Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Method Development and Validation of Tamsulosin Hydrochloride by using UV-Spectrophotometric Method
*Corresponding author: Swathi Mukurala, Department of pharmaceutical Analysis, Vishnu Institute of pharmaceutical Education and Research, Narsapur, Medak, 502313, Telangana.
Received: August 08, 2023; Published: August 15, 2023
DOI: 10.34297/AJBSR.2023.19.002638
Abstract
For the quantitative determination of tamsulosin HCl by using UV-visible spectrophotometric method, which is a Simple, Accurate, Precise, and Sensitive Spectrophotometric Assay method has been developed and validated. The absorption peak of tamsulosin HCl in 0.1N NaOH was at 279nm. The Beer’s law was followed over the concentration range of 100-500g/ml. In this concentration range, the correlation coefficient, which was found to be 0.999, demonstrated good linearity, accuracy, and precision. The percentage recovery figures were discovered to be within the limitations, demonstrating the accuracy of the approach. Statistical techniques were used to determine the LOD and LOQ. The percentage RSD values were under 2. The efficacy of this method is demonstrated by the Validation parameters, which were tested in accordance with ICH criteria. The proposed approach for estimating the dosage of tamsulosin HCl in pharmaceutical dosage form was found to be straightforward, exact, accurate, swift, economical, and repeatable.
Keywords: Tamsulosin HCl, 0.1N NaOH, Spectrophotometry
Introduction
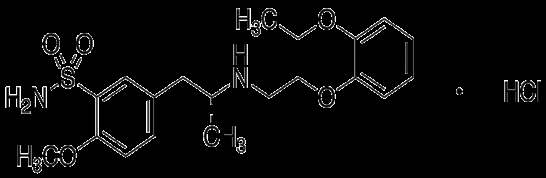
Tamsulosin Hydrochloride is an alpha-1, blocker in blood vessels that is usually administered in conjunction with diuretics to treat hypertension when other therapies are ineffective. It is used in the treatment of prostatic hyperplasia, chronic prostatitis, urinary retention, and help with the passage of kidney stones IUPAC name 5-[(2R)-2-[[2-(2-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenenesuphonamide hydrochloride[1-2].
Tamsulosin Hydrochloride having molecular formula C20H29ClN2O5S and molecular weight 444.97g/mol. It is freely soluble in DMSO (Dimethyl Sulfoxide), Methonal & NaOH but not soluble in water [1,2]. The Tamusolin HCL medication is available in combination with dutasteride or finasteride [3]. Moreover it is official in European Pharmacopeia [4].
Few analytical techniques, such as potentiometric titration HPTLC [5], RP-HPLC [6], HPLC [7], LC [8] and UV Spectrophotometry [9], are available, according to literature survey.
The developed method was evaluated by UV Spectrophotometric method for the determination of tamsulosin hydrochloride in formulation drug and found to be simple, specific, stable, quick, accurate, exact, trustworthy, less expensive, and time-saving (Figure 1).
Materials and Methods
Instrumentation and Materials
U.V. visible double beam spectrophotometers SL 210 Elico with Spectra treat software having path length 1cm U.V. matched quartz cells were used. Tamsulosin Hydrochloride sample and Standard was provided by our College management.
Method Development
Solvent selection: Different solvents were studied at the beginning of the technique development for this drug, including water, methanol, 0.1N NaOH, 0.1N HCl, and acetonitrile. Tamsulosin HCl was found to be easily soluble in 0.1N NaOH after solubility studies were conducted to determine the best solvent for the determination of the drug. Due to its better solubility and repeatable readings of maximum absorbance, 0.1N NaOH was utilized for all of the dilutions [12].
Preparation of Stock Solution (1000μg/ml): Accurately weighed about 100mg of Tamsulosin Hydrochloride and transferred to 100ml volumetric flask. Dissolved in Diluent and make up the volume to 100 ml, with 0.1 NaOH.
Standard Solution Preparation: (10μg/ml): Pipette 10ml of the above stock solution into a 100ml volumetric flask, adding 0.1 N NaOH to the desired concentration. Mix thoroughly. Pipette 1ml of the aforementioned standard solution into a 10 ml volumetric flask, and then 0.1 N NaOH to the desired strength. Mix thoroughly (10μg/ml).
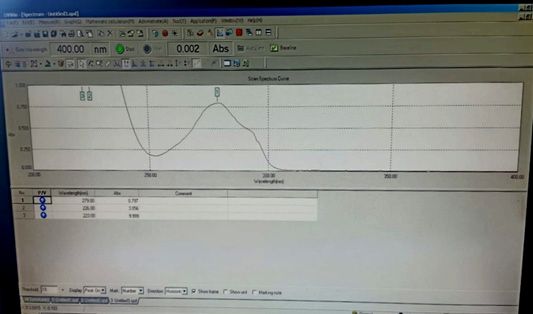
Selection of Wavelength: Using a suitable blank, the absorbance of the solutions containing Tamsulosin HCl was calculated in the visible range 200-400nm. Tamsulosin HCl solution in 0.1N NaOH was found to have a maximum at 279nm (Figure 2).
From the (Figure 2) Tamsulosin Hydrochloride spectra of wavelength maxima was found for quantification were 279nm (λ max).
Sample Solution Preparation: Tamsulosin HCl of the commercial formulation (veltam tab) of 20 tablets was accurately weighed, and an average weight was determined. The tablets were ground into a fine powder, and then 100mg of Tamsulosin HCl was weighed, transferred, and sonicated for 30minutes with frequent shaking and transferred into a 100mL volumetric flask together with 70ml of 0.1N NaOH. NaOH was used to create volume. The aforementioned solution was sonicated for ten minutes. A 100mL volumetric flask was filled with water after pipetting 10mL of the aforementioned solution into it. As stated above, make a 10g/ml concentration solution.
Procedure [13,14]: Applying the formulas, place the standard solution and sample solution in the UV Visible Spectrophotometric equipment, measure the absorbance of Tamsulosin HCl, and determine the % Assay.
Method Validation of the proposed Analytical Method
Linearity of Test Method: The ability of an assay method to yield test results that are directly proportional to the drug concentrations in samples within a certain range is known as linearity. The use of single-point calibrations is justified by linearity. Regression line’s correlation coefficient was found to be 0.999 (Table 1).
Precision
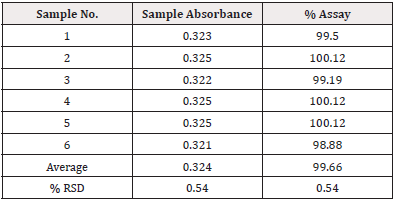
Repeatability (Intra-day precision): Six samples that were made by spiking raw Tamsulosin HCl over a less span of time that were used to calculate the test method’s intra-day precision. By carrying out the assay in accordance with the test method, the precision of the test procedure for 10mg was assessed (Table 2).
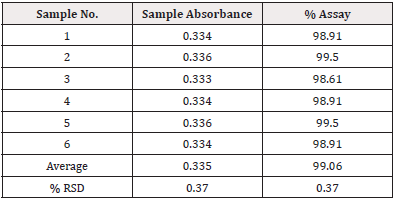
Intermediate Precision (Inter-day precision): Six samples that were made by spiking raw Tamsulosin HCl between days (in next three days). that were used to calculate the test method’s intra- day precision. By carrying out the assay in accordance with the test method, the precision of the test procedure for 10mg was assessed (Table 3).
Accuracy
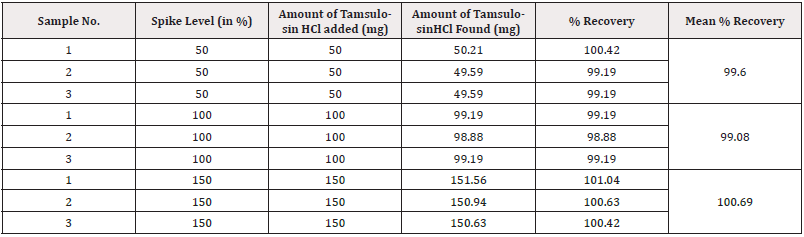
To determine the accuracy of the test method samples were prepared by spiking Tamsulosin HCl raw material with the equivalent amount of placebo at 50%, 100% and 150% of the target concentration. Three samples were prepared at each concentration level. The average % recovery of Tamsulosin HCl was found to be within the limits. The results were summarized in (Table 4).
Specificity and Selectivity
The analyte should have no interference from other extraneous components and be well resolved from them. The specificity of the method was evaluated regarding interference due to presence of any other excipients. The result is mentioned in Figure 3.
Limit of Detection [LOD] & Limit of Quantification [LOQ]
The limits of detection and quantitation, LOD and LOQ, were calculated by use of the equations LOD = 3.3σ/S and LOQ = 10σ/S, where σ is the standard deviation of the blank and S is the slope of the calibration plot.
Results and Discussion
Linearity of Test Method
(Table 1, Figure 3)
summary of the findings.
Precision
Repeatability (Intra-day precision): (Table 2)
Intermediate Precision (Inter-day precision): (Table 3)
Acceptance Criteria: The % RSD from the six sample preparations should be no more than 2.0%. The individual % Assay should not less than 98.0% and not more than 102.0%.
Accuracy
(Table 4)
Acceptance criteria: The mean recovery at each level should not be less than 98.0% and not more than 102.0%.
Specificity and Selectivity
(Figure 4)
Limit of Detection [LOD] & Limit of Quantification [LOQ]
These two parameters are required for assay validation as per ICH Q2A guidelines. Limit of detection and limit of quantitation of calibration curve were calculated which was based on the standard deviation of y-intercept of regression line (SD) and the slope (S) of the calibration curve at levels approximating the LOD and LOQ, LOD = 3.3 (SD/S) and LOQ =10 (SD/S). LOD and LOQ of calibration curve of drug prepared in water were found to be within the limits.
Thus, the developed Spectrophotometric method was found to be rapid, simple, precise, accurate, and economic and can be adopted for routine estimation of Tamsulosin HCl in pharmaceutical formulations in quality control laboratories.
Conclusion
Tamsulosin HCl dosage estimation in pharmaceutical dosage forms was successfully developed and verified using an effective UV Visible Spectrophotometric technique. The method’s max wavelength was found to be 279 nm and was developed using 0.1N NaOH as the solvent. Regarding linearity, precision, accuracy, sensitivity, and specificity, the approach was validated. The procedure was developed in accordance with the ICH definition and guidelines. By examining commercial formulations, accuracy was examined, and it was discovered that the percentage of recovery was within acceptable bounds. It can be concluded that the procedure was very accurate as a result. Low percentage Relative Standard Deviation (RSD) values for the intraday and inter-day ranges were obtained. This demonstrated that the method’s precision was deemed to be of high quality and accurate.
Hence the method can easily and conveniently adopt for the estimation of Tamsulosin HCl for bulk and pharmaceutical dosage form.
References
- (1996) The Merck Index, Maryadele JO Neil, Edns, 12th, Published by Merck Research Lab, Division of Merck & Co White House Station, NJ USA. pp. 9217, 1549.
- (1996) Martindale, Reynolds James, 31 Edition, Published by Royal society of Pharmaceutical Society London. pp. 784,932,952.
- Shrivastava A, Gupta VB (2011) A Review on Various Analytical Methods on Some Alpha Adrenergic Antagonists. Curr Pharma Anal 7: 27-41.
- (2006) European Directorate for the Quality of Medicines. 5th, 5(7). European Pharmacopoeia pp. 5121
- Bari S B, Bakhshi A R, Jain P S, Surana S J (2011) Development and Validation of Stability-Indicating HPTLC Determination of Tamsulosin in Bulk and Pharmaceutical Dosage Form, Chromatography Research International Article ID 893260 pp. 1-6.
- Richa Kumari, P P Dash, V K Lal, A Mishra (2010) RP–HPLC method for the estimation of Tamsulosin Hydrochloride in Tablet Dosage Form. Indian J Pharm Sci 72(6): 785-787.
- Zhang Z, Yang G, Liang G, Liu H, Chen Y (2004) Chiral separation of tamsulosin isomers using HPLC using cellulose tris (3,5-dimethylphenylcarbamate) as a chiral stationary phase. J Pharm Biomed Anal 34: 689-693.
- Srinivasu MK, Rao BM, Vittal TV, Rajendra Kumar P, I Gopi Chand, et al. (2005) A validated chiral LC method for the enantioselective analysis of tamsulosin hydrochloride and its enantiomer (S)-5-[2-[[2-(0-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxy benzenesulfonamide, monohydrochloride on amylose based stationary phase. Indian Drugs 42: 230-237.
- Rajesh S Jadhav, Jagdish V Bharad (2017) Analytical Method Development and Validation for Estimation of Tamsulosin Hydrochloride by UV-Spectroscopic method. International Journal of Chem Tech Research 10(5): 740-747.
- Michel, Martin C, Walter Krauwinkel, Mirjam Kuipers (2005) The pharmacokinetic profile of tamsulosin oral controlled absorption system (OCAS®). European Urology Supplements 4(2): 15-24.
- Michael E, Schartz IS, Krull (2004) Analytical method development and Validation. 3rd, London: John Wiley & sons pp. 25-46
- Sirisha P, G Ashwini, V Mohan Goudet, JVC Sharma, Ch B Praveena Devi, et al. (2019) Method development and validation for estimation of tamsulosin in bulk and pharmaceutical dosage form by UPLC. IOSR J Pharm 9 (2): 1-7.
- (2005) ICH: Q2 (R1), Validation of analytical procedures: text and methodology.
- (1996) ICH: Q2B, Analytical validation-methodology.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.