Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Testing For Dissolved Oxygen Levels, pH, and Nitrate and Phosphate Concentration in a Water Sample from the American River
*Corresponding author: Ashwin Sidhu, California Northstate University College of Medicine, 9700 W. Taron Dr., Elk Grove, CA, USA. ORCID ID: 0000-0001-7531-0095.
Received: August 07, 2023; Published: August 14, 2023
DOI: 10.34297/AJBSR.2023.19.002637
Abstract
This project conducts research on the clam species, Corbicula fluminea. Results were obtained from the collection of the water from the American River. This research is significant because the Asian clam is an invasive species, and it is essential to understand how chemical factors encourage its population growth. In other words, the clam species is studied to better understand why it can survive and reproduce at a high rate. Specifically, in the experiment, the water of the American River was studied. The water samples from the American River were collected in October and March and different methods were used to conduct testing on the water samples. The methods used were pH meter, dissolved oxygen probe, nitrate and phosphate kits, and spectrophotometry. Since the results from each water sample were different, conclusions were made about what causes the change in water conditions. The effects of the water were applied to the Asian clam, Corbicula fluminea, to see how they can survive and live in such water conditions. For example, the phosphate and nitrate in the water provides benefits to the clams, however, too much of either can have detrimental effects on the clam species. These quantities of chemicals in the water are crucial to this project because they impact the survival of the Asian clam or Corbicula fluminea. From experimentation, it was found that the water in the American River has normal conditions, which allows the clam species, Corbicula fluminea, to thrive.
Keywords: Corbicula fluminea, Invasive species, American river, Dissolved oxygen, Nitrate testing
Introduction
The American River is a river that runs in California from the Sierra Nevada Mountain Range to its connection with the Sacramento River. The American River is further connected to the San Francisco Bay watershed by the Sacramento River. The main source of water for the river is the melting snow from the Sierra Nevada and its many tributaries, such as the North Fork American River and the South Fork American River [1]. The American River is dammed to ensure flood control and for irrigation. In addition, the American River is a home to various wildlife and fish. One of the more widespread and invasive marine life in the river is the clam species, Corbicula fluminea. The clam species has no natural predators and finds itself competing with native species for space and nutrients in the river. This can cause many problems for native plant life and the ecosystem.
The Asian clam, or Corbicula fluminea, is an invasive species that exhibits rapid growth in freshwater ecosystems around the world. The Asian clam, as stated by its name, is indigenous to Eastern Asia but has spread throughout the world. The Asian clam, a bivalve mollusk, has spread to areas, such as Africa, Europe, and the United States. In fact, the Asian clam is considered one of the worst invasive species in the United States. The Asian clam is associated with many damages and needs extensive management. For instance, the clam species generates around $1 billion of damages per year around the world [2]. The reason for its invasive nature is the fact that the clam species grows rapidly, has a short lifespan, has early sexual maturity, has an extreme dispersal capacity, and high fecundity [3].
There are certain aspects of the clams that make them efficient in reproducing and dispersing throughout waterways. Asian clams are hermaphroditic, which means that they can self-fertilize. When clams fertilize there are thousands of larvae, even though the survival rate is very low. The nutrient rich environment protects the larvae, and the water allows the larvae to disperse easily throughout the water. When the clams are juveniles, they can anchor to hard surfaces are subjected to even more dispersal [4]. This explains how the clams are to spread throughout the waterways and become invasive species that grow rapidly.
Asian clams live in high density populations in their aquatic habitats. To survive, the Corbicula fluminea need well oxygenated rivers or lakes. Also, they can live in a specific pH range of water as well as nitrate and phosphate concentration. Nitrate concentration is important to track in waterways because high nitrate levels leads to algae growth and poor fish health. Also, phosphorus is a very important element for the marine environment. Phosphate helps with the development of bones and shells. Thus, phosphorous in waterways helps marine life develop and grow. However, phosphate levels in the American River, must be strictly controlled, because levels that are too high can cause uncontrollable algal blooms. Also, high levels of phosphate can hinder the shell growth of coral and other invertebrates. Furthermore, for this project, it is important to understand that oxygen concentration in water depends on several factors. Some factors include the salinity of the water, temperature, and depth.
Throughout the experiment, the river water sample will be studied to determine the living conditions of the Asian clams in the American River. In this experiment, spectrophotometry with the water in the American River will be used. Furthermore, the concentration of nitrate in the water will be explored using a spectrophotometer. Spectrophotometry is a method that allows for the measurement of a chemical substance’s absorbance of light [5]. During experimentation, it is important to know that solutions containing nitrates absorb UV light at 220 nanometers (nm). By conducting the experiment, it will be explained why the clams are able to live and reproduce at such high rates. In other words, by studying the water that the clams live in, in can be determined how the Asian clams act as invasive species. By understanding the invasive nature of the clams, actions can be taken to prevent or lessen the effects of the clams in the ecosystem.
Procedure and Materials
This research project had multiple parts. The parts consisted of retesting the dissolved oxygen levels, pH levels, and nitrate and phosphate levels of the newly collected sample of water. Also, another part of the research consisted of using the spectrophotometer to get a more accurate reading for nitrate level in the water sample from the American River. The main materials needed for this project were the dissolved oxygen meter, pH meter, nitrate test kit, phosphate test kit, the spectrophotometer, and the water sample from the river. Of course, personal protective equipment was needed as well.
Dissolved Oxygen Testing
To conduct the dissolved oxygen level measurement for the water, the dissolved oxygen meter was obtained and zeroed out before it could be used. The system used for the dissolved oxygen probe was LabQuest. Distilled water was used to “zero out” the dissolved oxygen meter. First, the dissolved oxygen level of distilled water was obtained (6.6mg/L). Next, the dissolved oxygen level of the river water sample was found using the meter (7.8mg/L).
pH Testing
Second, pH meter was obtained and calibrated using the pH calibration buffers. There were three calibration buffers corresponding to three different pH: 4, 7, and 10. The pH calibration buffer for a pH of 4 was a pink color. Also, the pH calibration buffer for a pH of 7 was a yellow color, while the calibration buffer for a pH of 10 was blue. Once the pH meter was calibrated, the pH of the water sample and tap water were taken. First, the pH of the lake water was tested (7.22). Next, the pH meter was used to get the pH of the tap water (7.34). In addition to the pH the pH meter also gave the temperature of the water sample.
Nitrate and Phosphate Testing Using Kits
Third, the nitrate test and phosphate test were conducted on the water sample from the river as well as distilled water. To begin this testing, the nitrate test kit and phosphate test kit were obtained. The nitrate test kit and phosphate kit were from the brand, Aquarium Pharmaceuticals (API). To completely and successfully do the tests, the directions on each kit were followed and the final color of the test tube was compared to the color cards. In other words, once the directions on the nitrate specific kit were followed, the test tube was a yellow color which corresponded to a specific concentration of nitrate in the river water (2.5mg/L). Once the phosphate test was done, the test tube was a bluish-green color, which corresponded to a specific concentration of phosphate in the river water (0.25mg/L).
Nitrate Testing Using Spectrophotometer [6]
Finally, the nitrate concentration was determined by spectrophotometry in an attempt to get more accurate results. Nitrate calibration standards were prepared with the volume of each standard at 50mL. Throughout this part, distilled water was used to dilute each standard. Six total standards were created using sodium nitrate (NaNO3) and distilled water. The first standard was 1M NaNO3, which was already pre-prepared, so it just had to be poured into a beaker. Using the 1M NaNO3 and distilled water, the equation M1V1=M2V2 was used to created standards of 0.5 M NaNO3, 0.25 M NaNO3, 0.125 M NaNO3, and 0.05 M NaNO3. The last standard was made using NaNO3 powder. Calculations were done as to how many grams of NaNO3 powder was needed to make 0.002M NaNO3. Water was used to ensure the total volume to be 50mL for each standard. After, 1mL of 1M HCl was added to all six standards. Next, the spectrophotometer was set up to test all samples at 220nm and 275nm. It was important to blank with distilled water before every run. All the samples were tested in quartz cuvettes and the absorbance values were compiled on the computer.
Data Collection and Data Processing
The water samples were collected from the American River. The data for the spectrophotometry component of the project was processed on the computer with the absorbance values being automatically transferred to excel. Excel was used to precisely input the data from the spectrophotometer.
Data/Results
pH Testing
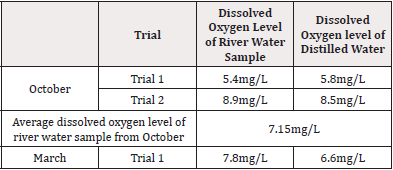
Table 1 shows the pH of the lake water that was obtained from the pH meter. The water sample collected in October shows the pH to be 7.97, while the pH of the water sample collected in March was 7.22. The pH of the river water seems to be lower according to the pH meter. pH meter most likely provided a more accurate reading of the pH. It is necessary to use the pH meter instead of the pH strips because the strips were not as accurate. For example, possible error could be not correctly interpreting the color of the strips. It is important to note that the pH differs between the months. While the temperature of the water is an important aspect of living conditions for the clams, the temperature of the water is reported as basically the room temperature. It will be ideal to take the temperature of the water at the source (Table 1).
Dissolved Oxygen Testing
Table 2 shows the dissolved oxygen levels of the lake water sample and distilled water. For the water sample from October, two trials were done to ensure accurate results and the average was taken for the dissolved oxygen level in the river water. Despite the two trials, the results were completely different. Trial1 gave the dissolved oxygen level of river water to be 5.4mg/L and the dissolved oxygen level of distilled water to be 5.8 mg/L. Trial 2 gave the dissolved oxygen of the river water sample to be 8.9mg/L, while the dissolved oxygen level of distilled water was 8.5mg/L. The dissolved oxygen concentration of water should be around 8mg/L. In comparison, the river water sample from March gave results between the two from October. The dissolved oxygen level of river water was 7.8mg/L, while the dissolved oxygen level of distilled water was 6.6mg/L. The distilled water acted as a control (Table 2).
Nitrate and Phosphate Testing Using Kits
Table 3 shows the nitrate concentration in the lake water and distilled water. The nitrate concentration level stayed the same. Specifically, the nitrate concentration level was 2.5mg/L in both October and March. However, these are just estimates because the kits are not the best tools to use (Table 3).
Also, Table 3 shows the phosphate concentration levels in the lake water sample and distilled water. According to the data, the phosphate concentration level went down in March when compared to October. The river water sample had a higher concentration of phosphate than the distilled water. Specifically, the lake water sample had 1.5mg/L of phosphate and distilled water had 0mg/L of phosphate. In comparison, the March water sample has 0.25mg/L concentration of phosphate (Figure 1).

Figure 1: This shows the nitrate and phosphate test being conducted. The yellow solution represents the nitrate test, while the bluish-green solution represents the phosphate test. The nitrate concentration on the card that corresponded to the color in the test tube was 2.5mg/L. The phosphate concentration was 0.25 mg/L.
Nitrate Testing Using Spectrophotometer
Table 4 shows the absorbance values for the six standards. The six standards are shown with the ID4-9. Also, in Table 4 the absorbance values for the blanks is shown. Each standard was tested at two wavelengths. The two wavelengths were 220nm and 275nm. From the table it seems that the concentration of nitrate in the river water sample is less than 0.002 M. However, there was not enough information to get an exact value (Table 4).

Table 4: Nitrate concentration determination in river water using spectrophotometer and absorbance values.
Scheme 1: Amount of sodium nitrate, in grams, needed to make 0.002 M sodium nitrate from
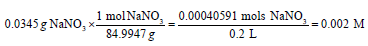

Based on the calculations, 0.0272grams of sodium nitrate powder was added to 200 mL of water. Hence, the 0.2L in the calculation comes from the 200mL of water. In other words, 200mL is equal to 0.2L. The conversion from moles to grams was needed, so it would be known how much of sodium nitrate was needed for the standard.
Discussion
The purpose of this research was to get a better understanding of the Asian clam and the habitat it lives in. Specifically, this project was conducted to get information on the water conditions that the clams live in. Using the information obtained, conclusions were able to be made about the environment of the American River and how it affects the clams.
When the pH of the river water sample from March was taken, the pH came out to be 7.22. This is in comparison to the pH of the water sample from October. As shown by Table 1, the sample from October gave a pH reading of 7.97. The water samples in both months is above 7, so the samples are not exactly neutral. In fact, the pH of a neutral substance at room temperature is 7 [7]. There can be many reasons for this change in pH. One likely reason for this small decrease in pH between the two months is possible discharge. The drainage of acid sulfate soils leads to the increase of acidity in water. For instance, river acidity can increase when there was an extremely dry weather [8]. The fall in California was dry and there was not much rain. Since the season was dry, the rain in the winter and spring may have caused runoff into the river that may have slightly lowered the pH of the river water. While the pH changed very slightly, and the water is still considered “safe,” further reduction in pH of the water can have hazardous effects on the habitat of the clam. For example, the Asian clam “can tolerate pH as low as 5.4 [and] can survive wide temperature ranges between 2-36℃”3. The Asian clam can survive a pH as low as 5.4. So, the pH change of the river water shows no reason for concern. However, it is possible that the pH of the river water will continue to decrease. If the pH of the water does get lower and lower, governmental regulations will need to be implemented to save the river water from dropping low enough to kill aquatic life.
Furthermore, the dissolved oxygen of the river water in March came out to be 7.8mg/L. This is shown in Table 2 and it can be compared to the dissolved oxygen in the water sample collected in October. In October, the dissolved oxygen concentration was 7.15mg/L, on average, between the two trials. For instance, “oxygen represents 21% of the gases in the atmosphere but in the marine environment, its concentration is only 4-8 ppm.”6 The data in Table 2 is in mg/L, but Parts Per Million (ppm) is the same. Thus, the dissolved oxygen level in the water sample can be compared to the information given in parts per million. Based on this information, the dissolved oxygen level in the American River is normal. Even though the dissolved oxygen is on the higher side of the normal conditions, the clams are still able to thrive in the ecosystem. It is essential that the dissolved oxygen is normal because organisms, such as the Asian clam, all need a supply of oxygen for respiration and metabolism. Therefore, oxygen concentration in the water can affect the ecosystem severely.6 One possible reason for the difference in dissolved oxygen level is the temperature. It is known that the solubility of oxygen decreases as water temperature increases. This makes sense because the climate was hotter in October than it was in March this past year. Thus, the higher temperature in the October led to a lower solubility of oxygen in the American River. Nonetheless, the dissolved oxygen in the river allows the clams to grow and reproduce without any obstructions from chemical factors.
Phosphorus and nitrate are critical for the success of marine environments. Phosphorous and nitrate play important roles in the development and survival of plants and marine life. According to Table 3 and 4, the nitrate concentration is normal in the water. In fact, the Environmental Protection Agency has set the Maximum Contaminant Level of nitrates at 10mg/L (or parts per million) [9]. The value shown in Table 3 gives a nitrate concentration of 2.5mg/L in both October and March. So, the nitrate levels are normal. If the nitrate levels were not normal in the American River, this would suggest the presence of contaminants in the water. Some possible contaminants may include pesticides, or other inorganic and organic compounds that could cause health problems to humans, as well as the clams and other marine life. Also, the phosphate levels in the water sample were normal as well. The “acceptable levels of phosphate are between 0.1 and 3.0ppm.”6 As shown in Table 3, the phosphate concentration in October was 1.5mg/L and the concentration in March was 0.25mg/L. In other terms, the phosphate levels were 1.5ppm and 0.25ppm, respectively. Thus, this confirmed that the phosphate level was normal in the American River.
The errors in this experiment were very limited. One major error could have been the inaccurate measurement of the standards. If the measurements were not correct, then the absorbance values obtained from the spectrophotometer would not be accurate. Also, the kits do not give very accurate readings. The spectrophotometer was used to get better readings for the nitrate concentration. While the results did give an estimate range for the nitrate concentration, an exact value was not given. Nonetheless, the minor errors did not limit experiment or ability to make conclusions.
Conclusion
Throughout the experiment, the habitat and environment of the Asian clam was studied by conducting experiments for pH of water, dissolved oxygen levels of water, nitrate levels, and phosphate levels. The Asian clam needs specific water conditions to live. In fact, it is due to the water conditions and ecosystem that makes the clam an invasive species. Without the many resources available to it, the clam would not be able to act as an invasive species. Between the months October and March, it seems that the water changed in many aspects, such as phosphate concentration, pH, and dissolved oxygen. There are numerous possible reasons for the results that occurred. However, one reason is possibly a dry fall season, that lead to the buildup of acidic soil that may have runoff when it rained in the winter and spring. Another reason for the differences in the values of dissolved oxygen was due to the temperature differences between the two months. October was a hotter month than March this past year. Overall, the pH, dissolved oxygen, and nitrate and phosphate levels in the water show no reason for concern, which is why the C. fluminea can spread throughout the Sacramento waterways.
Acknowledgement
Thank you to California North state University College of Health Sciences for providing the necessary materials as well as the mentorship in the completion of this research project.
Conflict of Interest
None.
References
- (2018) The American River. About the American River.
- Araujo R, Moreno D, Ramos MA (1993) The Asiatic clam Corbicula fluminea (Bivalvia: Corbiculidae) in Europe. American Malacological Bulletin: 39-49.
- Sousa R, Antunes C, Guilhermino L (2008) Ecology of the invasive Asian clam Corbicula fluminea in aquatic ecosystems: an overview. International Journal of Limnology 44: 85- 94.
- McMahon RF (2000) Invasive characteristics of freshwater bivalve Corbicula fluminea. Nonindigenous freshwater organisms: vectors, biology, and impacts.
- Zumdahl SS, Zumdahl SA (2015) Chemistry: An Atoms First Approach, Hybrid Edition, 2nd ed.; Cengage Learning: Boston, MA: A16-17.
- Austin RN (2013) Chemistry 108b Laboratory Manual; Bates College: Lewiston, ME, Chemical Reactivity in the Marine Environment.
- Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, et al. (2014) Campbell Biology, 10th ed.; Pearson Education: Glenview, IL: 51-52.
- Saarinen TS, Kløve B (2012) Past and future seasonal variation in pH and metal concentrations in runoff from river basins on acid sulphate soils in Western Finland. Journal of Environmental Science and Health. Part A, Toxic/hazardous Substances & Environmental Engineering 47(11): 1614-1625.
- (2018) Benton Franklin Health District. What are nitrates?





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.