Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Reduced C1q/Tumor Necrosis Factor-Related Protein9 Expression Promotes Hcy-Induced VSMCs Migration via Negative Regulating Endoplasmic Reticulum Stress
*Corresponding author: Professor Minghao Zhang, Department of Pathophysiology, School of Basic Medical Sciences, Ningxia Medical University, 1160 Shengli Street, Yinchuan, Ningxia 750004, China.
Received: May 22, 2023; Published: June 02, 2023
DOI: 10.34297/AJBSR.2023.19.002545
Abstract
To provide a theoretical basis for the prevention and treatment of hyperhomocysteinemia (HHcy), the current study aimed to investigate the mechanism underlying the effect of homocysteine (Hcy) on inducing the migration of vascular smooth muscle cells (VSMCs) via inhibited C1q/Tumor necrosis factor-related protein9 (CTRP9) expression negative regulating endoplasmic reticulum stress (ERs). Therefore, the overexpression and interference plasmid of CRRP9 were constructed, transfected into VSMCs cells, and administered Hcy stimulation cells. At the same time, endoplasmic reticulum stress inhibitor (4-PBA) and endoplasmic reticulum stress activator (TM) intervention cells were administered. The migration ability of VSMCs at 0h and 48h was assessed by wound-healing assays. The protein expression of CTRP9, DNA methyltransferase 1 (DNMT1), ERs marker GRP78 and AFT6a in VSMCs were detected by western blot. Furthermore, DNMT1 inhibitor 5-Azc was given to intervene cells to observe the effect of DNMT1 on CTRP9 expression. The results show that Hcy can down-regulate CTRP9 protein expression and stimulate VSMCs migration. Overexpression of CTRP9 can delay VSMCs migration caused by Hcy. Meanwhile, activation of ERs simultaneously with overexpression of CTRP9 can inhibit VSMCs migration. Interference with CTRP9 has achieved the opposite results. It is suggested that CTRP9 down-regulation promotes Hcy induced VSMCs migration, and ERs plays an important role in this process. In terms of mechanism, interference CTRP9 can activate ERs, while overexpression of CTRP9 can inhibit ERs. Meanwhile, the expression of DNMT1 is up-regulated by Hcy, and the expression of CTRP9 can be up-regulated by inhibiting DNMT1. In summary, the results of this study suggest that reduced C1q/Tumor necrosis factor-related protein9 expression promotes Hcy-induced VSMCs migration via negative regulating endoplasmic reticulum stress. The up regulation of DNMT1 expression induced by Hcy plays an important role in this process, and CTRP9 may be regulated by methylation.
Keywords: C1q/Tumor Necrosis Factor-Related Protein9; Endoplasmic Reticulum Stress; Hcy; VSMCs Migration; DNA Methyltransferase1
Introduction
Atherosclerosis (As) is an important underlying disease of ischemic cardiomyopathy and stroke [1]. The migration of vascular smooth muscle cells (VSMCs) plays an important role in the pathogenesis of atherosclerosis [2]. Hcy, an independent risk factor for AS, can induce the migration of VSMCs to cause atherosclerosis [3,4], but the mechanisms remain unclear. Endoplasmic reticu lum stress (ERs) can lead to a disorder of cellular metabolism [5]. CTRP9 is a novel endothelium-dependent and NO-mediated vasodilator [6]. There is a common molecular basis between CTRP9 and ERs, but whether CTRP9 has a regulatory effect on ERs remains unclear. Meanwhile, previous studies have found that CTRP9 expression is downregulated in the process of proliferation, migration and phenotypic transformation of VSMCs induced by Hcy, but its mechanism remains unclear [7].
This study aims to reveal the association between CTRP9 and ERs and their effects on Hcy-induced VSMCs migration in vitro. Therefore, we established an in vitro VSMCs model of Hcy stimulation. The current study explored whether CTRP9 may play a functional role in Hcy-induced VSMCs migration via regulating ERs, and the possible mechanism of CTRP9 protein expression reduction.
Materials and Methods
Cell transfection
The sequences of CTRP9 overexpression plasmid, GFP, Si-NC, and Si-CTRP9 were obtained from Genepharma (Shanghai, China) and infected as previously described [8] and they were transiently transfected into the cells using Lipofectamine 2000 (Life Technologies, Gaithersburg, MD, USA) following the manufacturer’s instruction. The transfection efficiency was detected by qRT-PCR and western blot, then the cells were collected for downstream analysis.
Cell culture and treatment
Human VSMCs were purchased from the BeNa Culture Collection (BNCC; Suzhou Bena Chuanglian Biotechnology Co. Ltd.) and cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin solution (Beijing Solarbio Science & Technology Co., Ltd.) at 37˚C in an incubator with 5% CO2. VSMCs were divided into the normal Control (Hcy-free), Hcy (100μmol/L), Hcy+Si-NC, Hcy+Si-CTRP9, Hcy+Si-CTRP9+4-PBA (10 mmol/L, ERs inhibitor, MCE, HY-A0281), Hcy+GFP, Hcy+CTRP9, Hcy+CTRP9+TM (0.5μmol/L, ERs agonist, MCE, HY-A0098) groups. All cells used were between passages 3 and 7. Prior each experiment VSMCs were induced with 100μmol/L Hcy for 48h.
Wound healing assay
A wound healing assay was carried out to evaluate the cell migration ability of Hcy-treated VSMCs. Briefly, 5×103 VSMCs in a volume of 100μl/well were seeded in 6-well plates and were allowed to reach 80-90% confluency. Subsequently, a linear scratch wound was made at the center of the cell monolayer using a 200μl tip. Cells were induced by Hcy and transfected CTRP9 overexpression plasmid, GFP, Si-NC, and Si-CTRP9. Following incubation for 48 h, images of the migrated cells were captured, and their number was calculated under an image acquisition system microscope (Olympus Corporation).
Western blot analysis. To detect the changes in the protein levels in VSMCs, western blot analysis was carried out using specific antibodies [9]. Total proteins were isolated from cells using a whole protein extraction kit (Nanjing KeyGen Biotech Co., Ltd.), while protein concentration was determined using the SimpliNano ™ Biochrom Spectrophotometer (Biochrom, Ltd.). The protein samples (20μl/lane) were separated by SDS-PAGE and were then transferred onto a PVDF membrane (MilliporeSigma). Following blocking with 5% non-fat milk in PBS with Tween-20, the membrane was incubated at 4˚C overnight with the following antibodies: Anti-CTRP9 (dilution, 1:1,000, no.DF9407), anti-GRP78 (dilution, 1:1,000, no.AF5366), anti-AFT6a (dilution, 1:1,000, no.DF6009), anti-DNMT1 (dilution, 1:1,000, no.DF9407) and anti-β-actin (dilution, 1:1,000, no.AF7018. all from Affinity.). Following washing, the membranes were incubated with the corresponding horseradish peroxidase-conjugated IgG (anti-rabbit, no. ZB2301 or anti-mouse, no. ZB2305, dilution, 1:5,000. ZSGB-BIO) for 4h. Finally, the protein bands were visualized using chemiluminescence (ECL; Nanjing KeyGen Biotech Co., Ltd.).
Results
CTRP9 negatively regulates ERs and promotes Hcy induced VSMCs Migration
The results of cell scratch experiment showed that there was no difference in cell migration ability among all groups at 0h. At 48h, the scratch area was significantly reduced, and the percentage of wound healing increased in the Hcy group. It is suggested that Hcy can induce VSMCs migration, which is consistent with previous findings [10]. After interference with CTRP9, the scratch area was further reduced, and the wound healing percentage was further increased. While interfering with CTRP9, 4-PBA was administered to inhibit ERs, which showed a significant increase in scratch area and a decrease in wound healing percentage. After CTRP9 is overexpressed, the opposite result is obtained (Figure 1). These results suggest that CTRP9 low expression promotes Hcy induced VSMCs migration, and the effect is related to the negative regulation of CTRP9 on ERs.
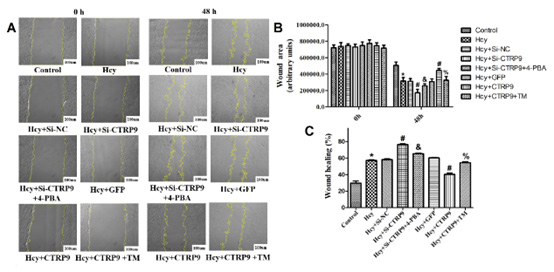
Figure 1: A. Results of the scratch test; B. The change of cell scratch area in each group; C. Changes in the percentage of cell wound healing in each group. Data are represented as the mean ± SD of three independent experiments (n=3). *P < 0.05, compared with Control group. # P < 0.05, Compared with Hcy group. % P < 0.05, compared with Hcy+CTRP9 group. & P < 0.05 compared with Hcy+Si-CTRP9 group.
DNMT1 inhibits the expression of CTRP9 and activates ERs
To further explore the mechanism of VSMCs migration induced by Hcy, we detected the expression of CTRP9 and ERs marker proteins GRP78 and ATF6a. The results showed that Hcy could down-regulate the expression of CTRP9 Figure 2A and up-regulate the expression of GRP78 and ATF6a Figures 2B,2C, suggesting that Hcy can inhibit CTRP9 and activate ERs. After interference of CTRP9, GRP78 and ATF6a protein expression increases obviously, while overexpression of CTRP9 results in the opposite result, suggesting that CTRP9 can negatively control ERs activation. Further, in order to observe the specific mechanism of Hcy down-regulation of CTRP9, the protein expression of DNMT1 was detected, and 5-Azc was used to inhibit DNMT1, and the expression of CTRP9 was observed. The results showed that Hcy could up-regulate the expression of DNMT1. After interference with CTRP9, the expression of DNMT1 is significantly increased. While interfering with CTRP9, 4-PBA was administered to inhibit ERs, and the expression of DNMT1 is decreased. After overexpression of CTRP9, the opposite result is obtained Figure 2D. Furthermore, after 5-Azc intervention, the protein expression of DNMT1 was significantly decreased, and the protein expression of CTRP9 was significantly increased Figure 2E, suggesting that 5-Azc inhibited the expression of DNMT1, and DNMT1 could down-regulate the expression of CTRP9, and there was negative feedback regulation between DNMT1, CTRP9 and ERs (Figure 2).
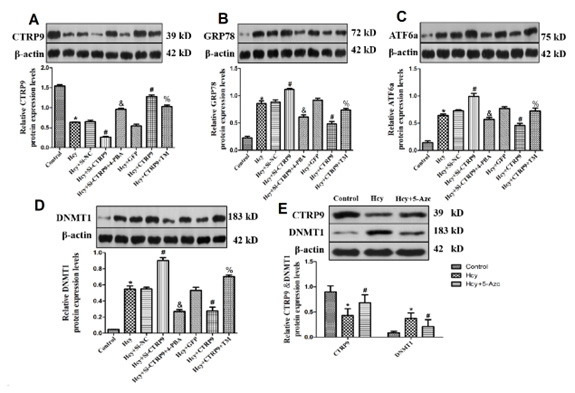
Figure 2A-2E: A. Changes in CTRP9 protein expression; B. Changes in GRP78 protein expression; C. Changes in ATF6A protein expression; D. Changes in DNMT1 protein expression; E. Effects of 5-Azc on the expression of CTRP9 and DNMT1 proteins. Data are represented as the mean ± SD of three independent experiments (n=3). *P < 0.05, compared with Control group. #P < 0.05, Compared with Hcy group. % P<0.05, compared with Hcy+CTRP9 group. & P < 0.05 compared with Hcy+Si-CTRP9 group.
Discussion
Atherosclerosis (As) is a chronic compensatory arterial inflammatory response associated with changes in the composition of blood vessel walls and is the main cause of cardiovascular disease (CVD). It is considered to be the common pathological basis of most cardiovascular diseases such as myocardial infarction, stroke, and peripheral artery disease [1].
VSMCs are highly specific cells located in the middle layer of the arterial wall and are the main source of macrophage-like cells and foam cells in As plaques [11,12]. VSMCs are one of the active cells in the plaque proliferation system of As [13,14]. Literature has shown that 70% of plaque components in the plaque formation of As are composed of VSMCs and their derivatives [15,16], and 40% of foam cells, which constitute an important part of lesions, come from VSMCs. They are called smooth muscle derived foam cells [17,18]. VSMCs migrate from the vascular media into the vascular intima and subendothelial layer, then phagocytic lipids, and finally form foam cells, which are the main initial link in the formation of As [19,20]. Although there have been many studies on As, the mechanism of VSMCs migration is not very clear.
Homocysteine (Hcy), also known as homocysteine, is a kind of sulfur-containing amino acid, which is the intermediate product of methionine metabolism. Normally, plasma Hcy levels are very low; When the plasma Hcy concentration is higher than 15 μmol/L, it is called hyperhomocysteinemia (HHcy). Evidence of evidence-based medicine shows that HHcy is an independent risk factor for As [3,4], and Hcy induces As through a variety of pathway interactions and correlations. Every 5 μmol/L increase in plasma Hcy is equivalent to a 0.5 mmol/L increase in cholesterol, and the vascular risk increases by about 1/3 [21]. Studies have shown that Hcy induces the proliferation and migration of VSMCs in rats by down-regulating the expression and activity of metal matrix proteinase 2/9 (MMP-2/9) and tissue inhibitor of metalloproteinase 2(tTIMP-2) in VSMCs, promoting the degradation of the extracellular matrix such as basement membrane, destroying the physiological barrier of VSMCs migration [22]. By promoting the expression of concave protein- 1, Hcy inhibits the activity of endothelial nitric oxide synthase (eNOS) and the production of NO, activates the expression of PI3K and p-Akt, and induces the proliferation and migration of cultured thoracic aorta smooth muscle cells of SD rats in vitro, leading to As [23,24]. These studies indicate that Hcy can affect the extracellular matrix, destroy vascular basement membrane, and activate growth factors and related genes in VSMCs, ultimately promoting the proliferation and migration of VSMCs. At the same time, our research group also found in previous studies that Hcy can induce VSMCs migration and promote the occurrence of As, but the detailed mechanism remains to be further studied. Exploring the possible molecular mechanism of Hcy induced VSMCs migration will provide theoretical basis for the prevention and treatment of hyperhomocysteinemia (HHcy) and Hcy induced As.
The endoplasmic reticulum (ER) is a key site of posttranscriptional modification of lipid synthesis, and ER dysfunction is associated with many disorders of lipid metabolism, including As [5]. When ERs occurs, binding immunoglobulin protein (BiP/Grp78) and the corresponding stress sensor on the endoplasmic reticulum requires inositol-requiring enzyme 1α(IRE1α), Isolation of protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6α(ATF6α), Downstream signalling cascades are activated, and three signalling pathways IRE1α/XBP-1, PERK/eif2α/ATF4 and ATF6α are activated, leading to the occurrence of a variety of diseases. There have been some reports on ERs induced by Hcy. Dall’Acqua S, et al. [25] found that in cultured vascular endothelial cells and vascular smooth muscle cells, Hcy induced ERs activated sterol regulatory element binding proteins, which led to increased gene expression of cholesterol/triacylglycerol biosynthesis and uptake, leading to intracellular cholesterol accumulation. Zhang D, et al. [26] fed CBS-/- mice with a high methionine diet induced the formation of HHcy and led to As and found that the expression levels of ERs marker protein GRP78/94 and pho-PERK were increased in atherosclerotic plaques. These results indicated that ERs-mediated abnormal lipid metabolism was involved in the formation of As caused by Hcy.
C1q/Tumor necrosis factor-related protein9 (CTRP9) is a novel adipocyte factor, which belongs to the tumor necrosis factor-associated protein family (CTRP). Studies have shown that CTRP9 is closely related to cardiovascular diseases such as atherosclerosis, vascular calcification, pulmonary artery sclerosis and reverse heart re-modelling [27]. Studies in human umbilical vein endothelial cells have demonstrated that CTRP9 induces dilatation of the vascular ring in vitro through an adiponectin receptor 1/AMPK/eNOS-dependent NO-mediated signalling pathway [28]. In a mouse model of arterial injury, the administration of the adenovirus mediated CTRP9 gene can significantly reduce the intima/media area ratio after arterial injury, inhibit neointima proliferation, and accelerate re-endothelialisation [29]. In macrophages, CTRP9 protects against atherosclerosis by promoting cholesterol efflux to reduce the formation of foam cell in virtue of inducing autophagy in an AMPK/ mTOR signalling pathway-dependent manner [30]. It is suggested that CTRP9 plays a protective role in both endothelial cells and macrophages. At the same time, pro-inflammatory cytokines are important regulatory factors that inhibit the expression of CTRP9 [31], and pro-inflammatory cytokines play an important regulatory role in the apoptosis induced by ERs [32], suggesting that there is a common molecular basis between CTRP9 and ERs, but whether CTRP9, as an adipocyte factor, has a regulatory role in ERs remains unclear. It is unclear whether CTRP9 also plays a protective role in VSMCs migration of As.
Therefore, it remains unclear whether Hcy, an intermediate product of the methionine acid cycle, is responsible for regulating ERs through CTRP9, which in turn causes VSMCs migration. To gain a better understanding of the relationship between CTRP9 and ERs, targeting these factors could pave a new path towards identifying potential targets for preventing and treating AS.
The study’s findings indicate that the CTRP9 protein expression is decreased during the migration of VSMCs caused by Hcy. Additionally, ERs are present in VSMCs. The overexpression of CTRP9 can hinder ERs and decrease the migration of VSMCs induced by Hcy. Conversely, interfering with CTRP9 has the opposite effect, indicating that CTRP9 has a protective role in the migration of VSMCs induced by Hcy. This role is closely related to CTRP9’s ability to inhibit ERs in VSMCs. Further research revealed that Hcy inhibits CTRP9 through DNMT1 expression’s upregulation. DNMT1 is the primary regulatory enzyme that controls the methylation level in the promoter region of a gene. This suggests that CTRP9 may be subject to epigenetic regulation, such as methylation regulation in its promoter region. This discovery makes our follow-up research even more exciting.
To aid in the prevention and treatment of hyperhomocysteinemia (HHcy), this study has uncovered the molecular mechanism by which Hcy induces the migration of VSMCs. Specifically, it inhibits CTRP9 expression and negatively regulates ERs. These findings provide a valuable theoretical foundation.
Acknowledgment
Thanks to Ningxia Medical University, Key Research and Development Project of Ningxia Hui Autonomous Region Natural Science Foundation (2021BEG03093), and Research Fund Project of Ningxia Medical University (XT2022028).
Author Contributions
ZMH designed the experiments. WXY, MX, and ZY performed the experiments and analyzed the data. WXY wrote the manuscript. All authors have read and approved the manuscript.
Funding
This study was supported by grants from the Key Research and Development Project of Ningxia Hui Autonomous Region Natural Science Foundation (2021BEG03093), and the Research Fund Project of Ningxia Medical University (XT2022028).
Availability of Data and Materials
The analyzed data and material sets generated during the present study are included in this published article.
Ethics Declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Ethics Committee of Ningxia Medical University (NO. 2020-548) and the reporting follows the recommendations in the ARRIVE guidelines.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
References
- Fan JL, Watanabe T (2022) Atherosclerosis: Known and unknown. Pathol Int 72(3): 151-160.
- Grootaert MOJ, Bennett MR (2021) Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res 117(11): 2326-2339.
- Fanapour PC, Yug B, Kochar MS (1999) Hyperhomocysteinemia: an additional cardiovascular risk factor. WMJ 98(8): 51-54.
- Guieu R, Ruf J, Mottola G (2022) Hyperhomocysteinemia and cardiovascular diseases. Ann Biol Clin (Paris) 80(1): 7-14.
- Yu XH, Zhang DW, Zheng XL (2019) Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog Lipid Res 73: 65-91.
- Du Y, Zhang S, Yu H, Wu Y, Cao N, et al. (2019) Autoantibodies Against β1-Adrenoceptor Exaggerated Ventricular Remodelling by Inhibiting CTRP9 Expression. J Am Heart Assoc 8(4): e010475.
- Sun FX, Zeng Y, Zhang MH (2020) The Changes of CTRP9 Expression during the Proliferation, Migration and Phenotypic Transformation of Vascular Smooth Muscle Cells Induced by Homocysteine. J Mol Cell Cardiol 43(11): 1115-1119.
- Guo W, Zhang H, Yang A, Ma P, Sun L, et al. (2020) Homocysteine accelerates atherosclerosis by inhibiting scavenger receptor class B member1 via DNMT3b/SP1 pathway. J Mol Cell Cardiol 138: 34-48.
- Zhang MH, Wang XY, Bai B, Zhang R, Li Y, et al. (2016) Oxymatrine protects myocardial injury against sepsis via inhibition of TNF-α/p38-MAPK/caspase-3 signalling pathway. Molecular Medicine Reports 14(1): 551-559.
- Zhang MH, Wang XY, Li F, Gong J, Xian Y, et al. (2020) MiR‑145 alleviates Hcy‑induced VSMC proliferation, migration, and phenotypic switch through repression of the PI3K/Akt/mTOR pathway. Histochem Cell Biol 153(5): 357-366.
- Bennett MR, Sinha S, Owens GK (2016) Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 118(4): 692-702.
- Grootaert MOJ, Bennett MR 92021) Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res 117(11): 2326-2339.
- Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z (2019) Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16(12): 727-744.
- Zhu JM, Liu B, Wang ZY, Wang D, Ni H, et al. (2019) Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 9(23): 6901-6919.
- Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, et al. (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6): 628-637.
- Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA (2014) Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129(15): 1551-1559.
- Susanne F, Birgit F, Robert L, Essmann F, Schulze-Osthoff K, et al. (2014) Trans differentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115(7): 662-667.
- Chaabane C, Coen M, Bochaton Piallat ML (2014) Smooth muscle cell phenotypic switch: implications for foam cell formation. Curr Opin Lipidol 25(5): 374-379.
- Wolf MP, Hunziker P (2020) Atherosclerosis: Insights into vascular pathobiology and outlook to novel treatments. J Cardiovasc Transl Res 13(5): 744-757.
- Qi YX, Han Y, Jiang ZL (1097) Mechanobiology and vascular remodelling: from membrane to nucleus. Adv Exp Med Biol 1097: 69-82.
- Held C, Sumner G, Sheridan P, McQueen M, Smith S, et al. (2008) Correlations between plasma homocysteine and folate concentrations and carotid atherosclerosis in high-risk individuals: baseline data from the Homocysteine and Atherosclerosis Reduction Trial (HART). Vasc Med 13(4): 245-253.
- Meng LP, Liu LB, Zhou CZ, Pan S, Zhai X, et al. (2016) Polyphenols and polypeptides in chinese rice wine inhibit homocysteine-induced proliferation and migration of vascular smooth muscle cells. J Cardiovasc Pharmacol 67(6): 482-490.
- Bao XM, Zheng HC (2015) Effect of homocysteine on proliferation and migration of vascular smooth muscle cells in rats and its possible mechanism. Shandong Med 55(44): 25-27.
- Ji X, Wang X, Yue XL (2017) Effects of eNOS, CAV1 and PI3K/Akt signalling pathways on homocysteine promoting migration and proliferation of vascular smooth muscle cells in rats. Chinese General Practice 20(12): 1469-1473.
- Dall'Acqua S, Bolego C, Cignarella A (2011) Vasoprotective activity of standardized Achillea millefolium extract. Phytomedicine 18(12): 1031-1036.
- Zhang D, Chen Y, Xie X, Liu J, Wang Q, et al. (2012) Homocysteine activates vascular smooth muscle cells by DNA demethylation of platelet-derived growth factor in endothelial cells. J Mol Cell Cardiol 53(4): 487-496.
- Yu XH, Zhang DW, Zheng XL (2018) C1q tumor necrosis factor-related protein 9 in atherosclerosis: Mechanistic insights and therapeutic potential. Atherosclerosis 276: 109-116.
- Liu Q, Zhang H, Lin J, Zhang R, Chen S, et al. (2017) C1q/TNF-related protein 9 inhibits the cholesterol-induced Vascular smooth muscle cell phenotype switch and cell dysfunction by activating AMP-dependent kinase. J Cell Mol Med 21(11): 2823-2836.
- Li J, Zhang P, Li T, Liu Y, Zhu Q, et al. (2015) CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages. Biochem Biophys Res Commun 58(4): 890-895.
- Zhang L, Liu Q, Zhang H, Wang XD, Chen SY, et al. (2018) C1q/TNF-Related Protein 9 Inhibits THP-1 Macrophage Foam Cell Formation by Enhancing Autophagy. J Cardiovasc Pharmacol 72(4): 167-175.
- Zhang H, Gong X, Ni S, Wang Y, Zhu L, et al. (2019) C1q/TNF-related protein-9 attenuates atherosclerosis through AMPK-NLRP3 inflammasome singling pathway. Int Immunopharmacol 77: 105934.
- Li W, Cao T, Luo C, Cai J, Zhou X, et al. (2020) Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol 104(14): 6129-6140.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.