Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Non-bilayer Lipid Phases Modulate the Structure and Functions of Mitochondrial Membranes: Pharmacological Relevance
*Corresponding author: Edward S Gasanoff, Science Department, STEM Research Center, Chaoyang Kaiwen Academy, No.46, 3rd Baoquan street, Beijing 100018, China.
Received: July 27, 2023; Published: August 10, 2023
DOI: 10.34297/AJBSR.2023.19.002632
Abstract
This review is an attempt to elucidate the contemporary understanding of the role of non-bilayer lipid phases in mitochondrial bioenergetics. It is based on the critical review of biochemical and biophysical concepts on the structure and functions of energy transducing membranes reported over sixty years of experimental and computer simulation studies of model and native mitochondrial membranes. In this review, the non-bilayer lipid phases are presented as indispensable elements of structural dynamics and remodelling of mitochondrial membranes. The wealth of published data on the kinetic coupling of the electron transport chain with the ATP synthase is thoroughly examined to reach the conclusion that the kinetic coupling resolves the issues of chemiosmotic theory driven by the H+ gradient in bulk solutions across the cristae membrane. It is emphasised that the H+ movement in kinetic coupling does not induce fluctuations in pH in bulk solutions in the matrix and intermembrane space, thus averting unphysiological conditions on both sides of the cristae membrane. New tentative details in the mechanism of mitochondrial ATP synthesis are proposed to suggest that the increase in proton density on the crista inner membrane surface next to Fo subunit of ATP synthase breaks the ionic bond between the phosphate groups of cardiolipin and conserved lysine residues in the rotor of ATP synthase. This event triggers the formation of cardiolipin inverted micelles, which not only transport protons into the matrix, but also induce the rotation of ATP synthase rotor. It should be noted that the transport of protons to the matrix across the crista membrane in cardiolipin inverted micelles that induce rotation of ATP synthase rotor is a new hypothesis that shall be tested in future studies. This review may promote development of novel pharmaceuticals to enable restoration of healthy cristae morphology and rejuvenation of mitochondrial functions in aging and disease.
Keywords: Non-bilayer lipids; Kinetic coupling of the electron transport chain; Inverted micelle protons carrier; Mitochondrial disfunctions in aging and disease
List of Abbreviations: ATP: Adenosine Triphosphate; ADP: Adenosine Diphosphate; LPM: Lateral Pressure Model; FSM: Flexible Surface Model; TM: Thylakoid Membranes; IMM: Inner Mitochondrial Membrane; OMM: Outer Mitochondrial Membrane; NMR: Nuclear Magnetic Resonance; CL: Cardiolipin; PE: Phosphatidylethanolamine; PC: Phosphatidylcholine; MGDG: Monogalactosyldiacylglycerol; Cyt c: Cytochrome c; SS: Szeto-Schiller Tetrapeptides; ETC: Electron Transport Chain; CTI and CTII: Cytotoxins CTI and CTII; DANTE: Delay Alternating with Nutation for Tailored Excitation; DCCD BPF: Dicyclohexylcarbodiimide-Binding Protein; mPTP: Mitochondrial Permeability Transition Pore; OXPHOS: Oxidative Phosphorylation; FCCP: Carbonyl Cyanide p-Trifluoro Methoxy Phenyl Hydrazone; ROS: Reactive Oxygen Species; OPA1: Dynamin Protein Mediating Membrane Fusion at the IMM; MICOS: Mitochondrial Contact Site and Cristae Organizing System
Introduction
The dynamic structure and function of the lipid phase of biological membranes has been an important area of research in the field of membrane biochemistry and biophysics since the beginning of the previous century. A very successful Singer-Nicolson fluid mosaic model of the cell membrane structure published in 1972 describes the lipid phase of a biological membrane as a bilayer of lipids with the characteristically low permeability to water and water soluble substances [1,2]. A bilayer of lipids with membrane proteins and glycoproteins embedded in a lipid phase serves as a barrier that selectively regulates the movement of substances across the membrane of cells and organelles, supports transmembrane signal transduction and facilitates intercellular communication. These processes uphold regulatory effects upon the direction and rate of cellular metabolism. However, a bilayer model of biological membrane does not take into account the highly dynamic and intricate network of inner mitochondrial membranes with the continuously changing shape of the membrane structure that triggers membrane fusion and fission. In addition, a lipid bilayer model does not consider the most abundant lipids of the inner mitochondrial membrane, cardiolipin and phosphatidylethanolamine, which due to their conical shape tend to form non-bilayer lipid structures.
To harmonize the existence of conically shaped non-bilayer lipids within the framework of the fluid bilayer mosaic membrane model, three successive membrane models, lateral pressure model (LPM), flexible surface model (FSM) and dynamic exchange model (DEM), were developed to complement the fluid bilayer mosaic model by taking into account the existence of non-bilayer lipids in biological membranes. According to LPM, the functional state of membrane proteins is supported through the forces of increased lateral pressure in the hydrophobic region of lipid alkyl chains and decreased lateral pressure in the region of lipid headgroups which are caused by the presence of conical non-bilayer lipids [3,4]. It has been proposed that in the absence of lateral pressure, caused by non-bilayer lipids, the membrane-embedded proteins become less functional [3,4]. According to FSM, non-bilayer lipids, due to their conical shape, modulate the protein energetics via variations in the curvature and elastic energy [5,6]. Overall, both LPM and FSM emphasize that non-bilayer lipids increase the dynamics of proteins embedded in membranes via the enhancement of membrane structural plasticity [4,6,7]. It has been recently proposed that changes in membrane dynamics and shape caused by the presence of non-bilayer lipids determine the changes in the functional properties of membrane proteins [8]. It should be noted that according to LPM and FSM non-bilayer lipid phases exist in the bilayer membrane only locally and transiently [4,9]. Both LPM and FSM propose that non-bilayer lipid phases cannot exist persistently inside the bilayer membrane and cannot associate with the bilayer membrane.
The latest membrane model, DEM, postulates that in the membranes with lipids of high non-bilayer inclination, the bilayer and non-bilayer lipid phases may coexist in dynamic equilibrium be tween the two phases [10,11]. According to DEM, membrane-embedded proteins push the conically shaped lipids into the bilayer packing. This has been experimentally shown in vitro using assemblies of cardiolipin with cytochrome c oxidase [12] and monogalactosyldiacylglycerol with light-harvesting complex II from thylakoid membranes [13]. DEM also postulates that transient structures, mostly including non-bilayer lipids, segregate and bud out from the bilayer membrane when a sizeable membrane patch free of proteins is exposed to water [14-17].
DEM suits particularly well in explaining the homeostasis of the energy-converting membranes [10,11]- thylakoid membrane (TM) and inner mitochondrial membrane (IMM). Both TM and IMM contain high percentage of non-bilayer lipids which play key roles in the dynamics and organization of the extended and continuously changing network of membranes of various structures which is built via membrane fusion, fission and intermembrane exchange of lipids accomplished and mediated by the transient formation of non-bilayer lipid phase [9,15,18-21]. The inner aqueous volume of TM and IMM is abundant in proteins [17,22-24]. Some of those proteins like lipocalin bind to membrane lipids [25-28], forming non-bilayer lipid phases, presumably associated with lipids extruded from the membrane. These lipocalin-non-bilayer lipid structures are at least partly surrounded by water while may still be closely associated with the membrane [9]. Removal of ‘excess’ lipids from the energy converting membranes may be needed to tighten the proteins of the photosynthetic and/or electron transport chain to facilitate the migration of the excitation energy [29]. On the other hand, the lipocalin-non-bilayer lipid structures may rejoin the bilayer membrane network of TM or IMM when the remodelling and extension of the membrane network occurs. Isolated granum and stroma TMs placed in a proper aqueous environment spontaneously form extended and interconnected membrane networks made of narrow membrane channels rich in proteins embedded in the bilayer membrane [30]. Thus, DEM proposes the coexistence of the bilayer and non-bilayer phases in the TM and IMM and serves to explain the dynamic homeostasis of the energy-transducing membranes in which the equilibrium may shift towards the bilayer or non-bilayer phase depending on the energy needs of cell [9]. Thus, the percentage of non-bilayer phase in the energy-transducing membrane may serve as an indicator of the metabolic rate, energy demand and the state of health and disease [31,32].
In this article, we critically review the important experimental and theoretical research data that became available over the past sixty years on the dynamic structure of non-bilayer lipid phases in model and fully functional mitochondrial membranes. We also thoroughly review the experimental results which became recently available on the effects of non-bilayer phases on the functional activities of mitochondrial membranes. Finally, we propose the roles that non-bilayer lipid phases may play in the structure-function relationship underlying molecular mechanisms in bioenergetic processes in inner mitochondrial membranes.
Lipid Phase Polymorphism in Model Membranes
It has been determined by small-angle X-ray diffraction by V. Luzzati’s team in 1967 that the formation of the body-centered cubic phase and hexagonal phase is triggered by divalent cations in hydrated soaps with carbonyl groups serving as polar heads [33]. This was the first report on non-bilayer structures formed by lipid- like organic compounds. By the early 1970s, it has been shown that hydrated lipids form not only a lamellar phase but also a variety of non-bilayer phases [15,33-35]. Formation of non-bilayer phases has been explained by the conical shape of non-bilayer lipids [15,35]. It was determined by 31P-NMR that cardiolipin (CL) and phosphatidylethanolamine (PE), non-bilayer lipids in IMM, form the HII phase when hydrated in the presence of divalent cations [35-37]. Hydrated monogalactosyldiacylglycerol (MGDG), a non-bilayer lipid of TM, also adopted the HII phase as determined by X-ray diffraction [38]. By the end of the previous century, there was an abundance of data showing that non-bilayer lipid phases can be easily generated in model membranes made of lipids isolated from TM [14]. Freeze-fraction electron microscopy data have revealed that keeping lipids isolated from TM with co-solutes of sugars or betaine [39] or treating TM lipids with a non-cryogenic low temperature [40] triggers the formation of an HII phase. Coexistence of both bilayer and hexagonal phases was revealed by molecular dynamics simulation of TM lipids [16].
In late 1970s and early 1980s 31P-NMR spectroscopy has been widely used in studies of polymorphic phase behaviour of lipids isolated from mitochondrial membranes. Dispersion of PC isolated from rat liver mitochondrial membrane retained the lamellar phase [41], but the dispersion of PE isolated from the same membranes underwent a lamellar-to-HII transition in the 10-37°C temperature range [41]. The 31P-NMR spectra of a dispersion of total lipid mixtures from the rat liver mitochondrial membrane revealed a coexistence of bilayer and non-bilayer lipid phases [41]. Model PC membranes enriched with CL or PE in solution with divalent cations contained a hexagonal HII phase coexisting with a lamellar phase [36,37]. By the late 1980s, the existence of non-bilayer lipid phases has been widely documented, but the perception that non-bilayer lipids, which are probably present in all biological membranes, are generally arranged in a lamellar phase in lipid membranes persisted until the beginning of the 21st century [42].
From the early 1980s, research groups in biochemical and biophysical membranology have begun employing a range of powerful biophysical methods to elucidate whether membrane-active proteins from natural sources can induce polymorphic transitions in model membranes [43-46]. Several cationic proteins were found to trigger bilayer to non-bilayer transitions in PC membranes enriched with CL [43,46]. One of them is cytochrome c (Cyt c), a small 12 kD water-soluble peripheral protein of IMM that is involved in the respiratory chain as an electron carrier [46]. Cationic Cyt c is rich in lysine residues, and it specifically binds to CL to induce non-bilayer lipid structures [43,47] and trigger pore formation [48] in CL-containing model membranes. It has been recently discovered that in non-polar environment, Cyt c forms nanospheres with CL which possess lipoperoxidase activity [49]. Cyt c peroxidase is activated by reactive oxygen species [50]. In the oxidized form, Cyt c can readily trigger apoptosome assembly [51]. It is possible that the bilayer to non-bilayer transition triggered by the Cyt c binding to CL, which in turn facilitates the peroxidase activity, plays a crucial role in initiating the intrinsic apoptosis pathway [52]. Mitochondrial creatine kinase is another potential cationic protein which may trigger non-bilayer phase formation in CL containing membranes. It has been shown that C-terminal lysine residues of creatine kinase drive its binding to membrane phospholipids [53]. Separate studies have shown the formation of the creatine-kinase induced CL clusters [54] and segregation of CL in phospholipid monolayers [55], which allow one to suggest that creatine kinase may trigger the formation of non-bilayer phase in CL segregated areas of membrane. The mitochondria regenerating Szeto-Schiller (SS) tetrapeptides have been shown to rejuvenate mitochondrial function and promote tissue regeneration in aging [56]. The SS tetrapeptides are made of basic lysine and hydrophobic amino acid residues, and they specifically target CL in model and mitochondrial membranes. The SS tetrapeptides are being studied as potential therapeutic agents to treat mitochondrial disfunction and diseases associated with aging [56]. It has been suggested that the SS tetrapeptides bound to cardiolipin in the IMM increase lipid packing to support tighter cristae curvature [1] that increases efficiency of the electron transport chain (ETC) in energy generation to enable restoration of healthy cristae morphology in aged mice (Figure 1) that empowers robust ATP production. It makes sense to investigate if the SS tetrapeptides can induce the formation of non-bilayer lipid phases in IMM as the regulated lipid polymorphism in IMM could be an important step in the process underlying the rejuvenation of aging mitochondria.
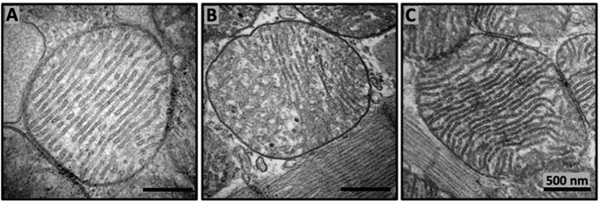
Figure 1: The SS-31 tetrapeptide restores mitochondrial cristae morphology in aged mice. Transmission electron micrography images from 6 months old (A), 26 months old (B), and 26 months old murine cardiac mitochondria treated with the SS-31(C). The subcutaneous daily 1mg/kg dosage administration of SS-31started at the age of 24 months [56]. This figure is modified from [114].
Cytotoxins CTI and CTII, membrane-active cationic proteins of 7 kD from the Central Asian cobra venom, have been widely used in studies to probe the structural organization of model lipid membranes [21,45,57-67]. Both cytotoxins phenocopy membranotropic properties of C8 subunit of the F0 sector in bovine ATP synthase [9,61]. Both cytotoxins avidly bind to membranes enriched with acidic phospholipids, but do not interact with pure PC membranes [63,67]. It has been shown that CTII decreases the angular anisotropy of the spin- label EPR spectral signals in lipid films enriched with acidic phospholipids, while CTI demonstrates the same ability only in lipid films enriched with CL, but not with other acidic phospholipids [59]. Both CTI and CTII trigger aggregation of liposomes and dehydration of the membrane surface [45,57-61]. CTII induces intermembrane exchange of lipids and membrane fusion in membranes enriched with any kind of acidic lipids, while CTI is able to do the same only in membranes enriched with CL [45,57-61,65-68]. 31P- and 1H-NMR spectroscopy studies of the large unilamellar liposomes made of either PC+10mol% CL or PC+10mol% PS and treated with either CTI or CTII have shown that CTII induces the formation of non-bilayer structures and increases membrane permeability of both PC+10mol% CL and PC+10mol% PS liposomes, while CTI induces formation of non-bilayer structures and increases membrane permeability only in PC+10mol% CL liposomes (Figure 2) [59,61].
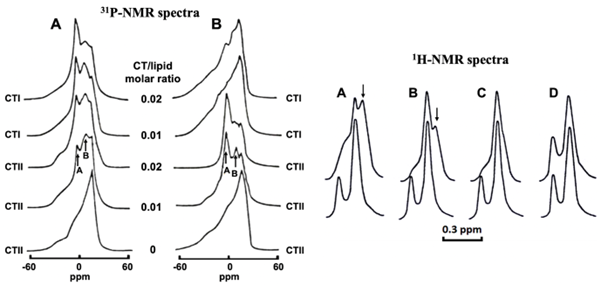
Figure 2: Cytotoxins induce the formation of non-bilayer structures and increase membrane permeability in the membranes of large unilamellar liposomes. 31P-NMR spectra on the left derived from large unilamellar liposomes of PC+10mol% CL (A) and PC+10mol% PS (B) treated with cytotoxins at given CT/lipid molar ratios. The signal A belongs to phospholipids with rapid isotropic mobility that exchange with lamellar phase within 10–2–10–4 s. Signal B belongs to phospholipids with immobilized movement that do not exchange with lamellar phase within 10–2–10–4 s. 1H-NMR spectra derived from the N+(CH3)3 groups of PC in large unilamellar liposomes untreated (lower spectra) and treated (upper spectra) with cytotoxins and composed of PC+ 10 mol% CL (A and B) or PC+ 10 mol% PS (C and D) in the presence of K3[Fe(CN)6]3 and treated with either CTII (A and C) or CTI (B and D) at a cytotoxin/lipid molar ratio of 0.02. Arrows in A and B point at non-bilayer phase signals. Disappearance of the low field signals in the upper spectra A, B and C is the result of increased membrane permeability to K3[Fe(CN)6]3. This figure is modified from [59].
A molecular mechanism of translocation of cobra cytotoxins via the plasma membrane, OMM and IMM has been suggested (Figure 3) based on the results of a recent study on the interaction of CTI and CTII with model outer mitochondrial membrane (OMM) employing 31P- and 1H-NMR spectroscopy along with molecular dynamics and Autodock simulation [60] which were analyzed in conjunction with the results of previous studies on CTI and CTII interaction with model cell membrane and IMM [59,61,69]. According to the suggested mechanism, cytotoxin binds via its hydrophobic and basic residues to the cell membrane surface and at the same time the cytotoxin attracts the plasma membrane of a neighbouring cell to form an intermembrane junction in the form of an inverted micelle with cytotoxin in its center (Figure 3A), which is an intermediate step in the process of membrane fusion. After fusion of cell membranes linked by an intermembrane junction, the cytotoxin settles inside of the inverted micelle in a single cell membrane (Figure 3B). Due to the high surface curvature, the inverted micelle is not stable and it transforms to the bilayer to release cytotoxin into the cytosol, where the cytotoxin targets CL on the surface of OMM and then forms an intermembrane junction between OMM and the lysosomal membrane (Figure 3C). After the fission between OMM and the lysosomal membrane, the cytotoxin settles in the inverted micelle in OMM and then eventually gets into the intermembrane space (Figure 3D), where the cytotoxin forms another intermembrane junction between OMM and IMM (Figure 3E). After the fission between OMM and IMM, the cytotoxin settles in the inverted micelle in IMM (Figure 3F) and from there the cytotoxin may translocate into matrix. At very low concentrations, cytotoxin enhances the plasticity of IMM and facilitates the formation of intra-cristae membrane compartments which increase ATP synthase activity [9,61]. However, at higher concentrations, cytotoxin disrupts mitochondrial membrane integrity and function [46,64].
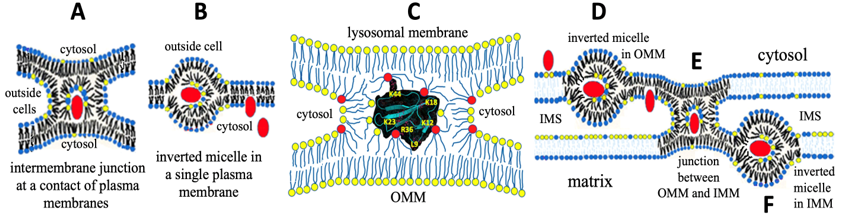
Figure 3: Translocation of cytotoxins via the plasma membrane, OMM and IMM. A - Intermembrane junction with CTII (red) formed at a contact of two plasma membranes has phospholipids immobilized by CTII which are responsible for 31P-NMR signal B in Figure 2. B - After fusion of cell membranes, CTII settles in an inverted micelle in a single membrane and due to the high surface curvature, the micelle transforms to the bilayer to release CTII into the cytosol. C - Intermembrane junction with CTII at a contact of OMM and lysosomal membrane. Amino acid residues (a.a.r.) of CTII (given in cartoon representation), K23, R36 and K12 bind to OMM surface and L9 penetrate to the hydrophobic region of OMM. A.a.r. K44, K18 and a.a.r. K5, K35 on another side of CTII attract acidic lipids of the neighboring lysosome to form an intermembrane junction. D - After fission between OMM and lysosomal membrane, CTII settles in the inverted micelle in OMM, then CTII is released into the intermembrane space (IMS). E - CTII via attraction to CLs on OMM and IMM forms another intermembrane junction between OMM and IMM. F - After fission between OMM and IMM, CTII settles in the inverted micelle in IMM. At high CTII concentrations, these events disrupt mitochondrial membrane integrity and function, but at very low concentrations CTII facilitates mitochondrial energetics. In A, B, D, E and F the head groups of CLs are colored yellow and the head groups of PCs are colored blue. In C the head groups of CLs are colored red and the head groups of PCs colored yellow. This figure is modified from [59,60].
Melittin is a small membrane-active peptide from bee venom of 2.84 kD with anticancer properties [70-72]. Melittin is probably the only cationic membrane-active peptide that can bind to the pure PC membrane, which exposes N+(CH3)3 groups to the solution to shield cationic peptides away from the membrane surface [73,74]. Melittin binds and perturbs the membrane structure enriched with acidic phospholipids with higher avidity than binding and perturbing pure PC membrane [70-72]. This observation explains the higher cytotoxicity of melittin to cancer cells, which have acidic lipids on the outer surface of the plasma membrane, than to healthy cells, which have mostly PC molecules on the outer surface of the plasma membrane [71]. It is believed that melittin, similarly to snake venom cytotoxins, has evolved from the body’s membrane-active proteins that regulate the functions of plasma membranes and membranes of organelles through the induction of changes in the dynamics and structure of the lipid phase of the membrane [67,72]. Similarly, to cytotoxins, melittin triggers the formation of non-bilayer lipid phase in cardiolipin-containing membranes [70,72], which at high melittin concentrations disrupts mitochondrial membrane integrity and function, but at low concentrations it is believed that melittin may increase mitochondrial membrane plasticity to rejuvenate mitochondrial functions [70]. Interestingly, short-chain alcohols facilitate melittin’s membranotropic activity by making the membrane surface interfaces more conducive for melittin action [72].
Structural and Functional Roles of Non-Bilayer Lipid Phases in Mitochondria
Non-Bilayer Lipid Phase in Intact Mitochondria
The first report on non-bilayer lipid phase in biological membranes was made more than 40 years ago. It was revealed by 31P-NMR spectroscopy that phospholipids in intact rat liver mitochondria at 37°C coexist as bilayer and non-bilayer phases [41]. About a decade later, a fraction of bovine liver mitochondrial proteolipids, which predominantly contained CL molecules bound to proteins of Fo subunits of ATP synthase, was isolated [75]. 31P NMR spectrum of this sample of mitochondrial proteolipids had a symmetrical slightly broad line shape with the signal peak at 6 ppm (hereafter called the 6ppm signal). The 6 ppm 31P NMR signal has been previously observed in PC+CL multilamellar dispersions treated with CTII [76] and aqueous CTII-lipid complexes [77]. Computational simulation studies suggested that the 6ppm signal is derived from non-bilayer packed CL molecules electrostatically interacting with lysine and arginine residues of CTII [77]. As for the mitochondrial proteolipids, it was concluded that the 6ppm signal derives from the non-bilayer packed CL molecules which are immobilized by a force of electric attraction to the conserved lysine residues of Fo subunit [75]. It should be noted that about a decade after the work in [75], non-bilayer lipids arranged in hexagonal packing were discovered by small-angle neutron scattering in os motically shocked rat heart mitochondria [78]. At about the same time, it was also discovered that two cytotoxins CTI and CTII from cobra venom phenocopy proteins of mitochondrial ATP synthase Fo subunit in forming protein-lipid oligomers through binding to CL molecules [59,61,64]. Cytotoxins CTI and CTII were employed to probe the structure and dynamics of cauliflower and bovine mitochondrial membranes [59,61,64]. Both samples of intact cauliflower and bovine mitochondria treated with CTI and CTII at 18°C (cauliflower) or 15°C (bovine) generated the 31P-NMR spectrum with two non-bilayer signals superimposed over the lamellar signal [59,61,64]. One non-bilayer signal had a resonance at 0ppm and another at 6ppm. Application of DANTE (delay alternating with nutation for tailored excitation) train of saturation pulses at the highfield peak of the lamellar signal revealed that non-bilayer signal at 0 ppm derives from the lipids with rapid isotropic movement which exchange with the lipids in lamellar phase within the 31P-NMR time scale, while the signal at 6 ppm derives from the lipids that do not exchange with the lipids in lamellar phase in the 31P-NMR time scale [58,79]. To elucidate further details of the molecular mechanism of cytotoxins, interactions with mitochondria, intact mitochondria and model membranes of phospholipid composition of IMM treated separately with cytotoxins CTI and CTII were investigated with a set of biophysical methods including 31P-NMR, 1H NMR, 2H-NMR, EPR of oriented lipid films, luminescent quenching spectroscopy and differential scanning calorimetry [59,61,79]. It was determined that the 6 ppm signal derives from non-bilayer organized CL molecules immobilized by binding to cytotoxins and located in the intermembrane junctions between the OMM and the IMM [59]. It was also suggested that non-bilayer arranged phospholipids responsible for the 6ppm signal could be immobilized by binding to mitochondrial proteins which have binding sites for CL similar to those of CTI and CTII [59,61,79]. Docking simulation analysis revealed that CTI and CTII share CL binding sites with the dicyclohexylcarbodiimide-binding protein (DCCD BPF), which is the C8 rotor protein that is a part of the Fo sector embedded in IMM in bovine mitochondrial ATP synthase. The C8 rotor subunit of the Fo sector plays a key role in moving protons from the inter-cristae space to the matrix [80]. Model membranes with the phospholipid composition of IMM were treated with DCCD-BPF similarly to the treatment of the same model membranes with CTI and CTII, and it was demonstrated that DCCD-BPF and cytotoxins share similar membranotropic properties [59,61,79]. When DCCD-BPF was reconstituted in model membranes with phospholipid composition of IMM, the 6 ppm 31P-NMR signal was observed. It was established that the 6ppm signal derives from immobilized phospholipids that do not exchange with the phospholipids of the lamellar phase which suggests that the 6 ppm phospholipids are CL molecules immobilized by binding to DCCD-DPF [59,61,79]. It should be noted that the mobility of CL molecules immobilized by binding to DCCD- DPF or to cytotoxins is faster than the mobility of annular lipids which do not generate 31P NMR signal as they move slower than 31P-NMR time scale 10-2 - 10-4 s. This finding prompted the use of CTI and CTII as proteins that model the membranotropic effects of DCCD-DPF [60]. Further experiments have shown that at low concentrations, CTI and CTII trigger the increase in ATP synthesis in intact mitochondria and that the increase in ATP synthesis parallels the increase in intensity of the 6ppm signal [59-61,79]. Interestingly, the C8 rotor subunit of the Fo sector has been recently proposed as a key component of the mitochondrial permeability transition pore (mPTP) [81]. In this regard, one can conclude that the ability of DCCD-BPF to generate non bilayer lipid structures may play a key role in the formation of mPTP that could be linked to the generation of non-bilayer structures involving immobilized CL molecules.
Role of Non-Bilayer Phospholipids in Cristae Remodelling and Function
The non-bilayer lipids, CL and phosphatidylethanolamine (PE), play an essential role in the dynamics and structural assembly of mitochondrial membranes [82]. In mammalian mitochondria, PE constitutes about 40% of lipids in OMM and IMM, while CL constitutes about 3% and 20% of lipids in OMM and IMM, respectively [83]. Both CL and PE exert asymmetrical lateral pressures in lipid polar heads and in lipid hydrophobic alkyl chain areas to generate mechanical stress necessary for creating numerous folds in IMM and invaginations in cristae tubular membranes [84]. Both non-bilayer phospholipids contribute greatly not only to the maintenance of functionally active states of the respiratory chain proteins, but also to the overall membrane morphology of mitochondria [82].
The effects of CL and PE towards supporting the structural and functional stability of IMM, such as generating membrane potential and facilitating the maximal activity of electron transport chain (ETC) proteins, are different [85]. The activity of cytochrome c oxidase and the membrane potential of IMM decrease in the lack of PE, but in the lack of CL, PE exerts a destabilizing effect on the respiratory chain super-complexes [85]. The stable membrane potential, efficient activity of cytochrome c oxidase, and structural strength of the respiratory chain super-complexes requires CL [85]. CL is the main structural element in the dimerization of the ATP synthases. Moreover, CL acts as a glue in stabilizing ribbon-like assembly of ATP synthase dimers, which is a signature feature affecting the overall morphology and organization of cristae membranes [86]. Apart from binding to proteins of the ETC, CL also binds to the transporters of ADP-ATP, pyruvate, phosphate carriers and to cyt c, which cannot function efficiently without CL while deficiency of PE does not affect the functioning of these proteins [87]. It is a common understanding that one of the main roles of CL is to preserve elastic bonds between ATP synthase dimers and oligomers and keep the tight associations between the proteins of Respiratosomes, while PE primarily serves to supplement CL in maintaining the plasticity and structural integrity of the curved area of IMM [85]. The high angle conical shape of CL with four highly flexible alkyl chains and two phosphate groups suits very well for the arrangement of tight, strong but flexible bonds between ATP synthase complexes in dimeric and oligomeric forms of the enzyme [64,79] and proteins of the respiratory chain super-complexes in the highly dynamic lipid phase of IMMs [85].
Mitochondrion is an organelle which continuously changes its morphology and cristae are the most dynamic structures in IMM which remodel their architecture in a timescale of seconds in response to changes in energy demand which depend on metabolic rate, physiological states of cells and tissues and the states of health and disease [88-91]. Cristae act as ‘sub-organelles’ connected through slit-like structures, called crista junctions, to the inner boundary membrane. Electron tomography revealed that there is a dynamic interaction between the cristae junction and cristae [92,93]. Disappearance of cristae and formation of new cristae, a step in cristae membrane remodelling, occurs in several seconds according to data obtained by advanced optical microscopy [88,91,94]. Dynamics of cristae remodelling affects the membrane potential of an individual crista, Ca2+ homeostasis, OXPHOS and apoptosis [90,91]. Cristae act as independent bioenergetic sub-organelles, each having different rates of ETC activitiy and ATP synthesis in different parts of the IMM [95]. Cristae also have a higher membrane potential (ΔΨm) than the inner boundary membranes, plus individual cristae differ in their membrane potential ΔΨm [96]. Treatments with different mitochondrial stressors, oligomycin and carbonyl cyanide p-trifluoro methoxy phenyl hydrazone (FCCP), differently affected heterogeneity in ΔΨm of cristae [96]. A laser-induced depolarization of a specific crista does not spread to neighbouring cristae in the same mitochondrion [96], a finding that may have a profound implication as the dysfunction in an individual crista is not spreading through the entire mitochondrion. A protection from the spread of dysfunction could be executed by cristae junctions, which provide electrical insulation and maintain ΔΨm of individual cristae in a mitochondrion even when neighbouring cristae are depolarized [96]. It has been proposed that the ΔΨm heterogeneity of cristae may have a functional importance [97]. Cristae, which differ in ΔΨm may have different functions including ATP synthesis, production of reactive oxygen species (ROS), which is crucial for macrophages [98] and release of mitochondrial DNA [97] (Figure 4). Since the cristae junctions seemingly control the ΔΨm level in cristae, it is conceivable that non-bilayer CL, a major lipid in the microenvironment of cristae junctions, plays a key role in maintaining a healthy ΔΨm level in cristae.
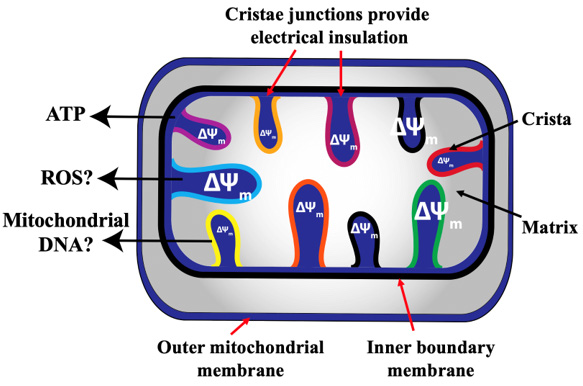
Figure 4: Heterogeneity of membrane potential ΔΨm of cristae. It has been recently reported that individual cristae within the same mitochondrion may have different ΔΨm [96,97]. This may lead to subsections of the same mitochondrion that produce ROS, release mitochondrial DNA and maintain ATP production [97]. This Figure is modified from [97].
ATP synthase, OPA1, mitochondrial contact site and cristae organizing system (MICOS), and the lipid microenvironment composed mainly of CL determine the rates of cristae junction formation and remodelling of cristae [89,91,95,99]. Abnormal rates of cristae remodelling caused by CL insufficiency results in a range of diseases [90,95,100]. Creation of new cristae is facilitated by CL’s non bilayer propensity [90,99,102]. Cristae membrane bending is regulated by the MICOS complex subunits (MIC60 and MIC10), which are surrounded by CL at cristae junctions [89]. The short and long forms of OPA1 bound to CL regulate the width of the cristae junction and the two phosphate groups of CL are considered mostly responsible for keeping proteins in cristae junctions tight [89]. It is believed that positive membrane curvature at the cristae tip is induced by ATP-synthase dimers surrounded by the CL predominant microenvironment in the inner leaflet of the cristae membrane [89]. Insufficiency in CL changes not only the cristae remodelling but also the setting of OXPHOS complexes [98], which may cause cancer, cardiovascular pathologies and neurodegeneration [88,99]. The abnormally swollen and highly interconnected cristae induced by the decline in CL molecules [103,104] lead to defects in heart and skeletal musculature [103].
Intermembrane exchange, membrane fusion and fission are the central processes in the remodelling of cristae architecture [104- 106]. This is evident from the multiple junctions with the inner boundary membrane and numerous cristae membrane interconnections. Decline in mitochondrial CL caused by aging and disease inhibits the dynamics of IMM fusion and fission, which leads to defects in cardiac and skeletal muscle [103] and cardiovascular and neurological disorders and cancer [103,107]. Reduced levels of CL in IMM reduces membrane coupling and efficiency of ATP synthase and respiratory ETC in muscle cells [108].
It has been well established that CL, a lipid in IMM with the highest non-bilayer propensity, drives membrane fusion and fission via triggering lipid phase transitions from bilayer to non-bilayer phase [59,61,76,109,110]. 31P-NMR spectroscopy observes dynamics of phospholipid phase polymorphism in the model and native IMM in the timescale 10-2 to 10-4 s [59-61]. The above-mentioned experimental data, along with the recent excellent review by Joubert and Puff that stresses the role of non-bilayer lipids in mitochondrial architecture and function [111] strongly suggest that the high inclination of CL to form non-bilayer phases in IMM is a key driving force that promotes rapid cristae remodelling.
Non-Bilayer Lipid, Cardiolipin, Facilitates ATP Synthesis in Cristae
Cristae’s morphology is highly dynamic, and it changes dramatically in response to changes in the metabolic state [111,112]. An individual crista is a self-sufficient bioenergetic sub-organelle of mitochondria that serves as a suitable surface area for clustering of ETC proteins and ATP synthase dimers which retain protons on the inner surfaces of the crista membrane [111-113]. Binding of CL on the inner surface of the crista membrane to the Fo sector of ATP synthase creates ATP synthase dimers in the apex of the crista with the highest membrane surface curvature in cristae [9,61,114]. The CL molecules mediate the dimerization of ATP synthases not only in the apex of the crista but also along the long axis of crista, which creates membrane raft-like structures with the high membrane surface curvature at the peak of the raft [113]. Each ATP synthase complex of the dimer sits on opposite sides of the raft’s peak [113]. Clustering of OXPHOS oligomers both in the apex of cristae and at the peaks of membrane rafts, which is mediated by CL binding to OXPHOS proteins, creates optimal conditions for kinetic coupling of the ETC proteins with ATP synthase in which the ETC proteins transfer protons along the inner cristae membrane surface to the ATP synthase dimers [113,115-117].
The idea of protons diffusing between the ETC proteins along the inner crista membrane surface was first suggested in 1961 [118] and it was convincingly corroborated one and a half decades later in the octane-water interface system [119]. About two decades later, the existence of a kinetic barrier for proton transfer from a membrane surface to bulk water was reported [120] and a few more years later a proton transfer across the interface under conditions of catalysis and driven by proton gradient on the membrane surface was demonstrated [121]. Four more years later, a metastable bond between protons and mitoplast surface [122] and protons from Brønsted acid bounded to the mitochondrial surface and serving as a substrate for ATP synthase was reported [123]. It has been also reported that cardiolipin binds to protons and transports H+ along the membrane surface to the ATP-synthase [124,125]. This transport generates an electric field along the membrane surface. The nano-level hydrodynamic effects induce rotation of ATP-synthase rotor and release of ATP into the solution [124]. In the last five years, a few more important studies were published. It was shown that the rate of ATP synthesis is determined by the lateral movement of protons along the membrane surface from proton pumps to ATP synthase [126] and that ATP synthesis in cristae membranes is driven by the kinetic coupling of the ETC with ATP synthase, but not by the proton gradient in bulk water [115], and finally it was suggested that ATP synthesis is driven by proton currents inside the coupling membrane [127]. The last study is of particular interest. It is possible that protons inside the coupling membrane could be found on the inner surface of an inverted micelle made of CL (Figure 5B) and formed in a membrane bilayer in a similar way suggested for an inverted micelle with cytotoxin in its inner surface (Figure 3F) [59,60]. Due to the high structural tension, the inverted micelle transforms to a bilayer and releases protons down the concentration gradient to the membrane surface on the matrix side. From there, protons are returned to the inter-crista space via proton pumps. Then when protons diffuse again along the membrane surface back to ATP synthase surrounded by CL, the conical shape of CL increases to trigger the formation of an inverted micelle with protons in the inner surface of the micelle which then again releases protons to the matrix. It is quite possible that the reversible polymorphic transitions from the bilayer membrane to non-bilayer micelles near the ATP synthase trigger rotation of the ATP synthase rotor that releases ATP into solution. Should the suggested transfer of protons inside an inverted micelle across the coupling membrane down the proton concentration gradient be proven true, this would account for another important role of non-bilayer lipid phase in ATP synthesis in cristae membranes. Overall, in the kinetic coupling of the ETC with ATP synthase, protons move along the inner surface of the crista membrane over ETC proteins to the ATP synthase.

Figure 5: An inverted micelle made of CL serves as a vehicle for the transport of H+ ions from intra-crista space to matrix. Concentration of H+ ions next to Fo subunit of ATP synthase increases the conical shape of CL molecules (A) and triggers the formation of a CL made inverted micelle (B) carrying H+ ions to be released into the matrix. Atomic structure of ATP synthase was reconstituted by Dr. S. V. Nesterov of Moscow Institute of Physics and Technology from PDB # 6b8h coordinates using the UCSF ChimeraX program [128,129]. The alpha- and beta-subunits of the catalytic domain are shown in yellow and orange, respectively; the c ring is given in blue. All other subunits are presented in grey. Polar heads of CL are shown in blue. Lipids with red polar heads represent other IMM lipids with two acyl chains. Alkyl chains of lipids are not drawn over the ATP synthase hydrophobic subunits for better visibility.
As we proposed, protons are transferred into matrix across the crista membrane inside the inverted CL micelle (Figure 5B). We further suggest that the formation of inverted CL micelles triggers the rotation of ATP synthase rotor. Thus, we support the concept of protons movement along the inner surface of the crista membrane to the ATP synthase dimers from where protons are transferred into matrix inside the inverted CL micelle and then returned to the inner crista membrane surface via proton pumps. The important point of the suggested movement of protons is that it does not affect pH in bulk waters across the crista membrane. The chemiosmotic theory driven by the proton gradient in bulk solution across the crista membrane has two issues. First, it would require a colossal number of H+ ions in solution in the intermembrane space to create a proton gradient across cristae membranes. Second, ATP synthesis coupled with the movement of protons from bulk solutions across the crista membrane would create pH fluctuations in solutions in the intermembrane space and matrix, which would affect the 3D folding and function of proteins and other organic substances in solution across cristae membrane.
In IMM, ATP synthases are arranged in dimers to increase curvature on the inner side surface of the crista membrane. ATP synthase dimerization creates maximal membrane curvature in the apex of the crista and at the peaks of rafts that leads to the electric charge redistribution in which protons are pushed into the region of maximum curvature where ATP synthase dimers are located [9,113]. This boosts the density of protons near the Fo subunit and increases the rate of proton translocation to matrix and the rate of ATP synthesis [9]. The boost in proton density enhances the neutralization of phosphate groups of CL that increases the conical shape of CL and membrane curvature to trigger the transformation of the bilayer packed CL molecules to the non-bilayer inverted micelles which may transport protons down the concentration gradient into the matrix (Figure 5A, B). As suggested above, the formation of the CL inverted micelles may trigger the rotation of ATP synthase rotor. It has been recently reported that CL selectively binds to the conserved lysine residues in the ATP synthase rotor [130]. An increased proton density at the maximal curvature of the inner crista surface between ATP synthase dimers neutralizes the negative charge of CL phosphate groups electrically bound to the conserved lysine residues of ATP synthase rotor. This event bears three consequences: 1) breakage of ionic bonds between CLs and the conserved lysine residues which offers higher mobility to ATP synthase rotor; 2) increase in the conical shape of CLs which facilitates formation of inverted micelles containing protons to be released down the concentration gradient to the matrix; 3) rotation of ATP synthase rotor triggered by the energy of bilayer membrane to inverted micelle transition. These three processes are reversible. The release of protons by the CL inverted micelles into the matrix decreases the proton density on the surface of CL polar heads that increases the negative charge density of CL phosphate groups and decreases the conical shape of CL to turn the CL molecules back to the lamellar phase. This returns the positions of CLs next conserved lysine residues of the ATP synthase rotor to reestablish ionic bonds. This increases the mass and reduces the kinetic energy of ATP synthase and stops its rotation. This could be seen as a dynamic equilibrium linking the rate of lipid polymorphism in cristae membranes driving OXPHOS efficacy on one side of the equilibrium and the cristae membrane lamellar phase dynamic ‘stillness’ on another side. The position of equilibrium is controlled by the energy demand that changes with the change in the physiological state of cells and organism.
It has been demonstrated in recent studies that creating compartments in the intra-crista space by forming intermembrane junctions between the inner surfaces of parallel crista membranes may further facilitate ATP synthesis in the crista as protons are ‘squeezed’ along the inner membrane surface of the compartments to further increase H+ ions density near the Fo subunits to promote transport of protons into matrix and to increase the rate of ATP synthesis [9,61,114] (Figure 6).
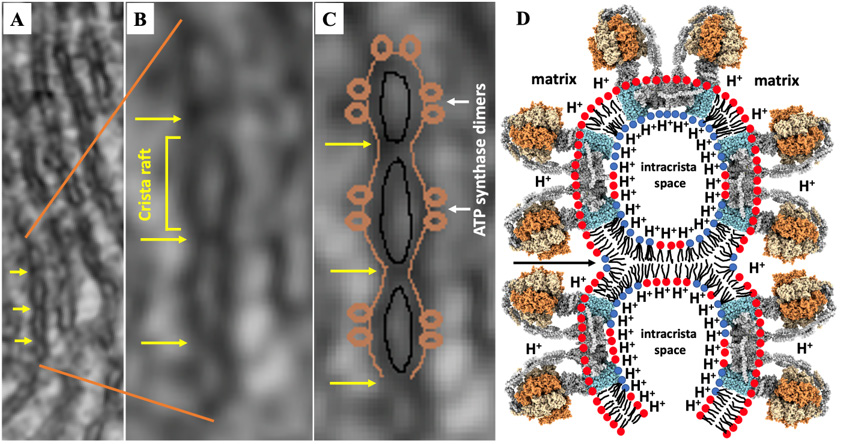
Figure 6: Intermembrane junctions increase the concentration of protons along the crista inner membrane surface. A, B and C - transmission electron micrography images modified from [114] show intermembrane junctions between the membranes of the crista (yellow arrows) that separate crista rafts. Schematic diagram of two crista compartments that form rafts on the compartments’ outer surface which are separated by intermembrane junctions - see black arrow (D). Reconstruction of ATP synthase and designation of lipids are described in Figure 5. For better visibility alkyl chains of lipids are not drawn over the ATP synthase hydrophobic subunits.
The initial step in the formation of an intermembrane junction in the intra-crista space, which is likely created between two areas of maximum curvature protruding into the intra-crista space, is driven by the attraction of CL molecules on the internal sides of the parallel membranes of the cristae to a cationic peptide on the surface of the opposite membrane of the crista via a mechanism described previously [9,60,61,114]. A cationic protein which serves as the initial point of intermembrane contact becomes the center of inverted micelles, which, due to the high tension of membrane curvature, may be transformed to the bilayer intermembrane junction and release the cationic protein into intra-crista space. CLs are found on the surface of lipid layers with high curvature [9,60,61,64,114]. Cationic proteins that may initiate the formation of the intermembrane junctions could be creatine kinase, cyt c, or even misfolded DCCD-BPF not incorporated into ATP synthase c-rings [9]. Intermembrane junctions may also serve to maintain an appropriate intra-crista space by preventing adjacent cristae membranes from being squeezed against each other. Appropriate intra-crista space is needed for the transport of nucleotides, phosphate group, and nucleotide carriers and substrates in the bulk solution [9]. A high dynamics of intermembrane junctions which undergo bilayer to non-bilayer transitions should be appreciated as they change reversibly in response to changes in proton density on the inner crista membrane surface. At high proton density bilayer phase transforms to the non-bilayer and at low proton density a reverse process takes place [9]. Due to the reversibility of the process, intermembrane junctions do not interfere with the transport of substrates and nucleotides from the bulk solution to intra-crista space. It has been also proposed that a channel linking the mitochondrial matrix to opposite sides of the crista may be created by the fusion of cristae membranes triggered by non-bilayer junctions [9]. Similar events occur when the lamellar structure of the cristae transforms to the tubular structure.
Conclusion
Analysis of the extensive experimental and theoretical data on non-bilayer lipids and non-bilayer lipid phases in model and mitochondrial membranes allowed us to conclude that the non-bilayer lipid phases are essential parts of fully functional mitochondrial membranes. The non-bilayer lipid phases are not only indispensable elements for maintaining the structural dynamics and remodeling of mitochondrial membranes, but they are also a centerpiece in the mechanism of ATP production in the IMM. Some important concepts discussed in this review have been presented in a recent major review [9] by the research group that included a corresponding author of this review. The previous review focuses on a comparative analysis of the structural and functional roles of non-bilayer lipid phases in thylakoid and inner mitochondrial membranes. In this review, we propose novel details in the mechanism of ATP synthesis in the IMM, thus we upgrade the mechanistic roles of non-bilayer lipid phases in mitochondria. We propose that the increase in proton density on the inner membrane surface of crista next to Fo subunit of ATP synthase weakens and breaks the ionic bond between the phosphate groups of CL and the conserved lysine residues of the ATP synthase rotor. This triggers the formation of the CL inverted micelles which not only transport protons into the matrix but also facilitate the rotation of ATP synthase rotor. Transport of protons to the matrix in a CL inverted micelle across the crista membrane is proposed for the first time and it supplements the kinetic coupling of the ETC with the ATP synthase. The kinetic coupling resolves the issues of chemiosmotic theory in which the movement of protons is driven by the gradient of H+ in bulk solutions across the cristae membranes. In the kinetic coupling, the movement of protons does not induce fluctuations in pH in bulk solutions in the matrix and intermembrane space. This precludes unphysiological conditions on both sides of the cristae membrane.
It should be noted that the transport of protons to the matrix across the crista membrane in cardiolipin inverted micelles that induce rotation of ATP synthase rotor is a new hypothetical concept that should be tested in future studies. We believe that this review will attract the attention of researchers to the phenomenon of non-bilayer lipid phases in energy transducing membranes. This should facilitate further research on the elucidation of new important details in the mechanism(s) of fundamental bioenergetic systems such as inner mitochondrial membranes.
Some parts of this review article have been published by us in preprints in Qeios [131,132].
Data Availability
All data obtained in this study are contained within the article.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
This research was funded by the start-up grant from the Chaoyang Kaiwen Academy and NIH grants GM08012 and RR08124.
Acknowledgment
E.S. Gasanoff is grateful to Professor G. Garab of Biological Research Centre, Szeged, Hungary, for productive comments and editing. E.S. Gasanoff also thanks Professor L.S. Yaguzhinsky of Belozersky Institute of Physico-Chemical Biology, M.V. Lomonosov Moscow State University, Moscow, Russia, and Dr. S.V. Nesterov of Moscow Institute of Physics and Technology, Dolgoprudny, Russia, for fruitful discussions that helped to clarify new details in the mechanisms of ATP synthesis presented in this manuscript. It is acknowledged that the movement of protons to the matrix across the crista membrane in the CL inverted micelles leading to the rotation of ATP synthase rotor is suggested by E.S. Gasanoff.
References
- Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175(4023): 720-731.
- Nicolson GL (2014) The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta 1838(6): 1451-1466.
- Epand RM (1998) Lipid polymorphism and protein-lipid interactions. Biochim Biophys Acta 1376(3): 353-368.
- van den Brink-van der Laan E, Killian JA, de Kruijff B (2004) Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666(1): 275-288.
- de Kruijff B (1997) Biomembranes - lipids beyond the bilayer. Nature 386(6621): 129-130.
- Brown MF (2012) Curvature forces in membrane lipid-protein interactions. Biochemistry 51(49): 9782-9795.
- Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG (2010) An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog Lip Res 49(4): 378-389.
- Bozelli JC, Aulakh SS, Epand RM (2021) Membrane shape as determinant of protein properties. Biophys Chem 273: 106587.
- Garab G, Yaguzhinsky LS, Dlouhý O, Nesterov SV, Špunda V, et al. (2022) Structural and functional roles of non-bilayer lipid phases of chloroplast thylakoid membranes and mitochondrial inner membranes. Prog Lip Res 86: 101163.
- Garab G, Lohner K, Laggner P, Farkas T (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci 5(11): 489-494.
- Garab G, Ughy B, Goss R (2016) Role of MGDG and non-bilayer lipid phases in the structure and dynamics of chloroplast thylakoid membranes. Subcell Biochem 86: 127-157.
- Rietveld A, van Kemenade TJJM, Hak T, Verkleij AJ, de Kruijff B (1987) The effect of cytochrome-c-oxidase on lipid polymorphism of model membranes containing cardiolipin. Eur J Biochem 164(1): 137-140.
- Simidjiev I, Stoylova S, Amenitsch H, Javorfi T, Mustárdy L, et al. (2000) Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci USA 97(4): 1473-1476.
- Williams WP (1998) The physical properties of thylakoid membrane lipids and their relation to photosynthesis. In: Siegenthaler PA & Murata N (Eds.), Lipids in Photosynthesis: Structure, Function and Genetics. Springer, Dordrecht, Netherlands pp. 103-118.
- Seddon JM, Templer RH (1995) Polymorphism of lipid-water systems. In: Lipowsky R & Sackmann E (Eds.), Handbook of Biological Physics. North-Holland pp. 97-160.
- van Eerden FJ, de Jong DH, de Vries AH, Wassenaar TA, Marrink SJ (2015) Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. Biochim Biophys Acta 1848(6): 1319-1330.
- Kirchhoff H, Hall C, Wood M, Herbstová M, Tsabari O, et al. (2011) Dynamic control of protein diffusion within the granal thylakoid lumen. Proc Natl Acad Sci USA 108(50): 20248-20253.
- Chernomordik L (1996) Non-bilayer lipids and biological fusion intermediates. Chem Phys Lipids 81(2): 203-213.
- Blumenthal R, Clague MJ, Durell SR, Epand RM (2003) Membrane fusion. Chem Rev 103(1): 53-69.
- Eicher B, Marquardt D, Heberle FA, Letofsky Papst I, Rechberger GN, et al. (2018) Intrinsic curvature-mediated transbilayer coupling in asymmetric lipid vesicles. Biophys J 114(1): 146-157.
- Kalita B, Utkin YN, Mukherjee AK (2022) Current insights in the mechanisms of cobra venom cytotoxins and their complexes in inducing toxicity: implications in antivenom therapy. Toxins 14: 839.
- Järvi S, Gollan P, Aro EM (2013) Understanding the roles of the thylakoid lumen in photosynthesis regulation. Front Plant Sci 4: 434.
- Kühlbrandt W (2015) Structure and function of mitochondrial membrane protein complexes. BMC Biol 13(1): 89.
- Pfanner N, Warscheid B, Wiedemann N (2019) Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol 20(5): 267-284.
- Malnoë A, Schultink A, Shahrasbi S, Rumeau D, Havaux M, et al. (2018) The plastid lipocalin LCNP is required for sustained photoprotective energy dissipation in Arabidopsis. The Plant Cell 30(1): 196-208.
- Levesque Tremblay G, Havaux M, Ouellet F (2009) The chloroplastic lipocalin AtCHL prevents lipid peroxidation and protects Arabidopsis against oxidative stress. Plant J 60(4): 691-702.
- Bugos RC, Hieber AD, Yamamoto HY (1998) Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem 273(25): 15321-15324.
- Grzyb J, Latowski D, Strzałka K (2006) Lipocalins - a family portrait. J Plant Physiol 163(9): 895-915.
- Joliot P, Verméglio A, Joliot A (1993) Supramolecular membrane protein assemblies in photosynthesis and respiration. Biochim Biophys Acta 1141(2): 151-174.
- Dlouhý O, Karlický V, Arshad R, Zsiros O, Domonkos I, et al. (2021) Lipid polymorphism of the subchloroplast - granum and stroma thylakoid membrane–particles. II. structure and functions. Cells 10(9): 2363.
- Chan DC (2020) Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol 15: 235-259.
- Kondadi AK, Anand R, Reichert AS (2020) Cristae membrane dynamics - a paradigm change. Trends Cell Biol 30(12): 923-936.
- Luzzati VA, Tardieu T, Krzywicki G (1967) Polymorphism of lipids. Nature 215(5102): 701-704.
- Luzzati V, Tardieu A (1974) Lipid phases: structure and structural transitions. Annu Rev Phys Chem 25(1): 79-94.
- Rand RP, Sengupta S (1972) Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta 255(2): 484-492.
- de Kruijff B, Morris GA, Cullis PR (1980) Application of P-31-NMR saturation transfer techniques to investigate phospholipid motion and organization in model and biological membranes. Biochim Biophys Acta 598(1): 206-211.
- Cullis PR, de Kruijff B (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta 559(4): 399-420.
- Shipley GG, Green JP, Nichols BW (1973) The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta 311(4): 531-544.
- Williams WP, Brain APR, Dominy PJ (1992) Induction of non-bilayer lipid phase separations in chloroplast thylakoid membranes by compatible co-solutes and its relation to the thermal-stability of photosystem-II. Biochim Biophys Acta 1099(2): 137-144.
- Semenova GA (1999) The relationship between the transformation of thylakoid acyl lipids and the formation of tubular lipid aggregates visible on fracture faces. J Plant Physiol 155(6): 669-677.
- Cullis PR, de Kruijff B, Hope MJ, Nayar R, Rietveld A, et al. (1980) Structural properties of phospholipids in the rat liver inner mitochondrial membrane. Biochim Biophys Acta 600(3): 625-635.
- Vereb G, Szöllösi J, Matkó J, Nagy P, Farkas T, et al. (2003) Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc Natl Acad Sci USA 100(14): 8053-8058.
- de Kruijff B, Cullis PR (1980) Cytochrome specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim Biophys Acta 602(3): 477-490.
- Tournois H, de Kruijff B (1991) Polymorphic phospholipid phase transitions as tools to understand peptide-lipid interactions. Chem Phys Lipids 57(2-3): 327-340.
- Gasanov SE (1988) Mechanisms of Fusogenic Actions of Central Asian Cobra Venom Cytotoxins Vc5 and Vc1. Lomonosov Moscow State University: Moscow, USSR, 1988.
- Yeagle FL (2016) Lipid–protein interactions in membranes. In Membranes of Cells, 3rd ed.; Academic Press: Storrs, CT, USA, pp. 291-334.
- Trusova VM, Gorbenko GP, Molotkovsky JG, Kinnunen PKJ (2010) Cytochrome c-lipid interactions: new insights from resonance energy transfer. Biophys J 99(6): 1754-1763.
- Bergstrom CL, Beales PA, Lv Y, Vanderlick TK, Groves JT (2013) Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc Natl Acad Sci USA 110(16): 6269-6274.
- Vladimirov GK, Vikulina AS, Volodkin D, Vladimirov YA (2018) Structure of the complex of cytochrome c with cardiolipin in non-polar environment. Chem Phys Lipids 214: 35-45.
- Yin V, Shaw GS, Konermann L (2017) Cytochrome c as a peroxidase: activation of the precatalytic native state by H2O2-induced covalent modifications. J Am Chem Soc 139(44): 15701-15709.
- Matsuura K, Canfield K, Feng W, Kurokawa M (2016) Metabolic regulation of apoptosis in cancer. Int Rev Cell Mol Biol 327: 43-87.
- Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA (2006) Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 45(15): 4998-5009.
- Schlattner U, Gehring F, Vernoux N, Tokarska-Schlattner M, Neumann D, et al. (2004) C-terminal lysines determine phospholipid interaction of sarcomeric mitochondrial creatine kinase. J Biol Chem 279(23): 24334-24342.
- Epand RF, Tokarska-Schlattner M, Schlattner U, Wallimann T, Epand RM (2007) Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J Mol Biol 365(4): 968-980.
- Maniti O, Lecompte MF, Marcillat O, Desbat B, Buchet R, et al. (2009) Mitochondrial creatine kinase binding to phospholipid monolayers induces cardiolipin segregation. Biophys J 96(6): 2428-2438.
- Szeto HH, Liu SY (2018) Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging. Arch Biochem Biophys 660: 137-148.
- Feofanov AV, Sharonov GV, Astapova MV, Rodionov DI, Utkin YN, et al. (2005) Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage. Biochem J 390(Pt 1): 11-18.
- Dubovskii PV, Ignatova AA, Alekseeva AS, Starkov VG, Boldyrev IA, et al. (2023) Membrane-disrupting activity of cobra cytotoxins is determined by configuration of the N-terminal loop. Toxins 15(1): 6.
- Gasanov SE, Shrivastava IH, Israilov FS, Kim AA, Rylova KA, et al. (2015) Naja naja oxiana cobra venom cytotoxins CTI and CTII disrupt mitochondrial membrane integrity: implications for basic three-fingered cytotoxins. PLoS One 10(6): e0129248.
- Li F, Shrivastava IH, Hanlon P, Dagda RK, Gasanoff ES (2020) Molecular mechanism by which cobra venom cardiotoxins interact with the outer mitochondrial membrane. Toxins (Basel) 12(7): 425.
- Gasanov SE, Kim AA, Yaguzhinsky LS, Dagda RK (2018) Non-bilayer structures in mitochondrial membranes regulate ATP synthase activity. Biochim Biophys Acta 1860(2): 586-599.
- Baker CD, Ball WB, Pryce EN, Gohil VM (2016) Specific requirements of nonbilayer phospholipids in mitochondrial respiratory chain function and formation. Mol Biol Cell 27(14): 2161-2171.
- Dubinnyi MA, Dubovskii PV, Utkin YN, Simonova TN, Barsukov LI, et al. (2001) An ESR study of the cytotoxin II interaction with model membranes. Russian Journal of Bioorganic Chemistry 27(2): 84-94.
- Gasanov SE, Kim AA, Dagda RK (2016) The possible role of nonbilayer structures in regulating ATP synthase activity in mitochondrial membranes. Biophysics (Oxf) 61(4): 596-600.
- Gasanov SE, Alsarraj MA, Gasanov NE, Rael ED (1997) Cobra venom cytotoxin free of phospholipase A2 and its effect on model membranes and T leukemia cells. J Membr Biol 155: 133-142.
- Gasanov SE, Gasanov NE, Rael ED (1995) Phospholipase A2 and cobra venom cytotoxin Vc5 interactions and membrane structure. Gen Physiol Biophys 14(2): 107-123.
- Gasanov SE, Dagda RK, Rael ED (2014) Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J Clinic Toxicol 4(1): 1000181.
- Gasanov SE, Rael ED, Martinez M, Baeza G, Vernon LP (1994) Modulation of phospholipase A2 activity by membrane-active peptides on liposomes of different phospholipid composition. Gen Physiol Biophys 13(4): 275-286.
- Zhang B, Li F, Chen Z, Srivastava IH, Gasanoff ES, et al. (2019) Naja mosambica mossambica cobra cardiotoxin targets mitochondria to disrupt mitochondrial membrane structure and function. Toxins 11(3): 152.
- Gasanoff ES, Liu Y, Li F, Hanlon P, Garab G (2021) Bee venom melittin disintegrates the respiration of mitochondria in healthy cells and lymphoblasts and induces the formation of non-bilayer structures in model inner mitochondrial membranes. Int J Mol Sci 22(20): 11122.
- Li J, Hanlon P, Gasanoff ES (2020) Interaction of bee venom melittin, a potential anti-cancer drug, with phosphatidylcholine membrane enriched with phosphatidylserine. EC Pharmacology and Toxicology 8(11): 119-129.
- Wang H, Qin H, Garab G, Gasanoff ES (2022) Short-chained alcohols make membrane surfaces conducive for melittin action: implication for the physiological role of alcohols in cells. Cells 11(12): 1928.
- Xu Y, Hanlon P, Rael ED, Gasanoff ES (2020) Bee venom melittin modulates phospholipase A2 activity by affecting substrate interface on the surface of phosphatidylcholine membrane. Ann Toxicol 2(1): 26-35.
- Talbot JC, Bernard E, Maurel JP, Faucon JF, Dufourcq J (1982) Melittin-phospholipid interactions: binding of the mono- and tetrameric form of this peptide, and perturbations of the thermotropic properties of bilayers. Toxicon 20(1): 199-202.
- Segal NK, Gasanov SE, Palamarchuk LA, Ius'kovich AK, Kolesova GM, et al. (1993) Mitochondrial proteolipids. Biokhimiia (Moscow, Russia) 58(11): 1812-1819.
- Gasanov SE, Salakhutdinov BA, Aripov TF (1990) Formation of nonbilayer structures in phospholipid membranes induced by cationic polypeptides. Biol Membr 7: 1045-1055.
- Gasanov SE, Gasanov EE (1988) An Asymmetric Enlargement of the Monolayer Surfaces Mechanism of Membrane Fusion and Membrane Fusion Mediated by Proteolipid Non-bilayer Structures; Nuclear Physics Institute UzSSR Academy of Sciences: Tashkent, USSR, 1988.
- Murugova TN, Gordeliy VI, Kuklin AI, Solodovnikova IM, Yaguzhinsky LS (2007) Study of three-dimensionally ordered structures of intact mitochondria by small-angle neutron scattering. Crystallography Reports 52(3): 521-524.
- Gasanov SE, Kim AA, Dagda RK (2016) Possible role of nonbilayer structures in regulating the activity of ATP synthase in mitochondria. Biofizika 61: 705-710.
- Fillingame RH (1980) The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem 49: 1079-1113.
- Mnatsakanyan N, Jonas EA (2020) ATP synthase c-subunit ring as the channel of mitochondrial permeability transition: Regulator of metabolism in development and degeneration. J Mol Cell Cardiol 144: 109-118.
- Ball BW, Neff JK, Gohil VM (2018) The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett 592(8): 1273-1290.
- Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, et al. (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173(6): 2026-2034.
- Khalifat N, Fournier JB, Angelova MI, Puff N (2011) Lipid packing variations induced by pH in cardiolipin-containing bilayers: The driving force for the cristae-like shape instability. Biochim Biophys Acta 1808(11): 2724-2733.
- Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, et al. (2012) Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol 423(5): 677-686.
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL (2011) Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J 100(9): 2184-2192.
- Shen Z, Ye C, McCain K, Greenberg ML (2015) The role of cardiolipin in cardiovascular health. Biomed Res Int 2015: 891707.
- Chan DC (2020) Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol 15: 235-259.
- Kondadi AK, Anand R, Reichert AS (2020) Cristae membrane dynamics - a paradigm change. Trends Cell Biol 30(12): 923-936.
- Colina-Tenorio L, Horten P, Pfanner N, Rampelt H (2020) Shaping the mitochondrial inner membrane in health and disease. J Intern Med 287(6): 645-664.
- Kondadi AK, Anand R, Hänsch S, Urbach J, Zobel T, et al. (2020) Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep 21(3): e49776.
- Frey TG, Mannella CA (2000) The internal structure of mitochondria. Trends Biochem Sci 25(7): 319-324.
- Frey TG, Renken CW, Perkins GA (2002) Insight into mitochondrial structure and function from electron tomography. Biochim Biophys Acta 1555(1): 196-203.
- Liesa M (2020) Why does a mitochondrion need its individual cristae to be functionally autonomous? Mol Cell Oncol 7(2): 1705119-1705119.
- Khosravi S, Harner ME (2020) The MICOS complex, a structural element of mitochondria with versatile functions. Biol Chem 401(6-7): 765-778.
- Wolf DM, Segawa M, Kondadi AK, Anand R, Bailey ST, et al. (2019) Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J 38(22): e101056.
- Mills E, O’Neill LAJ (2020) Not all Mitochondrial cristae are the same: hetero-potential in the inner mitochondrial membrane. Immunometabolism 2(1): e200003.
- Angajala A, Lim S, Phillips JB, Kim JH, Yates C, et al. (2018) Diverse roles of mitochondria in immune responses: novel insights into immunometabolism. Front Immunol 9: 1605.
- Stephan T, Brüser C, Deckers M, Steyer AM (2020) MICOS assembly controls mitochondrial inner membrane remodeling and crista junction redistribution to mediate cristae formation. EMBO J 39(14), e104105.
- Eramo MJ, Lisnyak V, Formosa LE, Ryan MT (2019) The ‘mitochondrial contact site and cristae organising system’ (MICOS) in health and human disease. J Biochem 167(3): 243-255.
- Anand R, Kondadi AK, Meisterknecht J, Golombek M (2020) MIC26 and MIC27 cooperate to regulate cardiolipin levels and the landscape of OXPHOS complexes. Life Sci Alliance 3(10).
- Itoh K, Nakamura K, Iijima M, Sesaki H (2013) Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23(2): 64-71.
- Acehan D, Vaz F, Houtkooper RH, James J, Moore V, et al. (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286(2): 899-908.
- Mannella CA (2020) Consequences of folding the mitochondrial inner membrane. Front Physiol 11: 536.
- Ge YF, Shi XJ, Boopathy S, McDonald J, Smith AW, et al. (2020) Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane. eLife 9: e50973.
- Schuster R, Anton V, Simões T, Altin S, den Brave F, et al. (2020) Dual role of a GTPase conformational switch for membrane fusion by mitofusin ubiquitylation. Life Sci Alliance 3(1): e201900476.
- Gao S, Hu J (2021) Mitochondrial fusion: the machineries in and out. Trends Cell Biol 31(1): 62-74.
- Prola A, Blondelle J, Vandestienne A, Piquereau J, Deni RGP, et al. (2021) Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci Adv 7(1): eabd6322.
- Aripov TF, Gasanov SE, Salakhutdinov BA, Rozenshtein IA, Kamaev FG (1989) Central Asian cobra venom cytotoxins-induced aggregation, permeability and fusion of liposomes. Gen Physiol Biophys 8(5): 459-473.
- Gasanov SE, Kamaev FG, Salakhutdinov BA, Aripov T.F (1990) The fusogenic properties of the cytotoxins of cobra venom in a model membrane system. Nauchnye Dokl. Vysshei Shkoly Biol Nauki 2: 42-50.
- Joubert F, Puff N (2021) Mitochondrial cristae architecture and functions: lessons from minimal model systems. Membranes 11(7): 465.
- Mannella CA (2006) Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta (BBA) Mol Cell Res 1763(5-6): 542-548.
- Nesterov S, Chesnokov Y, Kamyshinsky R, Panteleeva A, Lyamzaev K, et al. (2021) Ordered clusters of the complete oxidative phosphorylation system in cardiac mitochondria. Int J Mol Sci 22(3): 1462.
- Gasanoff ES, Yaguzhinsky LS, Garab G (2021) Cardiolipin, non-bilayer structures and mitochondrial bioenergetics: relevance to cardiovascular disease. Cells 10(7).
- Toth A, Meyrat A, Stoldt S, Santiago R, Wenzel D, et al. (2020) Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc Natl Acad Sci USA 117(5): 2412-2421.
- Weichselbaum E, Österbauer M, Knyazev DG, Batishchev OV, Akimov SA, et al. (2017) Origin of proton affinity to membrane/water interfaces. Sci Rep 7(1): 4553.
- Nesterov SV, Skorobogatova YA, Panteleeva AA, Pavlik LL, Mikheeva IB, et al. (2018) NMDA and GABA receptor presence in rat heart mitochondria. Chem-Biol Interact 291: 40-46.
- Williams RJ (1961) Possible functions of chains of catalysts. J Theor Biol 1: 1-17.
- Yaguzhinsky LS, Boguslavsky LI, Volkov AG, Rakhmaninova AB (1976) Synthesis of ATP coupled with action of membrane protonic pumps at the octane–water interface. Nature 259(5543): 494-496.
- Antonenko YN, Kovbasnjuk ON, Yaguzhinsky LS (1993) Evidence in favor of the existence of a kinetic barrier for proton transfer from a surface of bilayer phospholipid membrane to bulk water. Biochim Biophys Acta 1150(1): 45-50.
- Evtodienko VY, Antonenko YN, Yaguzhinsky LS (1998) Increase of local hydrogen ion gradient near bilayer lipid membrane under the conditions of catalysis of proton transfer across the interface. FEBS Lett 425(2): 222-224.
- Moiseeva V, Motovilov K, Lobysheva N, Orlov V, Yaguzhinsky L (2011) The formation of metastable bond between protons and mitoplast surface. Dokl Biochem Biophys 438: 127-130.
- Eroshenko LV, Marakhovskaya AS, Vangeli IM, Semenyuk PI, Orlov VN, et al. (2012) Brønsted acids bounded to the mitochondrial membranes as a substrate for ATP synthase. Dokl Biochem Biophys 444: 158-161.
- Kocherginsky N (2009) Acidic lipids, H+-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell's Nobel prize award. Prog Biophys Mol Biol 99(1): 20-41.
- Haines TH, Dencher NA (2002) Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett 528(1-3): 35-39.
- Sjöholm J, Bergstrand J, Nilsson T, Šachl R, Ballmoos CV, et al. (2017) The lateral distance between a proton pump and ATP synthase determines the ATP-synthesis rate. Sci Rep 7(1): 2926.
- Morelli AM, Ravera S, Calzia D, Panfoli I (2019) An update of the chemiosmotic theory as suggested by possible proton currents inside the coupling membrane. Open Biology 9(4): 180221.
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, et al. (2021) UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30: 70-82.
- Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, et al. (2018) UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27(1): 14-25.
- Duncan AL, Robinson AJ, Walker JE (2016) Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc Natl Acad Sci USA 113(31): 8687-8692.
- Tao Y, Gasanoff ES (2023) Structural and functional roles of non-bilayer lipid phase in mitochondria. Qeios 2023: 1-12.
- Han Y, Gasanoff ES (2023) The studies of lipid phase polymorphism in model membranes. Qeios 2023: 1-9.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.