth results from alterations in critical regulatory genes that control cell proliferation, differentiation, survival and overcoming numerous highly evolved cellular defence barriers. There are six hallmarks of cancer generally present in all type of cancers and proposed that together these hallmarks constitute an organizing principle that provides a logical framework for un derstanding the remarkable diversity of neoplastic diseases. Those hallmarks include.
1) independence of external growth signals,
2) insensitive to external anti-growth signals,
3) ability to avoid apoptosis,
4) ability to replicate indefinitely,
5) ability of mass of such cells to trigger angiogenesis and vascularise, and
6) ability to invade tissue and establish secondary tumours [2].
Cell proliferation is carefully balanced by cell division and cell death which gives the definite life span to a cell. This balance was disrupted in malignant transformation of the cell and a single faulty cell may give rise to the clone of identical cells expanding to a considerable size, producing a tumour. A tumour which is incapable of indefinite growth and does not invade the healthy surrounding tis sue extensively is known as benign. Tumour with property of continuous growth and progressive invasion is malignant tumour and the term cancer refers specifically to these malignant tumours. In addition to uncontrolled cell division, malignant tumours have the property of metastasis; in this process small clusters of cancerous cells dislodge from a tumour and invade into blood or lymphatic vessels and are carried to other tissues where they continue to proliferate leading to tumour formation. Different types of malignant tumours are classified according to embryonic origin of tissues from which they are originated [3-5] (Figure 1).
Carcinomas are the tumours derived from endodermal or ectodermal tissues or the epithelial lining of the internal organs. It is the most common (>80%) type of cancer. e.g., Renal cell carcinoma, lung carcinoma, carcinoma of pancreas etc. Leukaemia’s and Lymphomas are the malignant tumours of the hematopoietic cells of the bone marrow and accounts almost 9% of the cancer incidence, for example, acute myeloid leukaemia, chronic myleloid leukaemia, Hodgkin’s and Non-Hodgkin’s lymphoma, etc.
Sarcomas type of cancer arises from mesodermal connective tissue, for example, bone fat cartilage and share only 1% of cancer incidence, for example, soft tissue sarcoma, retinoblastoma, alveolar soft part sarcoma, etc. [3-5].
There are many risk factors causing the cancerous condition and these risk factors include exposure to chemicals or other substances, causing variation in DNA sequence. Environmental factors and genetic disorders play a crucial role in the pathogenesis of human cancers. Studies have shown association between a potential risk factor and an increased risk of cancer. Risk factor association could explain how they could actually cause cancer. Risk factors for developing cancer include the following, which have been most studied or suspected of development of cancer. Advance age, family history, gender, consumption of alcohol, carcinogenic substances, sedentary lifestyle, chronic inflammation, dietary habit, hormones, obesity, suppression of immune system, infecting agents, radiation exposure, sunlight, sustained consumption of tobacco. Limiting exposures to these risk factors may lower occurrence of certain cancers.
Physiology of Cancer Cell
Cellular physiology and metabolic state of a cancer cell is very much different from a normal cell at all aspects of cellular physiology. Uncontrolled cell division is the main feature of all cancer cells suggesting that cell cycle and apoptosis is abnormally regulated. In cancer apoptosis, the cellular programmed cell death is inhibited therefore cells divide but do not die leading to tumour formation. Non-physiological level of oxygen tension owing to the rapid proliferation of cancer cells is known as Hypoxia. Tumour cells rapidly exhausts the oxygen and nutrient supply from the normal vasculature and drives upregulation of the production of angiogenic factors triggering the vascularization of the tumour. Hypoxia induces a number of complex intracellular signalling pathways, for example, Hypoxia-Inducible Factor (HIF) pathway, PI3K/AKT/mTOR, MAPK and the NFĸB. These pathways are involved in cell proliferation, apoptosis, metabolism, migration, and inflammation regulating cancer survival. These continually dividing cells need a lot of energy to maintain cell division and feeding this large number of cells. Apart from increasing blood supply by forming new blood vessels, these cancer cells adapt for more energy. Cancer cells alter their metabolism and adapt to promote growth and survival of the tumour. Increase in glucose uptake, fermentation of glucose to lactate lead to up-regulation of the pentose phosphate pathway for compensating reduced energy yield in tumours. Tumour cells has ability to break down glucose by glycolysis at a very high rate than in normal tissues, even when oxygen is abundant. This is the Warburg effect and almost all cancer hallmarks could be the consequence of this effect. Warburg effect enables rapidly dividing tumour cells to generate essential biosynthetic building blocks e.g., nucleic acids, amino acids, and lipids from glycolytic intermediates for growth and duplication of cellular components during cell division [6]. Energy metabolism and cell death pathway is regulated by another very important cell organelle known as lysosome. Lysosome is a catabolic cell organelle having the hydrolytic enzymes. Cancer cells have a block in their programmed cell death and could be targeted for therapy through the release of lysosomal enzymes. Importantly, the pathway of lysosomal cell death can be efficiently triggered by many chemotherapeutic regimens [7].
Mechanism of Cancer Pathogenesis
Cancer is a disease with differential phenotypes with the tumour origin and type. Mitogens (growth signals) are essential factors for proper cell growth and proliferation, are transmitted via specific receptors. In cancer these mitogens and receptors are dysregulated leading to uncontrolled cell growth. Cancer, the last stage of tumour formation is a stepwise process:
After genetic or environmental abnormality cells lose their ability to maintain the balance between cell division and cell death, leading to uncontrolled cell growth. This uncontrolled cell division leads to the formation of benign tumour which looks like the tissue with normal cells from which it originated, has a slow growth rate and most importantly they do not invade surrounding tissues and they do not metastasize to other organs. Generally benign tumour needs no treatment if symptoms are not problematic. Major health concern starts when benign tumour transforms into metastatic form of tumour known as Cancer. Epithelial to Mesenchymal Transition (EMT) mediates cellular transformation of benign tumour cell into the cancer cell leads to the activation or upregulation of enzymes which degrades Extra Cellular Matrix (ECM). Degradation of ECM by matrix metalloproteases leads to the invasion of tumour cell into the other organ and metastasized to the new place. This invasion and metastasis are a multistep process.
Immunoglobulins and cadherins class of proteins are involved in the cell-to-cell adhesions while integrins link cells to the ECM. E-cadherin is the most important protein cells to each other and coupling of cells by E-cadherin transmits antigrowth signals known as contact inhibition. N-cadherin helps the cancer cell to slip through blood vessels during migration and its expression increased in migrating cancer cells. Epithelial to mesenchymal transition characterized by the reduced E-cadherin and increased N-cadherin level [8].
After metastasis cells start to grow at the new location and form a tumour known as cancer. These tumours need nutrients to survive; new blood vessels are formed to supply nutrient and energy, this process is known as angiogenesis. Angiogenesis is largely regulated by Vascular Endothelial Growth Factor (VEGF) and stimulates vascular endothelial cell growth, survival, and proliferation. VEGF is a group of 6 structurally related proteins that regulate the formation, growth and differentiation of multiple components of the vascular system, mainly blood and lymph vessels [9].
Carcinogen And Genetic Variations
Cancer is mainly caused by genetic variations caused by toxic chemicals, environmental factors, food etc. All those substances and factors that can lead to cancer are called carcinogens. Carcinogens induce malignancies by generating DNA lesions (i.e., adducts) that can result into the mutations if remain unrepaired. Carcinogens can be divided into three major categories: chemical, physical carcinogens and biological.
Chemical Carcinogens: Exposure to noxious chemicals considered as chemical carcinogens is the single most important contributor to the incidence of human cancer. Exposure of these carcinogens leads to the interaction of the chemical with key biological macromolecules. Interaction with DNA is most dangerous and lead to the variations at the DNA level by the chemical modification of DNA sequences. These changes are cumulative, ultimately leads to a profound change at DNA level causing serious threats to the cell cycle. Example: cigarette smoke and some pesticides, Benzene, Formaldehyde, Mustard Gas [10,11].
Physical Carcinogens: Physical carcinogens include solid physical products operating through pressure or other mechanism, foil, inhaled fibres, asbestos particles, hard and soft synthetic materials, and gels. Some physical carcinogens are naturally occurring, while others are synthetic and highly variable in their chemical structure. X-rays, ultraviolet rays, and heat among the other non-particulate physical agents are responsible for the carcinogenesis. Some physical carcinogens act in concert with other factors such as genetic and other environmental factors to produce cancer. For example, asbestos can cause cancer on its own; however, its carcinogenic potential increased when combined with exposure to cigarette smoke [12].
Biological Carcinogens: 17.8% cancers were caused by viral (12.1%), bacterial (5.6%) and helminthes (0.1%) infection. According to International Agency for Research on Cancer (IARC) infections of all viruses that fulfil the following criteria can be direct carcinogens. First, the viral genome or part of it can usually be detected in each cancer cell. Second, the virus can immortalize after the growth of target cells in vitro. Third, it expresses several oncogenes that interact with cellular proteins and have multifunctional properties leading to disruption of the cell-cycle checkpoints, inhibition of apoptosis and cell immortalization. Four different viruses have been described as direct carcinogens: human papillomavirus family, Human T-cell Lymphotropic Virus type 1 (HTLV-1) and the two herpes viruses: Kaposi sarcoma- associated herpes virus (KSHV) and Epstein-Barr virus (EBV). Infectious agents can be indirect carcinogens by causing chronic inflammation leading to the production of chemokines, cytokines, prostaglandins secreted by infected cells. It includes two hepatitis viruses HBV and HCV, Helicobacter pylori, Schistosoma haematobium, Opistorchis viverrini, and Clonorchis sinensis [13].
Genetic Basis of Cancer
Cancer is a genetic disease of uncontrolled growth and proliferation developed by a multi-step process that requires the accumulation of many genetic changes over time. Malignant cells have undergone mutations and epigenetic changes and maintain the transformed phenotype even when cultured or injected into immunologically tolerant experimental animals. These nonlethal genetic alterations involve activation of proto-oncogenes to oncogenes, deregulation of tumour suppressor genes and DNA repair genes. These changes are leading to initiation and progression of cancer. Most of the genetic changes in tumours are somatic brought about environmentally or randomly and a small proportion of all cancers are identified as inherited also referred as “genetic”.
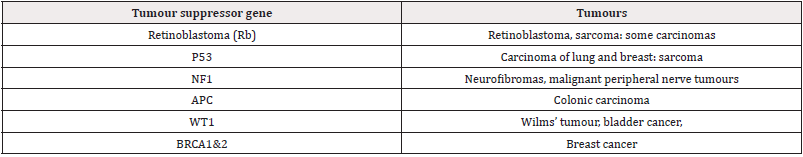
Tumour suppressor or anti-oncogenes, encodes the proteins that commonly control the fidelity of the cell cycle replication and inhibit cell proliferation and tumour development. Most of these ‘classical’ tumour suppressor genes have been characterized by the identification of germline mutations associated with the predisposition to human cancers. Tumour suppressor genes are recessive and therefore inactivation of both alleles is required. These genes are lost or inactivated by point mutations or deletion in both alleles of the gene. Loss of function of these genes liberates cells from growth restrictions and contributes to malignant transformation (Table 1).
The cumulative effect of genetic changes that inactivate tumour suppressor genes or activate proto-oncogenes is a mechanical failure in the balance between cell proliferation and cell loss because of differentiation or apoptosis leading to the clonal overgrowth of a specific cell lineage. Retinoblastoma susceptibility gene RB is the first recognized tumour suppressor gene which encodes a nuclear phosphoprotein that acts as a “gatekeeper” of the cell cycle. Deletion or point mutation leads to the inactivation of the RB; causing the protein to lose its regulatory capacity. P53 is the second recognized and well-studied tumour suppressor genes, expressed at low levels in normal cells and has regulatory role in the cell cycle, DNA synthesis and repair, transcriptional regulation and programmed cell death [14,15].
Oncogenes
Oncogenes were first discovered in cancer-causing viruses; however, they are present in all normal cells. More than 40 different highly oncogenic retroviruses have been isolated from a variety of animals. All of these viruses, like RSV, contain at least one oncogene that is not required for virus replication but is responsible for cell transformation (Cooper GM, book chapter). Example of retroviral oncogenes: abl(Mouse), ets(Chicken), fos(Mouse), jun(Chicken), rasH(Rat), rasK(Rat), src(Chicken) etc.. The unmutated wild-type allele of an oncogene is known as the proto-oncogene, and these proto-oncogenes converted to oncogenes by amplification, translocation, and point mutation of DNA.
Mutations in proto-oncogenes are typically dominant in nature and act through either overexpression or activating mutations. Oncogenes encode proteins that control cell proliferation, apoptosis which possess the ability to cause cellular transformation. The proteins encoded by oncogenes can be classified into six broad groups: transcription factors, chromatin remodelers, growth factors and its receptors, signal transducers, and apoptosis regulators [16]. Chromosomal translocations activate transcription-factor genes in lymphoid cancers, solid tumours, and certain sarcomas. Chromosome inversions and translocations are common cytogenetic increase or deregulate transcription of the oncogene. Most of the hematopoietic tumours and soft tissue sarcomas are initiated by the activation of an oncogene, followed by alterations in tumour suppressor genes and other oncogenes. In contrast, most carcinomas are initiated by the loss of function of a tumour-suppressor gene, followed by alterations in oncogenes and additional tumour suppressor genes [16]. Duration and aggressiveness of the tumour can be changed by introducing activated oncogenes or inactivated tumour-suppressor genes in mouse model of cancer.
Genetic Instability and Cancer
As mentioned earlier cancer is a genetic disease and different types of cancer has associated landscape of genomic variation. Some of the most common cancer is discussed below:
Breast Cancer
Breast cancer is among the most commonly diagnosed cancer and ranks second among causes for cancer related death in women. Breast cancer is most strongly associated with highly penetrant germline pathogenic variants in BRCA1 and BRCA2. BRCA1 (Breast Cancer susceptibility gene 1) and BRCA2 are tumour suppressor genes transcriptional regulated in response to DNA damage and contribute to DNA repair. 5-9% of all breast cancers are Hereditary in nature and estimated that the BRCA1 and BRCA2 gene mutations are responsible for ≈80% of the families with hereditary breast cancer. Apart from BRCA1 and BRCA2 there are many other genes which are associated with breast cancer, for example, TP53, ATM, PTEN, LKB1, HRAS1, NAT1, NAT2, GSTM1, CYP1A1, etc [17-19].
Cervical Cancer
Cervical cancer is one of the leading causes of death of women in developing countries. Epidemiological and laboratory studies have identified that infection of one of 15 high-risk Human Papillo- ma Virus (HPV) types as a necessary but not sufficient for the cervical cancer. Most HPV infection is transient and harmless and cleared by immune response but persistent infection with high-risk human papillomavirus can cause cancer. Genetic variations in the host genes involved in immune response pathways may be related to clearance of HPV, and HPV E6/E7 oncoproteins interacting or downstream genes may contribute to the outcome of HPV infection and cervical cancer. The most important among the known risk factors is the HLA class II DRB1-DQB1 haplotype, such as DRB1*1501- DQB1*0602 and DRB1*1301-DQB1*0603 associated with increased and decreased risk of cervical cancer, respectively [20-22].
Gastric Cancer
Gastric cancer is associated with poor survival rates leading to high proportion of global cancer mortality. Approximately 90% of gastric cancers are adennocarcinoma and it is well known that the development of gastric cancer is associated with underlying gastric diseases, Helicobacter pylori (H. pylori) infection and genetic susceptibility factors. Almost 10% of gastric cancer cases display familial clustering whereas only 1-3% of gastric carcinomas arise due to inherited gastric cancer predisposition syndromes. Co-segregation of germline E-cadherin (CDH1) mutations with early onset diffuse gastric cancer in families with an autosomal dominant pattern of inheritance (HDGC) show that gastric cancer is hereditary in nature. APC, a tumour-suppressor gene and is associated with colorectal cancer [23-25].
Oral Cancer
According to International Classification of Diseases, oral cancer is the cancer of the oral cavity and pharynx, including cancer of the lip, salivary glands, tongue, gum, floor and other areas of the mouth, nasopharynx, oropharynx, pharynx, hypopharynx, and other buccal areas. Oral cancer is frequently associated with Chromosomal alterations in chromosome 8. Chromosomal gains in 8q and losses in 8p are the common alterations present and have been shown to be involved in the lymph node metastasis of oral cancer. c-MYC (8q24) in chromosome 8 is frequently amplified in various cancers. Frequently deleted regions are 8p21, 8p22 and 8p23 [26- 28].
Lung Cancer
Lung cancer is one of the most common human cancers representing most common form of cancer death worldwide, and tobacco smoke is the major risk factor for this disease. Collaborative, genome-wide association studies have identified common gene variants involved in lung cancer. Three separate loci associated with lung cancer (5p15, 6p21, and 15q25) are identified including the genes that regulate acetylcholine nicotinic receptors and telomerase production. Mutations in Epidermal Growth Factor Receptor (EGFR) tyrosine kinase identified in patients with lung cancer who have never smoked. Prevalence of mutations in KRAS and P53 genes differ in patients with tobacco-associated lung cancer and patients who have never smoked [29-32].
Soft Tissue Sarcoma
Soft-tissue sarcoma is a heterogeneous group of neoplasms arising in mesenchymal tissue and comprises more than 35 histologic subtypes, often associated with distinctive clinicopathologic features. These types of sarcomas are rare, but generally aggressive tumours mainly affecting children and young adults. These types of tumours arise from muscle, connective tissue, supportive tissue and vascular tissue with high propensity for local recurrence. Soft-tissue sarcoma can be divided into two groups: Rhabdomyosarcoma (RMS) and Non-Rhabdomyosarcoma Soft-Tissue Sarcomas (NRSTS). Frequently mutated genes in soft tissue sarcoma included TP53 (17% of pleomorphic liposarcomas), PIK3CA (18% of myxoid/ round-cell liposarcomas) and NF1 (10.5% of myxofibrosarcomas and 8% of pleomorphic liposarcomas) [33-35].
Signal Transduction in Cancer
Cancer is not a single disease it is a syndrome with many disease phenotypes involving massive army of genes involved in it. During the course of tumour initiation and progression tumour cells acquire a number of characteristic alterations which allow them to proliferate, survive, and invade other tissues. All these cancers associated phenotypes are associated and dependent on each other and regulated by network of the genes. Cellular signalling pathways are interconnected to form complex signalling pathways. Cancer cells receive information from many different growth factor receptors and from cell-matrix and cell-cell contacts and then integrate this information via signal transduction and cellular signalling. This integrated network regulates protein synthesis and cell growth, cell architecture and polarity, motility, differentiation, and programmed cell death capacities. There are many pathways regulating these processes are dysregulated leading cancer progression and survival e.g. Src and FAK in cell motility and invasion, The Ras/MAP kinase pathway: Targets and scaffolds, PI 3-kinases and the regulation of cell growth, motility, Survival, Rho family GTPases etc. PI3K-Akt and Ras-ERK Pathways is an important signalling pathway responsible for cancer is described here [36,37].
The PI3K-Akt and Ras-ERK Pathways: The phosphatidylinositol 3-kinase-mammalian target of rapamycin (PI3K-mTOR) and ras-extracellular signal-regulated kinase (Ras-ERK) signalling pathways are the chief mechanisms for controlling cell survival, proliferation, differentiation, metabolism, and motility in response to extracellular signals. These pathways play key roles in the transmission of proliferative signals from membrane bound receptors. Activation of these pathways can induce cellular immortalization, proliferation, survival, metabolism of cancer cells and resistance to anticancer therapeutics, for example, epidermal growth factor receptor inhibitors and chemotherapy. The PI3K signalling pathway is dysregulated almost in all cancers due to the gene amplification, genetic mutations of PI3K gene and the components of the PI3K pathway. These pathways are activated by growth factors, polypeptide hormones, neurotransmitters, chemokines, and phorbol esters, which signal through their cognate RTKs (receptor tyrosine kinases) and GPCRs (G protein-coupled receptors). Many of the genes commonly mutated in cancer encode components or targets of the Ras-ERK and PI3K-Akt pathways. Normally these pathways are transiently activated in response to growth factor or cytokine signalling and ligand occupancy of receptors, but genetic changes can lead to constitutive signalling even in the absence of growth factors [38,39].
Cancer Therapy
Cancer is one of the biggest challenges and facing the absence of right effective drug for the treatment. Surgery is the most effective method of treatment for the localized primary tumours and associated regional lymphatics. With the emergence of radiation therapy and chemotherapy, surgery has become conservative for the cancer treatment. Chemotherapy and radiation therapy are only capable of killing a fraction of tumour cells and complementary to each other [40]. With the tremendous advances achieved in the understanding of cancer biology molecularly targeted cancer therapy come into the scene. Targeted cancer therapies are drugs or other substances that target specific molecules and block the growth and spread of cancer, for example, Monoclonal antibodies: Anti-HER2 (Trastuzumab), Anti-EGFR (Cetuximab), Anti-VEGF (Bevacizumab); Chemotherapy: Altretamine, Cisplatin, Carboplatin; Endocrine therapy: Tamoxifen, Fulvestrant, Aromatase Inhibitors; Small molecule: Sunitinib, Imatinib, Lapatinib, Erlotinib [41]. Tumour heterogeneity is the main factor behind limited response against targeted therapy therefore targeted cancer therapy remains challenged by a high failure rate and an extremely small proportion of patients benefited. Oncolytic viruses are the class of anticancer agent emerging as a promising tool for cancer therapy. These viruses replicate within, and ultimately lyse, cancer cells sparing normal cells. Many oncolytic viruses have been developed, for example, adenoviruses, herpesviruses (HSV), reoviruses, retroviruses and Vesicular Stomatitis Virus (VSV) and measles virus. US FDA has approved the oncolytic herpesvirus talimogene laherparepvec in advanced melanoma, a major breakthrough for the oncolytic virus as cancer therapeutics [42]. Immunotherapy is the treatment that uses body’s own immune system to fight cancer and this can be achieved by stimulating immune system to work harder or smarter to attack cancer cells or exogenously giving immune system components or proteins. Immunotherapy includes monoclonal antibodies, immune checkpoint inhibitors, cancer vaccines. Gene therapy (an experimental technique using genes to treat or prevent disease) is emerging as a field of cancer research which offers a number of potential treatments. This technique may allow doctors to treat a disorder by inserting a gene into a patient’s cells instead of using drugs or surgery. The term gene therapy includes a wide range of treatment types that all use genetic material to modify cells to cure cancer. Gene therapy of cancer includes transfer of genetic material through viral (or bacterial) and non-viral vectors into cancerous cell or the surrounding tissue to cause cell death or slow down the cancer growth. In recent development of gene therapy clustered regularly interspersed palindromic repeats (CRISPR) technique is used for human diseases including cancer [43].
Conclusion
During the last two decades and half, more than 2000 gene therapy clinical trials have been undertaken worldwide. Almost 95% of the trials were in early phases of development and 72% were ongoing [44]. The majority of gene therapies clinical trials identified targeted cancer diseases. Regulators are defining path for rapid access of those new therapies. Major steps forward are expected in the field of gene therapies in the future.
Acknowledgement
None.
Conflict of Interest
None.
References
- http://globocan.iarc.fr/Default.aspx.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646-674.
- (2000) Molecular Cell Biology, 4th, Harvey Lodish, Arnold Berk, S Lawrence Zipursky, Paul Matsudaira, David Baltimore, and James Darnell. New York: WH Freeman.
- Alberts B, Johnson A, Lewis J, Walter P, Raff M, et al. (2002) Molecular Biology of the Cell 4th Edition: International Student Edition.
- Cooper GM (2000) The Cell: A Molecular Approach, 2nd, The Cell: A Molecular Approach. Sunderland MA.
- (2015) Risk Factors for Cancer. Cancer Causes and Prevention.
- Zhang YJ (2010) Interactions of chemical carcinogens and genetic variation in hepatocellular carcinoma. World J Hepatol 2(3): 94-102.
- Canel M, Serrels A, Frame MC, Brunton (2013) VG E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 126(Pt 2): 393-401.
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56(4): 549-580.
- Ma L, Weinberg RA (2008) Micromanagers of malignancy: role of microRNAs in regulating metastasis. Trends Genet 24(9): 448-456.
- Miller EC, Miller JA (1981) Mechanisms of Chemical Carcinogenesis. Cancer 47(5 suppl): 1055-1064.
- Morando Soffritti, Franco Minardi, Cesare Maltoni (2003) Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th Hamilton (ON): BC Decker.
- Papillomaviruses H (2011) IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: IARC.
- Luca Grumolato, Stuart A Aaronson (2015) The Molecular Basis of Cancer (Fourth Edition).
- Mendelsohn J, Howley PM, Israel MA, Gray JW, Thompson CB (2008) The Molecular Basis of Cancer: Expert Consult-Online. Elsevier Health Sciences.
- Croce CM (2008) Oncogenes and Cancer. N Engl J Med 358(5): 502-511.
- Perou CM, Børresen Dale AL (2011) Systems biology and genomics of breast cancer. Cold Spring Harb Prospect Biol 3(2): a003293.
- de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WT, Oosterwijk JC, et al. (2002) Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet 39(4): 225-242.
- Nik Zainal S, Helen Davies, Johan Staaf, Manasa Ramakrishna, Dominik Glodzik, et al. (2016) Landscape of somatic mutations in 560 breast cancer whole genome sequences. Nature 534(7605): 47-54.
- Xiaojun Chen, Jie Jiang, Hongbing Shen, Zhibin Hu (2011) Genetic susceptibility of cervical cancer. J Biomed Res 25(3): 155-164.
- Eileen M Burd (2003) Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev 16(1): 1-17.
- Wolf JK and Ramirez PT (2001) The molecular biology of cervical cancer. Cancer Invest 19(6): 621-629.
- Yan Lu,Fang Lu, Sha Zeng, Suqing Sun, Li Lu, Lifeng Liu (2015) Genetics and gastric cancer susceptibility. Int J Clin Exp Med 8(6): 8377-8383.
- Chen Yang Yu, Hao Yan Chen (2015) Genetic Variations and Gastric Cancer. Gastrointest Tumours 2: 90-97.
- Hudler P (2012) Genetic aspects of gastric cancer instability. Scientific World Journal pp. 761909.
- Sunit Kumar Jurel, Durga Shanker Gupta, Raghuwar D Singh, Mrinalini Singh, Shilpi Srivastava (2014) Genes and oral cancer. Indian J Hum Genet 20(1): 4-9.
- Yong ZW, Zaini ZM, Kallarakkal TG, Karen Ng LP, Rahman ZA, et al. (2014) Genetic alterations of chromosome 8 genes in oral cancer. Sci Rep 4: 6073.
- Pérez Sayáns M, Somoza Martín JM, Barros Angueira F, Reboiras López MD, Gándara Rey JM (2009) molecular alterations associated with oral squamous cell cancer (Review). Oncol Rep 22(6): 1277-82.
- Chen HY, Yu S, Chen CH, Chang GC, Chen CY (2007) A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med 356(1): 11-20.
- Rosell R, Wannesson (2012) A genetic snapshot of small cell lung cancer. Cancer Discov 2(9): 769-771.
- E Telbany A, Ma PC (2012) Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer 3(7-8): 467-480.
- Brennan P, Hainaut P, Boffetta P (2011) Genetics of lung cancer susceptibility. Lancet Oncol 12(4): 399-408.
- Letson GD, Muro Cacho CA (2001) Genetic molecular abnormalities in tumours of the bone and soft tissues. Cancer Control 8(3): 239-251.
- Jain S, Xu R, Prieto VG, Lee P (2010) Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 3(4): 416-428.
- Cooper C S, Cornes P (1997) Molecular genetics of soft tissue sarcomas. In: Verweij J., Pinedo H.M., Suit H.D. (eds) Soft Tissue Sarcomas: Present Achievements and Future Prospects. Cancer Treatment and Research, vol 91. Springer.
- Sever R, Brugge JS (2015) Signal Transduction in Cancer. Cold Spring Harb Perspect Med 5(4): a006098.
- Martin GS (2003) Cell signalling and cancer. Cancer Cell 4: 167-174.
- Mendoza MC, Er EE, Blenis J (2011) The Ras ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6): 320-328.
- Asati V, Mahapatra DK, Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signalling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem 109: 314-341.
- Urruticoechea A, Alemany R, Balart J, Villanueva A, Viñals F, et al. (2010) Recent advances in cancer therapy: an overview. Curr Pharm Des 16(1): 3-10.
- Huang M, Shen A, Ding J, Geng M (2014) Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci 35(1): 41-50.
- Prafull K Singh, Juwar Doley, G Ravi Kumar, A P Sahoo, Ashok K Tiwari (2012) Oncolytic viruses & their specific targeting to tumour cells. Indian J Med Res 136(4): 571-584.
- Cross D, Burmester JK (2006) Gene Therapy for Cancer Treatment: Past, Present and Future. Clin Med Res 4(3): 218-227.
- Hanna E, Rémuzat C, Auquier P, Toumi M (2017) Gene therapies development: slow progress and promising prospect. Journal of market access & health policy 5(1): 1265293.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.