Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Use of Localized Human Growth Hormone/Testosterone Injections for Chronic Shoulder Pain: A Case Series, with 12-Month Follow-up
*Corresponding author: David Morrisette, Department of Physical Therapy, Medical University of South Carolina, 151 B Rutledge Avenue, Charleston, SC 29425, USA.
Received: June 19, 2023; Published: July 19, 2023
DOI: 10.34297/AJBSR.2023.19.002612
Abstract
Background: The objective of this case series was to investigate the feasibility and safety of a new injection technique for the treatment of chronic shoulder pain. Injections of recombinant human growth hormone and testosterone to the shoulder capsular ligaments in individuals with chronic shoulder pain who presented with proposed capsular pain and/or laxity were performed.
Case Presentation: Consecutive patients with chronic shoulder pain received the intervention of injections of recombinant human growth hormone and testosterone, followed by exercise. Outcome was measured at 12 months from the completion of the injections. A total of 23 consecutive patients attending pain management practice for chronic shoulder pain were recruited for the experimental treatment. Those who met the criteria during the physical examination and responded favorably to the diagnostic injections received injections of recombinant human growth hormone and testosterone in the areas discovered with the diagnostic injections. Individualized exercise therapy programs were followed after the injections. Outcomes were assessed at 12 months through the Shoulder Pain and Disability Index, the Shoulder Pain Score, the Mankowski Pain Sale, and the patent’s self-rated percent improvement. Of the 23 consecutive patients who met the inclusion critieria, 17 (19 shoulders) provided informed consent and 14 (16 shoulders) completed all aspects of the study. Those patients who completed the study reported a significant decrease in pain ratings (p<.0001), and a significant improvement in self-rated SPADI (p<.0001) and SPS (p<.0001). Of the patients who withdrew from the study, two underwent surgery prior to the 12-month time frame and one switched to another interventional therapy prior to the 12-month follow-up.
Conclusion: The injection procedure demonstrated safety and effects warranting further study.
List of Abbreviations: HGH: Human Growth Hormone; Testos: Testosterone; IGF-1: Insuline like Growth Factor; MPS: Manoski Pain Scale; SPDI: Should Pain and Disability Index; SPS: Shoulder Pain Scores
Background
Chronic shoulder pain is a common condition which involves long term pain and disability. Common problems associated with chronic shoulder pain involve tears and fraying of the rotator cuff tendons, the labrum, and involvement of the joint capsule. Most of these conditions are due to degeneration or injury to dense connective tissue, primarily composed of ground substance and collagen. Recent literature indicates that “micro instability” may play a role in the development of glenohumeral pathology including tendinopathy and labral lesions [1-3]. An intervention that would lead to intrinsic repair and strengthening of the involved dense connective tissues would be expected to have benefit in regard to pain and an improvement in function.
The Orth biologic/Regenerative Medicine revolution is expanding treatment for chronic pain. Chronic musculoskeletal conditions involving joint, tendon, ligament and cartilage are the primary targets. Regenerative medicine involves the use of bioactive cells or factors to stimulate healing and remodeling of pathological tissues. The most commonly studied procedures have been proliferative therapy [4-9], injections with platlet-rich plasma. [4,6,7,10, 11], and increasingly, stem cell injections [12] and other tissue engineering interventions [13].
An emerging area of regenerative medicine is the use of human growth hormones and other anabolic agents. A few numbers of both animal and human studies have been published, with somewhat mixed results regarding the efficacy of these hormones in facilitating injury repair on musculoskeletal tissues [6,11,14-18]. Demonstrating the ability for the use of potent anabolic agents to improve clinical outcomes in the management of diagnosed chronic musculoskeletal conditions can be a significant advancement in the treatment of these conditions.
In a previously reported study involving the treatment of chronic low back pain, localized injections of recombinant human growth hormone (rhGH) and testosterone were found to lead to an improvement in pain and function [19]. It was hypothesized that this was due at least in part to an improvement in the integrity and strength of the treated ligaments, tendons, and joint capsules. RhGH and testosterone are both endogenous anabolic hormones that stimulate protein synthesis [20,21] Research describing the mechanism of action of rhGH and testosterone has been previously described [19] It is felt that the localized injection of rhGH and testosterone interact synergistically at the injection site to stimulate protein synthesis, leading to formation of new collagen and ultimately mature connective tissue. In conjunction with the injections, controlled exercise would be expected to positively influence the remodeling of the dense connective tissue, while possibly preventing contractures.
Case presentations
This is a 12-month follow-up case series using a one-group pretest posttest design regarding patients seen in a private pain management clinic. This study is designed to provide preliminary data and determine if a randomized control trail is warranted in the future.
Participants received a standardized shoulder examination by an anesthesiologist/pain management physician. Participants had chronic shoulder pain, with the examination suggesting primarily capsular/tendon or labral involvement with or without associated acromioclavicular joint or rotator cuff involvement in the production of symptoms.
The study was approved by and performed under the auspices of the Institutional Review Board (IRB) of Roper St. Francis Healthcare, Charleston, SC, USA. Data were monitored by a Data Collection Site Monitoring Board and Adverse Experience Reporting Committee. All participants provided informed consent. The outcomes and intervention data used to support the findings of this study are restricted by HIPAA in order to protect patient privacy. Data are available from the primary author for researchers who meet the criteria for access to confidential data and with approval from the IRB of Roper St. Francis Healthcare. No external funding was involved with this case series.
Participants
Participants were 23 consecutive eligible adult participants, aged 18-85 with chronic shoulder pain, serving as a convenience sample during the interval of June 2009 through June 2011. Inclusion and exclusion criteria are listed in Table 1.
Five participants had undergone previous shoulder surgery, with one having undergone two previous surgeries. The surgeries included three for rotator cuff repair, two for arthroscopy and debridement including a SLAP lesion repair. Seven of the shoulders had a history of previous dislocations, and two participants had been diagnosed with hypermobility syndrome. The participants had received prior therapies including trigger point injections, subacromial and gleno-humeral joint steroid injections, physical therapy, chiropractic adjustments, and pharmacological pain management. The previoius interventions were not consistent across participants. Participant’s demographics are in Table 2.
The examination included active/passive range of motion in abduction and flexion, grip strength, Obrien’s Active Compression test, glenohumeral external rotation apprehension test, rotator cuff testing for weakness and/or pain, deltoid testing for weakness and/ or pain, passive horizontal adduction of the glenohumeral joint for acromioclavicular joint (ACJ) pain, palpation of the coracoid process, ACJ, bicipital groove, greater tuberosity, and bicep and triceps strength testing.
To be included in the cohort, the Obrien’s Active Compression test and/or the apprehension test demonstrated reproduction of familiar symptoms or aberrant motion. Local anesthetic injections were performed under fluoroscopic guidance in the region of tenderness and suspected pathology. When the ACJ or rotator cuff findings were positive, injections were performed at the ACJ capsule, coraco-clavicular ligament, or the region of the humeral attachment of the affected rotator cuff muscle. Subjective relief of symptoms by 50% or more and improved strength testing indicated continuation in the study. All patients included met this criterion./p>
Intervention
Each participant meeting the inclusion criteria received a series of injections of rhGH/testosterone to the same areas as the local anesthetic was injected. The injections were performed by a board-certified, hospital-affiliated anesthesiologist/pain management physician under fluoroscopic guidance.
The injections consisted of recombinant rhGH (Sandoz International, Holzkirchen, Germany, 3u/10 cc volume), aqueous testosterone (12.5 mg for females, 25 mg for males, per 10 cc volume), 5 cc of 1% preservative-free procaine, and 0.9% NaCl to a total volume of 10 cc. The injections were performed at 3–4-week intervals and averaged 3-4 in number. Approximately 3, 10 cc syringes were administered at each injection visit. The number of injections needed for each participant was determined by improvements noted in Obrien’s and apprehension tests, as well as the tissue feel during injections. It is noted by the primary author that healthy and healing ligament and tendon tissue presented with a dense feel to syringe pressure, while degenerative/weakened ligament or tendon tissue has more of a soft feel.
Once able, the participants performed graded resisted exercise as tolerated and range of motion activities. Individualized exercise programs included the following:
a) Pilates exercise with a certified instructor
b) Physical therapy for range of motion, motor control and resistance exercise
c) Self-administered treatment at a fitness center or home
d) Working with a personal trainer at an exercise facility
e) After completion of the injections, the participants were followed for 12-months.
Outcome Measures
The primary outcome was determined by the Shoulder Pain and Disability index (SPADI) [22]. Secondary outcomes were the Shoulder Pain Score (SPS), [23] the patients pain rating from 0-10 using the Mankowski Pain Scale (MPSI), [24] and the participants reported overall percentage change in presenting symptoms [25]. The SPADI has been used to measure self-rated function across a number of shoulder diagnoses [26-30] The questionnaire is based on a score of 100, with the minimal detectible change (MDC 95%) described as 18 points [31].
The Shoulder Pain Score assesses the patient’s self-rated shoulder pain. This pain score has been found to provide a reliable rating of pain and is useful to follow the improvement in pain from the initial visit to the post treatment interval [32].
The MPSI was used as the pain rating scale. It is a 0-10 pain rating scale that links written descriptors of the intensity of the pain to a numerical value. It has been shown to be a valid and reliable tool for pain measurement, as compared to the Faces Scale, the Visual Analog Scale, and the Numeric Rating Scale [24]. The minimal clinically important difference for the change score has not been determined, but it has been reported for the Numeric Rating Scale as two points [33].
Testosterone levels were measured as total testosterone, and rhGH levels measured as IGF-1 at the beginning of the study in order to determine if baseline blood levels were a positive variable related to the outcome measures.
Statistical Analysis
All analyses were performed using SAS version 9.4 (Cary, NC). Statistical significance was defined at α=0.05. Descriptive statistics were used to compare the characteristics of the study sample. Means and standard deviations were calculated to describe continuous data. Frequencies and percentages were calculated to describe categorical data. An intention to treat analysis was used, with the last value carried forward for the patients who did not complete the injections.
The primary outcome measure was a change in SPADI scores. Secondary outcome measures were changes in SPS and MPSI scores. Changes in scores from baseline were assessed for normality visually using QQ-plots and statistically using the Shapiro-Wilk test. All three instrument scores were found to be normally distributed: SPADI (p=0.3531), SPS (p=0.0880), and MPSI (p=0.1243). Changes in scores were tested using paired Student’s t-tests.
Results
Raw outcome data is reported in Tables 3, 4, and 5. Of the 23 eligible patients, six chose not to enter the treatment program. Figure 1. Of the remaining 17 eligible participants, all provided informed consent to enter the study. All phases of the study including the 12-month follow-up were completed by 14 participants (16 shoulders). Of the three who did not complete the study, one completed injection but started another injection protocol near the six-month follow-up. The patient did not feel that progression was quick enough. One underwent surgery prior to completing the injections, and one underwent surgery shortly after completing the injections. Both surgical procedures were performed because of continued pain and decrease function, related to the original complaint.
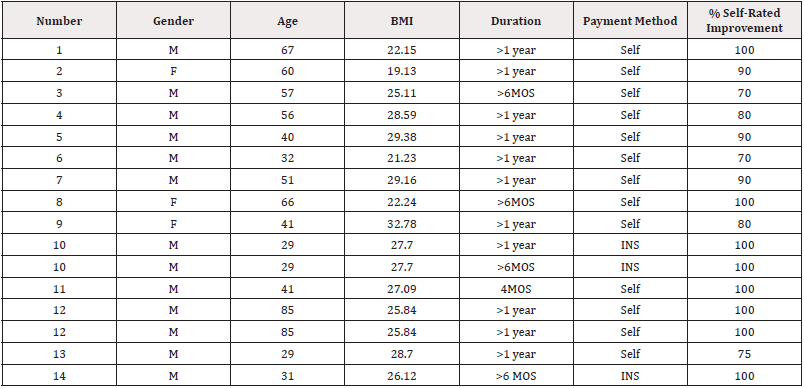
Table 3: Intake and post-treatment data.
Note*: Duration is the duration of shoulder pain for the present episode. The percent self-rated improvement is the patient’s perception of improvement at 12-months following the first injection. BMI is body mass index.

Table 4: Standardized Outcomes.
Note*: SPS is the Shoulder Pain Score. SPADI is the Shoulder Pain and Disability Index score. MPS is the Mankowski Pain Scale score. Side is the side of the body receiving injections.

Table 5: Human growth hormone, insulin-like growth factor and testosterone levels.
Note*: Mean HGh/inj and Test/inj volumes of HGh and testosterone used with injections. The patient reported events related to the development of shoulder pain. IGF-1 is the baseline level of insulin like growth factor with blood testing, and Testos is the base line level of testosterone. No correlation was seen with base line blood levels and results.
Across the19 shoulders (17 patients) at the 12-month follow- up, statistically significant mean decreases in Shoulder Pain and Disability Index, Shoulder Pain Score and pain severity (Mankowski Pain Scale) were found. There was also a significant improvement in the participants’ report of symptoms with an overall self-improvement of 76%.
The primary outcome measure of change in SPADI was found to significantly change from baseline, with a mean reduction in score of 31.8 points (95% Confidence Interval (CI): 20.7-43.0; p<.0001). Figure 2. The SPS also changed from baseline, with a mean reduction in score of 36.7 points (95% CI: 24.0-49.4, p<.0001). Figure 3. Finally, the MPSI showed a change from baseline, with a mean change in score of 3.1 points (95% CI: 2.0-4.2, p<.0001). Figure 4.
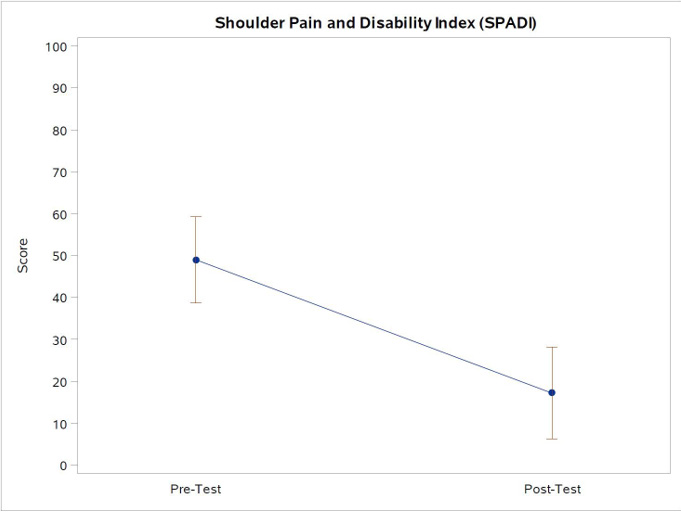
Note*: Figure 2 Shown above are the point estimates for the mean scores and 95% confidence limits for the SPADI scores, which shows a mean reduction of 31.8 points (95% CI: 20.7-43.0, p<.0001). The minimal detectable change is 18.1 points, with a minimal clinically important difference of 13.2 points (Schmitt & Di Fabio, 2004).
Figure 2: Shoulder Pain and Disability Index (SPADI) scores pre- and post-test.
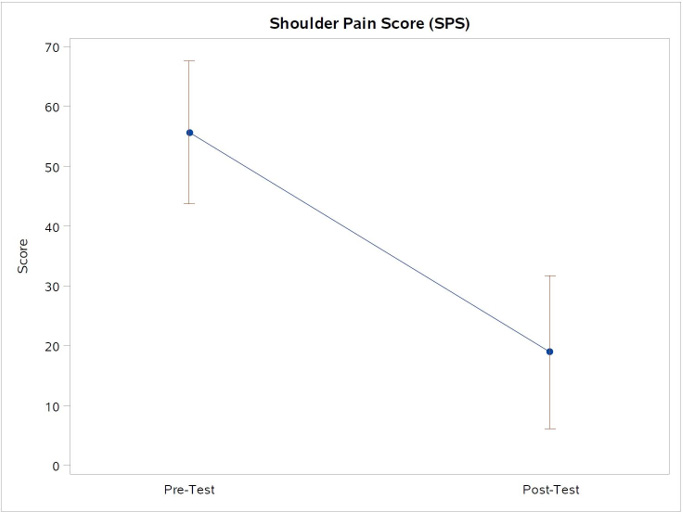
Note*: Figure 3. The point estimates for the mean scores and 95% confidence limits for the SPS scores, which shows a mean reduction of 36.7 points (95% CI: 24.0-49.4, p<.0001).
Figure 3: Shoulder Pain Scores (SPS) scores pre- and post-test.

Note*: Figure 4. The point estimates for the mean scores and 95% confidence limits for the MPSI scores, which shows a mean reduction of 3.1 points (95% CI: 2.0-4.2, p<.0001).
Figure 4: Mankowski Pain Scale Index (MPSI) scores pre- and post-test.
There were no significant correlations between study entry IGF-1 or testosterone levels and changes in the four outcome variables at 12-months. No adverse reactions related to the study were reported during the course of the treatment or at the follow-up sessions.
Discussion and Conclusiont
The positive outcome scores of the participants reveal a potential for rhGH and testosterone injections in conjunction with an exercise program to play a role in relieving pain and improving function in individuals with chronic shoulder pain due to degenerative disease and potential micro instability. These findings were found in a series of patients whose previous treatments failed to provide long-term benefit. Three participants in the study dropped out.
During the initial physical examination, 18 of the 19 shoulders (95%) tested positive during the apprehension test, and 17 of 19 (89%) tested positive for Obrien’s Test/Active Compression Test, indicating possible glenohumeral capsule laxity, apprehension at end range of motion, or superior labral involvement [34].
Positive findings during the physical examination with these two tests are, in the primary author’s opinion, paramount to the diagnosis of possible capsular or labral involvement versus other shoulder abnormalities. Two subjects revealed mild weakness of a rotator cuff muscle during examination and eight noted discomforts to acromio-clavicular joint testing. The symptomatic rotator cuff attachment on the humerus and/or the acromio-clavicular joint capsule and the coracoclavicular ligament were treated as well. The authors hypothesize that glenohumeral capsular laxity is a common occurrence that is under diagnosed and under treated. A sizeable percentage of chronic shoulder pain that has not responded to conservative treatments, or to arthroscopic surgery, may well have a component of capsular dysfunction, or degeneration of the ligamentous attachment at the enthesis.
Multiple studies found over the last ten years describe a variety of injection procedures for the treatment of musculoskeletal shoulder problems [35-44]. With the exception of the studies regarding adhesive capsulitis, the shoulder capsule was not reported as being involved.
This case series was designed as primarily an injection series. However, any interventional treatment of the shoulder should, in the author’s opinion, be followed by rehabilitation therapy or exercise to strengthen the shoulder musculature. If the surrounding musculature is not strengthened, the ligaments may not maintain their integrity. With this in mind, the participants were allowed to utilize any exercise program they wished, whether it be physical therapy, Pilates, individualized exercise, or trainer guided exercise, and agreed to do so following the injections.
The combination of recombinant human growth hormone and testosterone injections for chronic shoulder pain in patients diagnosed with a possible gleno-humeral capsular/labral involvement may hold promise of decreasing pain and restoring normal function in many of these individuals. Concurrent treatment of mild rotator cuff weakness/tendinosis and or acromoclavicular joint involvement was also undertaken, when indicated. Previous treatment options had not resolved the problem. We hypothesized and recent evidence suggests that the injection therapy with rhGH and testosterone stimulates the development of collagen formation that matures into healthy, functional connective tissue, resulting in strengthening of the chronically weakened ligaments and other dense connective tissues [45,46]. Limitations to this study include lack of randomization, lack of a control group, participants were not blinded to the treatment group, and the clinician was not blinded. This case series has shown that this line of investigation is safe and 84% of the subject shoulders completing the study. The results support a large-scale randomized controlled trial.
Authors Contributionst
MD provided concepts, screening of patients, consenting of patients, performed injections, tracked data, and and contributed to writing the manuscript.
DB performed the statistical analysis.
TR provided consultation to MD regarding examination and injections.
DM contributed to writing the manuscript and consulted with the case series.
Conflict of Interestt
None.
References
- Chambers L, Altchek DW (2013) Microinstability and internal impingement in overhead athletes. Clinics in Sports Medicine 32(4): 697-707.
- Mistry A, Campbell RSD (2015) Microinstability and internal impingement of the shoulder. Seminars in Musculoskeletal Radiology 19(3): 277-283.
- Reinold MM, Curtis AS (2013) Microinstability of the shoulder in the overhead athlete. Int J Sports Phys Ther 8(5): 601-616.
- Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, et al. (2015) Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared with Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 31(11): 2213-2221.
- Pourcho AM, Smith J, Wisniewski SJ, Sellon JL (2014) Intraarticular platelet-rich plasma injection in the treatment of knee osteoarthritis: Review and recommendations. American Journal of Physical Medicine and Rehabilitation 93(11): S108-S21.
- Dallaudière B, Pesquer L, Meyer P, Silvestre A, Perozziello A, et al. (2014) Intratendinous injection of platelet-rich plasma under US guidance to treat tendinopathy: A long-term pilot study. Journal of Vascular and Interventional Radiology 25(5): 717-723.
- Franceschi F, Papalia R, Franceschetti E, Paciotti M, Maffulli N, et al. (2014) Platelet-rich plasma injections for chronic plantar fasciopathy: A systematic review. British Medical Bulletin 112(1): 83-95.
- DeChellis DM, Cortazzo MH (2011) Regenerative medicine in the field of pain medicine: Prolotherapy, platelet-rich plasma therapy, and stem cell therapy-Theory and evidence. Techniques in Regional Anesthesia and Pain Management 15(2): 74-80.
- Topol GA, Reeves KD, Hassanein KM (2005) Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with chronic groin pain. Archives of Physical Medicine and Rehabilitation 86(4): 697-702.
- Baumgarten KM, Oliver HA, Foley J, Chen DG, Autenried P, et al. (2013) Human growth hormone may be detrimental when used to accelerate recovery from acute tendon-bone interface injuries. Journal of Bone and Joint Surgery - Series A 95(9): 783-789.
- Denaro V, Ruzzini L, Longo UG, Franceschi F, de Paola B, et al. (2010) Effect of dihydrotestosterone on cultured human tenocytes from intact supraspinatus tendon. Knee Surgery, Sports Traumatology, Arthroscopy 18(7): 971-976.
- Labusca L, Zugun Eloae F, Mashayekhi K (2015) Stem cells for the treatment of musculoskeletal pain. World J Stem Cells 7(1): 96-105.
- Meyer U, Meyer T, Handschel J, Wesmann HP (2009) Fundamentals of Tissue Engineering and Regenerative Medicine: Springer-Varlag.
- Baumgarten KM, Oliver HA, Foley J, Chen DG, Autenried P, et al. (2013) Human growth hormone may be detrimental when used to accelerate recovery from acute tendon-bone interface injuries. J Bone Joint Surg Am 95(9): 783-789.
- Gui TT, Pagkalos J, Tsiridis E, Narvani AA, Heliotis M, et al. (2009) Growth hormone: Does it have a therapeutic role in fracture healing? Expert Opinion on Investigational Drugs 18(7): 887-911.
- Lal SO, Wolf SE, Herndon DN (2000) Growth hormone, burns and tissue healing. Growth Hormone and IGF Research 10(SUPPL. B): S39-S43.
- Raschke M, Højby Rasmussen M, Govender S, Segal D, Suntum M, et al. (2007) Effects of growth hormone in patients with tibial fracture: A randomised, double-blind, placebo-controlled clinical trial. European Journal of Endocrinology 156(3): 341-351.
- Hedner E, Linde A, Nilsson A (1996) Systemically and locally administered growth hormone stimulates bone healing in combination with osteopromotive membranes: An experimental study in rats. Journal of Bone and Mineral Research 11(12): 1952-1960.
- Dubick MN, Ravin TH, Michel Y, Morrisette DC (2015) Use of localized human growth hormone and testosterone injections in addition to manual therapy and exercise for lower back pain: A case series with 12-month follow-up. Journal of Pain Research 8: 295-302.
- Demling RH (2005) The role of anabolic hormones for wound healing in catabolic states. Journal of burns and wounds 4: e2.
- Oh DM, Phillips TJ (2006) Sex hormones and wound healing. Wounds 18(1): 8-18.
- Breckenridge JD, McAuley JH (2011) Shoulder Pain and Disability Index (SPADI). Journal of Physiotherapy 57(3): 197.
- Winters JC, Sobel JS, Groenier KH, Arendzen JH, Meyboom De Jong B (1996) A shoulder pain score: A comprehensive questionnaire for assessing pain in patients with shoulder complaints. Scandinavian Journal of Rehabilitation Medicine 28(3): 163-167.
- Douglas ME, Randleman ML, DeLane AM, Palmer GA (2014) Determining pain scale preference in a veteran population experiencing chronic pain. Pain Management Nursing 15(3): 625-631.
- Cleland J, Gillani R, Bienen EJ, Sadosky A (2011) Assessing Dimensionality and Responsiveness of Outcomes Measures for Patients with Low Back Pain. Pain Practice 11(1): 57-69.
- Hill CL, Lester S, Taylor AW, Shanahan ME, Gill TK (2011) Factor structure and validity of the shoulder pain and disability index in a population-based study of people with shoulder symptoms. BMC Musculoskeletal Disorders 12: 8.
- Christie A, Hagen KB, Mowinckel P, Dagfinrud H (2009) Methodological properties of six shoulder disability measures in patients with rheumatic diseases referred for shoulder surgery. Journal of Shoulder and Elbow Surgery 18(1): 89-95.
- Tveitå EK, Ekeberg OM, Juel NG, Bautz Holter E (2008) Range of shoulder motion in patients with adhesive capsulitis; Intra-tester reproducibility is acceptable for group comparisons. BMC Musculoskeletal Disorders 9: 49.
- Angst F, Goldhahn J, Pap G, Mannion AF, Roach KE, et al. (2007) Cross-cultural adaptation, reliability and validity of the German shoulder pain and disability index (SPADI). Rheumatology 46(1): 87-92.
- Hill CL, Lester S, Taylor AW, Shanahan ME, Gill TK (2011) Factor structure and validity of the shoulder pain and disability index in a population-based study of people with shoulder symptoms. BMC Musculoskeletal Disorders 12: 8.
- Breckenridge JD, McAuley JH (2011) Shoulder Pain and Disability Index (SPADI). Journal of Physiotherapy 57(3): 197.
- Winters JC, Sobel JS, Groenier KH, Arendzen JH, Meyboom De Jong B (1996) A shoulder pain score: A comprehensive questionnaire for assessing pain in patients with shoulder complaints. Scandinavian Journal of Rehabilitation Medicine 28(3): 163-167.
- Michener LA, Snyder AR, Leggin BG (2011) Responsiveness of the Numeric pain rating scale in patients with shoulder pain and the effect of surgical status. Journal of Sport Rehabilitation 20(1): 115-128.
- Ravin T, Cantieri M, Pasquarello G (2008) Principles of prolotherapy. Denver, CO: American Academy of Musculoskeletal Medicine.
- Bron C, De Gast A, Dommerholt J, Stegenga B, Wensing M, et al. (2011) Treatment of myofascial trigger points in patients with chronic shoulder pain: A randomized, controlled trial. BMC Medicine 9: 8.
- Chang KV, Hung CY, Wu WT, Han DS, Yang RS, et al. (2016) Comparison of the Effectiveness of Suprascapular Nerve Block With Physical Therapy, Placebo, and Intra-Articular Injection in Management of Chronic Shoulder Pain: A Meta-Analysis of Randomized Controlled Trials. Archives of Physical Medicine and Rehabilitation 97(8): 1366-1380.
- Dimitroulas T, Hirsch G, Kitas GD, Klocke R (2013) Clinical outcome of ultrasound-guided steroid injections for chronic shoulder pain. International Journal of Rheumatic Diseases 16(4): 398-402.
- Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioǧlu K (2013) Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: A randomized controlled trial with 1-year follow-up. American Journal of Sports Medicine 41(11): 2609-2615.
- Bertrand H, Reeves KD, Bennett CJ, Bicknell S, Cheng AL (2016) Dextrose prolotherapy versus control injections in painful rotator cuff tendinopathy. Archives of Physical Medicine and Rehabilitation 97(1): 17-25.
- Alfredson H, Harstad H, Haugen S, Öhberg L (2006) Sclerosing polidocanol injections to treat chronic painful shoulder impingement syndrome-results of a two-centre collaborative pilot study. Knee Surgery, Sports Traumatology, Arthroscopy 14(12): 1321-136.
- Singh JA, Mahowald ML, Noorbaloochi S (2009) Intra-articular botulinum toxin A for refractory shoulder pain: a randomized, double-blinded, placebo-controlled trial. Translational Research. 153(5): 205-216.
- Kwon YW, Eisenberg G, Zuckerman JD (2013) Sodium hyaluronate for the treatment of chronic shoulder pain associated with glenohumeral osteoarthritis: A multicenter, randomized, double-blind, placebo-controlled trial. Journal of Shoulder and Elbow Surgery 22(5): 584-594.
- Simopoulos TT, Nagda J, Aner MM (2012) Percutaneous radiofrequency lesioning of the suprascapular nerve for the management of chronic shoulder pain: A case series. Journal of Pain Research 5: 91-97.
- Park KD, Nam HS, Lee JK, Kim YJ, Park Y (2013) Treatment effects of ultrasound-guided capsular distension with hyaluronic acid in adhesive capsulitis of the shoulder. Archives of Physical Medicine and Rehabilitation 94(2): 264-270.
- Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, et al. (2013) Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scandinavian Journal of Medicine and Science in Sports 23(5): 614-619.
- Vestergaard P, Jørgensen JO, Olesen JL, Bosnjak E, Holm L, et al. (2012) Local administration of growth hormone stimulates tendon collagen synthesis in elderly men 113(9): 1432-1438.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.