Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
In Vitro Pathogenicity of Pseudomonas Savastanoi Isolated from Olive Trees in Iraq on Fruits of Various Plant Species and Their Molecular Characterisation
*Corresponding author: Ghaith AA Al-obaidy, Department of Agricultural Science and Technologies, Erciyes University, Turkey.
Received: June 07, 2023; Published: June 09, 2023
DOI: 10.34297/AJBSR.2023.19.002557
Abstract
The present study was carried out to determine the pathogenicity of Pseudomonas savastanoi on carrots and some other fruits, and to characterize the isolates using molecular methods. Pathogenic Pseudomonas savastanoi bacterial samples were collected from olive trees with olive knot after a field survey of olive trees from around Duhok province in Iraq. The study period was determined to include the beginning of March until the end of May, after collecting the samples and transferring the samples to the laboratory and growing them on culture media. The samples were cultured on Blood agar, MacConkey agar and Cetrimide agar mediums for the diagnosis of Pseudomonas savastanoi and further studies. After activation, bacteria were inoculated on the carrots and other fruits of plants such as lemons, beans, local apples, and commercial apples. These fruits were subjected to the same environmental conditions of humidity, temperature, and incubation period. Results revealed that infection symptoms did not appear on the fruits after bacterial exposure. However, only some of the carrots were found to be infected after 13 days of incubation. On the other hand, when the incubation period was pro-longed to 20 days, all the carrots samples were infected. Bacteria were re-isolated from infected carrots and some basic microscopic, phenotypic and biochemical tests including oxidase, catalase and urease were performed on them. In addition, VITEK2 system were used for determining the isolates in species level. Also, PCR and RT-PCR and sequencing of 16S rRNA were performed to detect possible mutations.
Keywords: Pseudomonas savastanoi, 16S r RNA, RT-PCR, VITEK2, Sequencing
Introduction
A diversity of plants is possible to get infected by the gram-negative plant pathogenic bacteria such as Pseudomonas savastanoi [1]. Prior to DNA-relatedness studies, it was thought to be a set of strains of Pseudomonas syringae, but it has since been recognized as a distinct species. It is named after Savastano, a worker who demonstrated between 1887 and 1898 that bacteria were the reason for the occurrence of olive knots [2]. Pseudomonas savastanoi pv savastanoi, the pathogen that roots the illness caused by bacterial in olive, is of the most significant economic value [3]. On infected trees, galls can grow as a symptom; this is analogous to how the well-known crown gall pathogen, Agrobacterium tumefaciens, induces tumor formation by metabolizing indoleacetic acid. Both the soil and the air contain these microorganisms. Additionally, it also contaminates the roots, the germs are spread by wind to trees [4]. Every region that grows olives has olive knot disease. P. savastanoi v. savastanoi grows and spreads more readily in olive orchards in climates with warm temperatures and rainy autumn and spring [5]. Oleander knot is a common condition brought on by P. savastanoi pv. nerii. Manifestation of olive tuberculosis would happen when olive trees become infected with the olive tuberculosis bacteria [6,5]. The infection first manifests as numerous clusters and clumps on the terminal branches [4]. It then worsens when the major skeletal branches of the tree become affected [7]. Lumps are visible to the unaided eye; they resemble stones or pebbles hanging from a tree [7,6]. If the tree is not cared for, the infestation will have a really horrifying aspect [7]. This disease has a significant impact on olive tree productivity and can occasionally cause the tree to die completely [1]. P. savastanoi pv. Savastnoi Savastnoi is the reason to cause majority of plant infections. It is produced by wounds, particularly cracks from a late-frost event, hailstones, solifluction dust, actions of agriculture (including harvest a crop and trimming), and flower, leaf and blooming [2]. When the knot disease is growing (a few millimeters in diameter), with a pale larger excrescence that grows gradually, occasionally outreach the diameter with multiple centimeters, and eventually turn into brown or the colour of greenish brown [8-9].
Materials and Methods
On stems and branches of pomegranate trees in the primary growing zones, soft juvenile knots with smoother surfaces were randomly selected for the samples between 2021 and 2022. Fresh olive knots collected from orchards contained three isolates of olive around the city of Duhok in the Northern Iraq region. Every knot was handled separately. Young knots were cleaned with rushing water to get soil off of them and dust particles are cleaned by dipping them for two to three minutes in 25%(v/v) sodium hypochlorite. It continuously disinfected after being dried on sterile filter paper and rinsing with Sterile Distilled Water (SDW) with ethanol-soaked cotton. Low portions which were 5-10mm) of every knot were sterilized slicely using a sterilized scalpel. For 10 minutes, they were placed in a sterile Eppendorf tube with 1 ml of sterile saline (085% NaCl). On plates containing PVF-1 agar and King’s medium B (KB), tenfold serial dilutions were applied, and the plates were cultured at 26℃ for three days. Re-streaked onto fresh KB surfaces were single fluorescent or nonfluorescent representative colonies of the major morphological types of bacterial isolates and PVF-1 plates were grown for three days at 26℃ Pure single colonies were grown on KB slants and kept at 4℃ in 20% glycerol at -80℃ pending further identification. Two dangerous P. savastanoi isolates were produced from olive trees in the same area and a reference isolate (Hav-ran2b) used in an earlier study (Mirik and Aysan 2011) also offered for a basis of comparison.
Morphological Examination
Cetrimide agar swabs were cultured. The isolate was identified based on culture features, biochemical tests, and microscopic appearance following gram staining Baron, et al., (2007).
Microscope Examination
After staining with Gram stain, the bacterial isolates were studied microscopically using a bacterial smear to determine cell morphology and aggregation, as well as their interaction with Gram stain.
Biochemical Test
P. savastanoi was diagnosed using the following biochemical tests.
Catalase Test
Some part of the bacterial plant was moved to a clean slide of glass and placed on the MacConkey Agar medium. Using sterile wooden sticks, place a 3% drop of hydrogen peroxide. When gas bubbles were formed, it was confirmed that the results were positive [10].
Oxidase Test
A portion of the bacterial plant was transferred to a clean glass slide and placed on Mac-Conkey Agar media. Using sterile wooden sticks and two drops were added of oxidase detector over the filter paper, the appearance of dark violet signals a positive examination [10].
Molecular Study of the Genotype Content of Bacterial Isolates
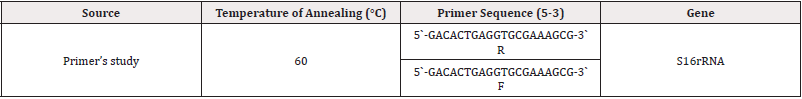
The solutions were prepared as instructed by the company (Korea) Bioneer for the Primers. To obtain a concentration of 100/ microliter, each primer solution was separately applied at a con centration of 10/microliter by taking 10/microliters of each stock buffer solution and adding 90/microliters. Deionized sterile distilled water Mix with Vortex and store the solutions at a temperature of-20℃, considering mixing the primer solution after exhaling it from the ice using Vortex for homogenization (Table 1).
Results
The samples were acquired at random by collecting young soft knots with smoother surfaces on stems and branches of pomegranate trees in main growing zones between 2021 and 2022. Three olive isolates were identified from fresh olive knots gathered from orchards in the Dohuk Governorate of Iraqi Kurdistan regions. Separate approaches were taken to each knot. According to the following diagram (Figure 1).
Young knots were first cleaned in running water to remove any soil or dust that had adhered to it. The surface was then disinfected by dipping it in 25% (v/v) sodium hypochlorite for a few minutes. They were also sterilized by being washed in Sterile Distilled Water (SDW), dried on sterile filter paper, and finally disinfected using cotton that had been soaked in ethanol. A sterile scalpel was used to aseptically cut small fragments (5-10mm) from each knot, which was then put in a sterile Eppendorf tube with 1ml of sterile saline (085% NaCl) and left to sit for 10 minutes. On plates containing King’s Medium B(KB) King, et al., (1954) and PVF-1 agar (Surico and La-vermicocca 1989), tenfold serial dilutions were applied, and the plates were cultured at 26℃ for three days. On fresh KB and PVF-1 plates, single fluorescent or nonfluorescent representative colonies of the most common morphological kinds of bacterial isolates were streaked.
Bacterial Infection of Fruits
The bacteria are inoculated by the cells to a group of fruits to determine whether the bacteria infect the fruits. All the following outcomes were obtained.
The use of Bacteria on Lemon Fruits
Inoculated lemon fruits were stored for 18 days at a humidity and temperature of 20℃. Where the outcome was negative, the lemon fruits did not become infected by the bacteria cells.
Infection of Bacteria on Carrot Fruits
Based to the results up to the ninth day, no illness symptoms had shown when the carrot pieces had been injected with bacteria under the same environmental conditions, including temperature and humidity. This is depicted in the figure below (Figure 2).

Figure 2: (A) Using the bacteria Pseudomonas savastanoi pv. savastanoi, carrots were sliced after a 13-day inoculation. Following a 20-day Pseudomonas savastanoi pv. savastanoi inoculation, carrots are shown chopped in (B).
As illustrated in the next image, a carrot-sized piece of mucous material was present where the infection first occurred after the thirteenth day but on the twentieth day.
Infection of Bacteria on Apples Local and Imported Apples Fruits
Despite exposing two different types of apple fruit to the bacteria Pseudomonas savastanoi pv. savastanoi and subjecting them to the same humidity and temperature conditions, there was no bacterial infection of the apple fruits even after twenty days. The following figure illustrates this.
Bacterial Contamination of Beans
As can be seen in the following image, after 20 days of infection with the bacterium used to infect the bean fruits under the same settings as the other fruits, no signs were visible on the fruits.
Molecular Study
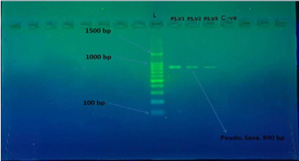
One of the most significant sources of injury in the orchards and fields around the world is Pseudomonas savastanoi pv. savastanoi strains, many of which are pathogenic FOR plant such as olive and carrot. When using the PCR& RT PCR gene test, the results revealed that the bacteria to which the carrot fruit was exposed is a bacterium Pseudomonas savastanoi pv. savastanoi, as illustrated in Figure 3.
Three Pseudomonas savastanoi pv. savastanoi isolates with the 16S ribosomal RNA gene were chosen for this study from the cut carrots that were infected. These isolates demonstrated that they could infect the under researched carrot fruit (Figure 3).
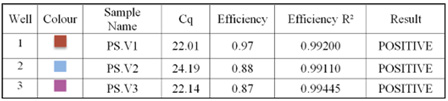
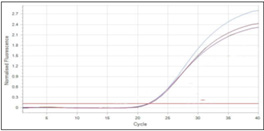
Using RT-qPCR, the ratios of the 16S ribosomal RNA gene to the housekeeping gene were determined. After the real time PCR runs were finished, the findings showed amplification of the examined genes as curves that represented the actual amplification status. The Real time PCR findings are displayed in Figures 4,5.
Alignment and Identification for 16S Ribosomal RNA Genes of Pseudomonas Savastanoi pv. Savastanoi Local Isolates Using the NCBI Database
The 16S ribosomal RNA genes were amplified using the PCR technique and delivered to Macrogen Company Korea for sequencing. And as the ensuing figure illustrates. use of the world’s website https://blast.ncbi.nlm.nih.gov/. Likewise, identify the variations between the nitrogenous base locations in the gene as illustrated in the following figure. It was discovered that mutations occur in some sections of the gene by the use of the BLAST® site Search and the use of gene sequencing. genes alignment with the NCBI Gene Bank. Sample is represented by Query, while a National Center for Biotechnology Information database is represented by Subject (NCBI).
Pseudomonas savastanoi pv. savastanoi has the two transition G>C coding -GGCCT, according to the 16S ribosomal RNA gene sequencing results. Additionally, the nitrogenous base sequences of the genes T>G, GA>AG, and A>C have changed. According to the Gene Bank, the subject of the 16S ribosomal RNA gene in NCBI under sequencing has a 98.54% compatibility with a portion of the 16S ribosomal RNA gene.
Discussion
It has been proven through the current work that it is possible to infect carrot plants with Pseudomonas savastanoi bacteria and the inability of this bacteria to infect other types of plants that were selected in this study, such as lemons, peas and apples, as shown by the results. P.s isolates and kept in the same environmental conditions of heat and humidity did not show any symptoms until the ninth day of inoculation, The first infection of carrot slices appeared on the thirteenth day in a small way, but on the twentieth day of inoculation, the infection appeared in a clear and obvious way and was in the form of a mucous patch the size of a carrot slice5. Various host plants, and among these plants was the carrot plant. The bacteria under study were isolated from the nodes formed in the inoculated sites on the carrot slices. The isolated bacteria were diagnosed by bio-chemical and physiological tests, and the results confirmed that the infection was caused by Pseudomonas savastanoi bacteria. In the province of Hatay and Turkey, P.S bacteria is one of the important problems that affect olive, pomegranate, myrtle and jasmine plants [10]. In severe infection cases, the bacterial infection causes the death of young seedlings. To confirm the infection with P.S bacteria (for the mentioned plants) [11], the diagnostic tests take a long time, which has benefited from the carrot plant. In developing a rapid method for bacterial growth (and this is indeed what was shown by the current study, as the carrot plant was an effective medium for the growth of P.s bacteria), swabs were taken from the mentioned and infected plants and carrot slices were inoculated from the isolated swabs, and the growth of the first typical node was observed within a week After 14 days of luck, the injury became clear and obvious [7], To confirm the pathogenicity of the isolated and developing bacteria on the carrot slices, isolates were taken from the carrot nodule and the olive plant (Gemlik variety) was inoculated, where a clear nodule growth was observed in the stabbing site two months after the operation [12]. P. S savastano using PCR& RT PCR gene tests and molecular methods, the results showed that the carrot slice method is a very simple and rapid technique to test the pathogenicity of P. S. pv.savastano.
Conclusions
In this study, during a field survey in olive orchards in northern Iraq, specifically in Dohuk Governorate. After collecting the samples and using them on a group of fruits designated for this experiment in this experiment, there were no negative results, all results were positive, as the results showed injury to the traction pieces only, and there was no injury to the rest of the fruits, and this is what was found to be the reason for discussing the results. The stages of the experiment began with the process of collecting samples from northern Iraq and identifying them from Dohuk Governorate, then exposing some fruits to bacteria and exposing these fruits inoculated with bacteria to the same environmental conditions. After infecting the fruits, bacteria were isolated from the infected fruits and biochemical tests were applied to them, then molecular tests were performed on these isolates such as PCR &RT-PCR and we also conducted a test on the DNA of the bacteria on the gene 16SrRNA.
Funding
None.
Conflict of Interest
None.
Acknowledgments
This article was derived from Master Thesis of Mr. Ghaith Adil ABDULHAMEED approved with 31 January 2023 date and 2023/02.06 numbered decision of Department of Agricultural Science and Technologies, Graduate School of Natural and Applied Sciences, Erciyes University.
References
- Schmitges FW, Radovani E, Najafabadi HS (2016) Attachment-1 copy 2.jpeg.pdf. Skelet Muscle 6(1).
- Disler RT, Gallagher RD, Davidson PM (2019) Factors impairing the postural balance in COPD patients and its influence upon activities of daily living. Eur Respir J 15(1).
- Brooks CF, Carrol KC, Butel JS, Mores SA (2007) Jawetz Melnick and Adelerg Medical Microbiology 24th
- Ramos C, Matas IM, Bardaji L, Aragón IM, Murillo J (2012) Pseudomonas savastanoi pv. savastanoi: Some like it knot. Mol Plant Pathol 13(9): 998-1009.
- Moreno Pérez A, Pintado A, Murillo J, Eloy Caballo Ponce, Stefania Tegli, et al. (2020) Host Range Determinants of Pseudomonas savastanoi Pathovars of Woody Hosts Revealed by Comparative Genomics and Cross-Pathogenicity Tests. Front Plant Sci 11: 973.
- Rodríguez Palenzuela P, Matas IM, Murillo J, Emilia López Solanilla, Leire Bardaji, et al. (2010) Annotation and overview of the Pseudomonas savastanoi pv. savastanoi NCPPB 3335 draft genome reveals the virulence gene complement of a tumour-inducing pathogen of woody hosts. Environ Microbiol 12(6): 1604-1620.
- Caballo Ponce E, Meng X, Uzelac G, Nigel Halliday, Miguel Cámara, et al. (2018) Quorum sensing in Pseudomonas savastanoi pv. savastanoi and Erwinia toletana: Role in virulence and interspecies interactions in the olive knot. Appl Environ Microbiol 84(18): e00950-18.
- Tegli S, Cerboneschi M, Libelli IM, Santilli E (2010) Development of a versatile tool for the simultaneous differential detection of Pseudomonas savastanoi pathovars by End Point and Real-Time PCR. BMC Microbiol 10: 156.
- Zouridis H, Hatzimanikatis V, Zhu K (2015) Regulation of G (1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. J Biol Chem 5(4).
- Dickinson M (2022) Phytopathogenic bacteria and plant diseases. B S Thind pp. 372.
- Mashiguchi K, Hisano H, Takeda Kamiya N, Yumiko Takebayashi, Tohru Ariizumi, et al. (2019) Agrobacterium tumefaciens Enhances Biosynthesis of Two Distinct Auxins in the Formation of Crown Galls. Plant Cell Physiol 60(1): 29-37.
- Morris CE, Lamichhane JR, Nikolić I, Stanković S, Moury B (2019) The overlapping continuum of host range among strains in the Pseudomonas syringae complex. Phytopathol Res 1(1).




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.