Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Microbial Contribution to Agricultural Development and Environmental Conservation
*Corresponding author: Michael Yu. Chernyshov, Siberian Institute of Plant Physiology and Biochemistry, Siberian Branch of Russian Academy of Sciences, Russia.
Received: November 21, 2023; Published: December 04, 2023
DOI: 10.34297/AJBSR.2023.20.002751
Abstract
Plausible knowledge is, no doubt, the only reliable ground of any treatment method and any strategy of treatment. An attempt has been made by the author to bring plausible knowledge of the sources of occurrence of HPV genotypes, which provoke cancer diseases, and knowledge of their epidemiological character - into a system. Not the methods of treatment themselves, but a systemic knowledge-based (and, so, methodologically organized) approach, which presumes systemic organization and systemic application of definite treatment methods, is recommended in the capacity of the way of proper organization of any treatment process in cancer cases. Moreover, a scheme implementing such an approach to prevention of HPV-induced cancer cases is described. The pattern of sequential development of understanding of the depth of HPV impact upon people (from the viewpoints of (i) provoking various forms of cancer and (ii) causing extensive morbidity and mortality) is described.
The types of viral sources, which reflect the human organism’s potential from the viewpoint of its capabilities to protect itself against HPV-conditioned cancer cases, have been identified and are described. Infecting people with highly oncogenic HPVs is gradually acquiring epidemiological character of its progression. In this connection, human health risks may grow. High-risk HPV genotypes, which cause definite cancer cases, have been analyzed. Knowledge-based treatment strategies have been brought into a system. A systemic knowledge-based approach bound up with constructing treatment plans is discussed.
Keywords: Carcinogenic impact of HPVs, Knowledge-based approach, Epidemic character of HPV impact progression, Cancer acquisition risk, Morbidity, Mortality
Abbreviations: ELISA: Enzyme-Linked Immunosorbent Assay; HR: High Risk; IR: Incidence Rate; PCR: Polymerase Chain Reaction; VA: Viral Agent; VLP: Virus Like Particle; VP: Viral Particle
Introduction
The Aspects of Problem Consideration: A Knowledge-Based Approach to Understanding and Treatment of Cancer Diseases and the Epidemiological Character of the HPV Impact
The issue of plausible knowledge is gradually becoming all the more principal issue in finding an efficient approach to treatment of any disease. Plausible knowledge and reliable biomedical grounds may form the basis of the treatment method. And it is ever more important that plausible knowledge would become the ground for any treatment strategy, which would ensure the remission.
In the present paper, carcinogenic impact of Human Papillomaviruses (HPVs) upon people and its epidemiological character are discussed from the viewpoint of a systemic knowledge-based approach to deep understanding and efficient treatment of cancer diseases. This approach formulated by the author presumes not simply application of treatment methods in each definite case, but application of a systemic knowledge-based (and, so, methodologically organized) approach, which presumes systemic organization and systemic application of an integrated system constructed of a sequential set of definite treatment methods organized into a sequence of treatment stages. This systemic knowledge-based approach is recommended below in the capacity of the key approach to the proper organization of any cancer treatment process.
The approach to be proposed implies not only application of biological methodological grounds in solving medical treatment problems, but also application of the systems science (systemological) approaches.
In turn, epidemiology is a developed branch of medicine. The issues bound up with modeling epidemics in medicine are known to be discussed from the 1970s [1]. Various stochastic models were proposed in the 1990s [2]. Furthermore, in the 1990s [3] and later, in the 2010s, ideas of prevention of epidemics in communities were discussed [4], and even immunity potentials of human organisms (preventing diseases and even epidemics) were analyzed with the aid of mathematical bioscience techniques [3]. Traditions of mathematical biology in the aspect of constructing epidemic mathematical models were retained and continued in the beginning of the XXI century [5-7] (epidemical models with varying infectivity). For example, multi-patch and multi-group epidemic models were constructed [5].
During a long time period since the 1990s, investigations of cancer etiology, cancer therapy (pharmacology, immunotherapy, etc.) and surgery were conducted in the aspect of epidemiology. The issues of prevention, survivorship and, in this connection, surveillance were widely discussed. Furthermore, in 2003-2023, various issues of HPV infecting of people were discussed also in connection with development of such infecting in the aspects of virology [8-11] and evolution of the papillomaviridae family [12].
With time, the specialists, who considered themselves involved in problems of medical epidemiology, started to speak about epidemics bound up with HPVs rather freely [13]. It was logical that epidemiology bound up with HPVs developed mainly in the medical aspect: vaccines, treatment of anogenital warts [14], safety measures for surgeons conducting ablation procedures [13,15,16], etc.
Progression of the most part of HPV-induced cancer cases could not be rigorously defined by specialists as epidemic in its character. Meanwhile, already in the 2000s, some of the specialists spoke about emerging (NB!) epidemics of human papillomavirus-induced cancers [13]. Such conclusions necessitated reliable scientifically assessed grounds [17,18].
In the 2000s, there appeared epidemiological classifications of HPV types, which caused cervical cancer. Already in 2003, an epidemiological classification of HPV genotypes bound up with cervical cancer was discussed in N Muñoz, et al., [19]. In 2006, a description of the epidemiology of genital HPV infection was given by H Trottier, et al., [20]. In 2007, EM Sturgis, et al., discussed an emerging epidemic of HPV-induced cancers [13].
Despite the absence of real HPV-conditioned cancer pandemics during the recent two decades, HPVs were discussed as the factors provoking the diseases capable of extensive human infecting and characterized by “a definite epidemiological potential” (2006- 2017) [4,13,20-24]. A review of the current knowledge of epidemiology, pathogenesis, and prevention of HPV infecting may be found in [23]. Noteworthy, over many years, HPV-conditioned cancer forms represented the main cause of cancer-related deaths in the world [25].
Furthermore, the epidemiological character of human papillomavirus infection was considered in several aspects, including the aspect of HPV pathogenesis [23]. It was shown that target cell cyclophilins facilitated HPV16 infecting [26], and vesicular trafficking of incoming HPV16 into the Golgi apparatus and into the endoplasmic reticulum required elevation of γ-secretase activity [27]. No wonder that L.Bruni, et al., (see the summary report of 2019) indicated the epidemiological character of HPV-infecting as to the proved fact [28]. Some results of an epidemiological investigation of the process of high-risk HPV infecting of the people characterized by cytological abnormalities (revealed in the processes of screening of cervical cancer cases) were published by W.You, et al., (2018) [29].
So, it was logical that during the time period from 2003 to 2023, the fundamental biomedical approaches were dominant in laboratory diagnostics of HPV-induced cancer cases in principle, and in ascertainment of the epidemiological character of HPV-infecting, which caused various cancer forms [4,8,9,13,21-24,29].
In the review publications of 2012-2022, two teams of talented researchers logically considered the impact of HPVs upon people as a kind of “global heavy burden of human papillomaviruses and the related diseases” [21,24,30-32]. Epidemiology of HPV-induced cancer diseases was discussed in A.Kombe, et al., (2021) [32].
Furthermore, the issues of HPV-induced morbidity and mortality were discussed [33,34]. The teams headed by T.R.Buchman, et al., (2016) and J.Y.Lei, et al., (2020) analyzed these issues in connection with vulvar, vaginal and cervical cancer cases provoked by HPVs, while emphasizing the improper impact of 2-, 4-, and 9-valent prophylactic HPV vaccines [35,36]. Moreover, in 2017, C. de Martel, et al., spoke about “the worldwide burden of the forms of cancer attributable to HPVs” [24]. This consideration included the details important from the viewpoint of understanding of substantial details, e.g., with respect to (a) HPV genotype, (b) country and even (c) site in the scrutinized country, where the corresponding cancer forms were spread [24].
A specific phenomenon, which implied co-circulation of several HPV genotypes in one site and was in 2007 interpreted as “integration”, was attributed to epidemiology [37]. Furthermore, the probability and even the estimated lifetime of acquiring HPVs were discussed already in 2014-15 [38,39].
In the present publication, the author wanted to encourage comprehensive and professional discussion of virally induced cancer problems in the world on the whole. The author is sure that validation of the novel treatment objectives, targets, and the corresponding novel surgical or therapeutic approaches are urgently needed.
The hypothesis: Deep understanding of possible approaches to diagnostics and treatment of HPV-induced cancer diseases (these approaches being based on the plausible and deep knowledge of the biomedical grounds of HPV-induced cancer cases) is the key issue in provision of adequate and efficient treatment of cancer diseases.
In the works of the author’s research team, it has been proved that highly oncogenic capsid proteins HPV6 L1, HPV11 L1, HPV16 L1 and HPV18 L1, which co-circulate in the social (sexual) sphere and in the environmental sphere (air-borne and water-borne spread of Viral Particles (VPs)), represent inductors of definite forms of cancer in people [40]. Despite all doubts, these HPV genotypes incur real risks bound up with cancer acquisition by the people and, in the perspective, may cause epidemics [13].
Problem statement: The requirement of multi-aspect biomedical safety of the people from various HPVs (on account of the degree of hazards represented by these Viral Agents (VAs)), necessitates development of a systemic knowledge-based approach to deeper understanding and more efficient treatment of such diseases. This approach implies: (a) obtaining plausible and deep knowledge about the causes and sources of occurrence of HPV DNA in the organisms; (b) elaboration and application of a complex of methods and techniques intended (i) for reliable detection of HPVs and (ii) efficient treatment of the organism.
Approaches and Methods
A Systemic Knowledge-Based Approach to Understanding and Treatment of Cancer Diseases
Obtaining plausible solutions of the problems, which are bound up with prevention of diverse cancer cases induced by highly oncogenic HPVs, implies application of the factor of knowledge. In other words, it presumes deeper understanding of (i) channels via which fresh and active HPVs get into the human organism (despite high potentials of its immune system [41,42]): (ii) genotypes, in which HPVs may be represented on the subcellular level and, so, can persist in the human organism; (iii) epidemiological problems, which get more aggravated with every next decade; (iv) facts of multidrug- resistance of HPVs; (v) resistance of HPV16 to alcohol-based disinfection (with ethanol and isopropanol), but sensitivity to hypochlorite and high concentrations of peracetic acid-silver-based disinfectant; etc. This knowledge indicates the necessity of new disinfection protocols for healthcare equipment. So, acquisition of plausible knowledge not only about a definite disease and about methods of its treatment, but also regarding possible sources (/ causes) of this disease and the aids of protection, represents a very important issue.
On the Way to a Universal and Transparent Knowledge-Based Treatment Approach
In the present investigation, which the author considers as research (not a review) one, methods of identification, analysis and comparison of a sequence of data obtained by the author (and integrated with the data obtained by the predecessors) have been applied to construct a systemic knowledge-based approach as a methodology of investigations, analyses and treatment. In this context, the approach itself (bound up with application of known medical treatment methods in each case) implies reconsideration. The approach proposed by the author allows one to reconstruct a set of methods good for treatment of a definite disease into a transparent knowledge-based universal treatment approach applicable to any disease of a given class (in our case, HPV-induced oncogenic diseases are implied).
HPV-induced oncogenic diseases represent a challenging direction of biomedical research. Diagnostics and treatment of such diseases are of interest in each definite case. Meanwhile, it so happened that, with time, the specialists acquired knowledge about (data and facts indicating to) the epidemiological status of such diseases. The total set of such data determined a definite contemporary level of biomedical knowledge related to the epidemiological status bound up with such diseases.
Techniques of clinical analyses of HPVs, which for a long time were applied in clinical practice, presumed either the Polymerase Chain Reaction (PCR-) analysis or the technique of Enzyme-Linked Immunosorbent Assay (ELISA). For a long time, the PCR analysis was a universal tool for detecting HPV agents (and, so, revealing the cancer cases, which might be attributed to HPVs). Obviously, the mechanisms of these two detecting techniques are different. ELISA has become an efficient additional technique in cases of doubt in the analytical results obtained with the use of PCR and the need for their verification.
In many cases, the above two techniques demonstrated incompatible results. Noteworthy, results of application of these two techniques were unequal from the viewpoint of revealing the epidemic character of the scrutinized disease. So, in the long run, it was found expedient to apply ELISA as a more advanced technology for detecting HPVs. The time optimality and the quality of diagnostics of cancer cases on preclinical and clinical stages were conditioned by the goal of their immediate and efficient treatment. It is absolutely logical that it is important to reveal and study a cancer case (i.e., to detect the total set of its symptoms) at an early (i.e., curable) stage.
Results
Understanding of the Epidemiological Status of Cancer Disease Progression
The global Incidence Rate (IR) of HPV-induced carcinogenic diseases was rather high during the recent 20 years. This was partially due to the fact that both the individuals with various prevalent forms of HPV-induced cancer and the sources of HPVs could not be timely and efficiently identified. On the first stage, this was understood in the aspect of necessity in advanced technologies bound up with detection of viruses.
Besides developing the technologies, it was quite important to trace the way of the patient from the stage of preclinical cancer case diagnostics to the stage of his clinical remission. Meanwhile, despite obvious symptoms easily revealed, many patients stayed out of clinics (because this was their will). The issue of preliminary identification and analysis of prevalent cancers in patients was not something unusual in clinical practice (say. on the stage of observational investigations).
Application of the author’s systemic knowledge-based approach to understanding and treatment of the corresponding diseases in clinical practice (in trials of cancer screening and later in prevention) has given the author an opportunity to practically construct a pattern of possible treatment and prevention.
The scheme conceptually demonstrating a systemic knowledge- based approach to understanding the case and a treatment approach to the disease, which presumes (i) tracing of the patient’s state by symptoms, (ii) clinical decision making, (iii) construction of the treatment and prevention plan, (iv) undertakings bound up with prevention of HPV-induced cancer cases, -is shown in Figure 1.
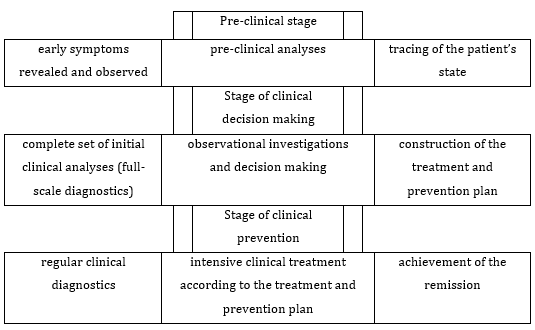
Figure 1: A scheme conceptually demonstrating a systemic knowledge-based approach to understanding the case and to the disease treatment, which presumes prevention of HPV-induced cancer cases.
All the stages of this treatment scheme surely presume avoidance of transition of the cancer disease into the metastatic phase, i.e., are oriented to reduction of the mortality percentage. Various early-observation approaches aid the available methods of handling screening-revealed cancers [43]. For example, in many earlier discussed case investigations, endoscopic analyses allowed the specialists to obtain current diagnoses needed.
On the Epidemiological Character of Development of HPV Influence Upon People
Detailed analysis of the problem in the aspect of the author’s systemic knowledge-based approach to understanding the case and to treatment of the disease has given the author an opportunity to understand the sequences in (a) growth of the medical knowledge (about the circumstances and/or known facts/factors indicating to the disease epidemic status, and, so, giving evidence of the contemporary level of the scientific knowledge) and, therefore, in (b) development of the forms of representing the data bound up with the influence of the circumstances and/or known facts/factors upon the conclusion on the epidemic status of the disease.
The influence of HPVs upon people has been sequentially (within the short, 24-year history) developing according to a logical scheme systematically shown in Table 1 below. Detailed consideration of these data may allow one to trace the character of such influence containing epidemiological elements. Indeed, growth of the numbers of morbidity and mortality in connection with HPVs may be noticed (Table 1).
Column 1 in Table 1 represents sequentially acquired knowledge of the circumstances, facts/factors indicating to the disease epidemic status. The sequence (up-down) of information in this column reflects the steps passed by medical specialists and scientists in understanding the problem, and, in this sense, reflects the contemporary level of world medical knowledge.
Column 2 in Table 1 represents sequentially formulated knowledge of the form of representing the data bound up with the influence of circumstances and/or known facts/factors upon the conclusion on the epidemic status of the disease. This may be, e.g., description of (i) the influence of HPVs, (ii) cancer cases. This form of representation reflects the level of understanding (by the specialists) of the influence of circumstances and facts/factors upon the situation.

Table 1: Sequential (historical) development of understanding of the depth of HPV impacts upon people from the viewpoints of (i) provoking various forms of cancer and (ii) causing extensive morbidity and mortality.
Table 1 contains the knowledge about the impact (upon people) of HPVs inducing hazardous cancer cases (diseases) characterized by a definite epidemiological potential [4,11,32,44].
The issues given in Table 1 were discussed with an explicit emphasis upon the epidemiological character of the cases (squamous cell carcinomas [8], other cancer cases [23] emerging epidemics of HPV-associated head and neck cancers [13] etc. Moreover, epidemiological classifications of human papillomavirus types provoking cervical cancer cases were proposed [19].
Regarding the data related to the growth of the number of HPVbound diseases the reader may be addressed to [22,32,45,46]. As far as data bound up with growth of the number of various cancer cases and diseases in connection with HPVs are concerned, we address the reader to [21,22,24,47-52]. The data bound up with growth of the number of mortality cases in connection with HPVs were discussed in [34,35, 53-55].
Urgent need of immediate prevention of HPV infecting and protection of the organism against HPV-induced cancer cases was discussed in [4,23,56-58] colorectal cancer. Moreover, some of these works considered cancer cases in the epidemiological aspect [58]. Conclusions were made on definite measures bound up with protection of the patients against HPV-induced cancer cases and their survival [23,55,59, 60].
About the Revealed Causes (Sources) of Carcinogenic Diseases Conditioned by Various HPV Genotypes
Note, abnormalities revealed in the investigation conducted by the World Health Organization were also bound up with cervical cancer, a typical form of the disease conditioned by various genotypes of HPV infection [61].
Analysis conducted by the author (the exposure characterization and assessment) has given an opportunity to identify 4 types of carcinogenic disease sources, which characterize the human organism’s potential in the aspect of its capabilities to protect itself against HPV-caused cancer cases (see Table 2). These are: (1) diseases conditioned by various HPV genotypes, (2) organism’s cytological abnormalities, (3) abnormalities bound up with molecular pathogenesis and (4) diseases conditioned by HPV genome variants or by various genetic mutations. It is important to understand that carcinogenic diseases or organism’s abnormalities are also conditioned by definite genotypes of HPVs.
Cellular (cytological) organism’s abnormalities (in comparison to the norm) were discussed in [29,62-68]. Such abnormalities have been revealed, e.g., in the processes of screening of many cervical cancer cases, which cause carcinogenic diseases due to weakness of the organism’s intracellular preventive mechanisms. Meanwhile, in many cases, the organism’s cytology and cellular (cytological) organism’s functions remain normal despite the impact of HPV16, HPV18 and HPV45 [63,64,67,69].
EM Venceslau, et al., discussed “detection of HPVs” (with the aid of primers MY09/MY11 and GP5+/GP6+) in patients with cytologic and/or colposcopic changes [65]. D Wolday, et al., discussed distribution of HPV genotypes in women having normal and abnormal cervical cytology [67]. W You, et al., discussed abnormal cytological findings revealed in the process of cervical cancer screening [29]. Two teams of researchers [66-67] considered the case of vaginal dysbiosis in connection with the risk of HPV-induced cervical cancer. J Norenhag, et al., described the case of cervical dysplasia [68].
The abnormalities bound up with molecular pathogenesis were discussed in [32,70,71]. The diseases conditioned by HPV genome variants or various genetic mutations were discussed in [72-85] (Table 2).
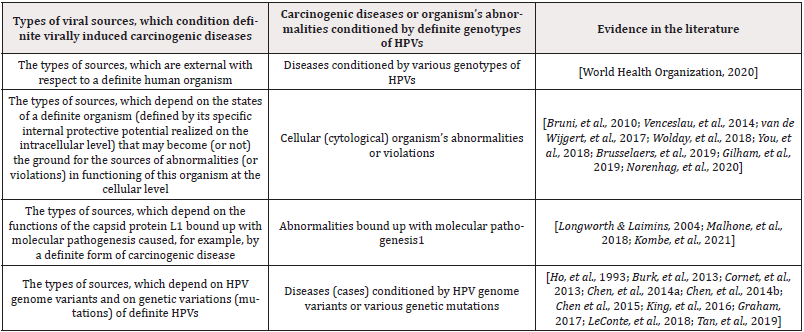
Table 2: The types of viral sources, which reflect the human organism’s potential from the viewpoint of its capabilities to protect itself against HPV-conditioned cancer cases.
Remarks to Table 2: 1-L1 was a subject matter of several therapeutic investigations bound up with HPVs because of the presence of high-affinity domains with the host responsible for stimulating the immune response [32,42,70] and, specifically, its ability to self-assemble into highly immunogenic, non-infectious Virus-Like Particles (VLPs) [32,71-73].
Discussion
Analysis of the results of this investigation has given the author an opportunity to state the following. The factor of knowledge has become the decisive one in all scientific spheres and in medicine [62]. This factor defines the state and the level of contemporary medicine. Absence of due high-level knowledge very often incurs deficit of positive results of any medical treatment. This, first of all, relates to immune-compromised patients, patients undergoing surgical operations, serious treatment errors.
Knowledge and understanding of all the principal issues are important in the stages of (a) pre-clinical investigation of the patient, (b) clinical observational investigations before the decision making, and (c) clinical treatment.
Understanding of the issues of cancer epidemiology may not be ignored. Knowledge of the epidemiological status of an HPV-induced disease is always a result of investigations oriented to various aspects bound up with progression of this disease. Promotion of deeper knowledge of (i) epidemiology of cancer diseases, as well as (ii) benefits of vaccination, might, in our opinion, form positive attitude of people (potential patients) to medicine in principle, to treatment methods, to cancer vaccines, etc. Furthermore, deeper understanding of virological and epidemiological aspects of diseases could scatter myths of spurious side effects of diseases and side effects of their treatment.
As noted above, already in 2007, specialists in HPV-related cancers started to speak about epidemics bound up with HPVs more freely [13]. It was logical that epidemiology bound up with HPVs even in recent years (2019-2023) developed mainly in the traditional medical aspect: Viral Agents (VAs), carcinogenic cases, application of vaccines, treatment of anogenital warts [14], safety measures for surgeons conducting ablation procedures [15,16], etc. Mortality worldwide owing to 36 cancers in 185 countries was discussed [34].
As it has been ascertained in our investigation, there are carcinogenic diseases or organism’s abnormalities conditioned by definite genotypes of HPVs and bound up with the cases conditioned by HPV genome variants and various genetic mutations. It is known that HPVs use the recombination-dependent replication mechanism for the vegetative amplification of their genomes in the differentiated cells [73]. HPV genome variants were discussed in [77]. Differences in the viral genome between the HPV-induced positive cervical cancer and the HPV-induced oropharyngeal cancer were studied (2018) [85]. Furthermore, distribution of HPV 16 E6 gene variants considered in screening of women was analyzed (2019), and its relationships with progression of cervical cancer and, hence, cervical lesions were understood (2019) [86]. Mutations may lead to elevation of cervical cancer risk worldwide [82]. Furthermore, in order to estimate the HPV prevalence in patients’ cancer diseases, meta-analysis was conducted in [87] (the case of gastric cancer), and the potential role of such meta-analysis from the etiological viewpoint was assessed.
Conclusions
Plausible knowledge is the only reliable ground of any efficient treatment method, any strategy of treatment. The issues of carcinogenic impact of HPVs upon people have been discussed above. An attempt to bring plausible knowledge of the sources of occurrence of HPVs, which provoke cancer diseases, as well as knowledge of their epidemic character, into a system has been undertaken. This approach presumes not simply application of treatment methods in each definite case, but involvement of a knowledge-based (and, so, methodologically organized) approach, which presumes systemic organization of the treatment process and systemic application of known treatment strategies and methods within the frames of an integrated methodological strategy. This knowledge-based approach is recommended by the author in the capacity of the key approach to the proper organization of any cancer treatment process. A scheme representing a systemic approach to prevention of HPV-induced cancer cases has been proposed (Table 1).
Furthermore, in the case of application of a systemic treatment strategy, the hazards possible for the people are seen in a more explicit form. Human health risks, which form the potential of progression of HPV-infecting, and which may cause epidemics, are seen as more concrete.
It is possible to draw the following additional conclusions. 1. On the whole, the author’s findings represent issues which seem to be well-known ones, in the aspect of systems science. These findings, which are based on a systemic knowledge- based approach, have uncovered the knowledge about the following types of sources of carcinogenic diseases bound up with HPVs: (1) globally conditioned sources, which are external with respect to a definite human organism, and which condition the diseases at the expense of (a) various HPV genotypes or (b) genetic variations (mutations) of HPVs; and (2) abnormalities in a definite organism, which are determined by its internal conditions: (i) cytological (intracellular level) abnormalities, (ii) molecular and (ii) genetic abnormalities (Table 2).
2. Many researchers have come to the conclusion on the epidemiological character of the carcinogenic impact of HPVs upon people. Indeed, infecting people with highly oncogenic HPVs is gradually acquiring epidemic character. The epidemic character of cancer diseases has been discussed above from the viewpoint of this systemic knowledge-based approach to understanding and treatment of diseases in principle. In this situation, a systemic knowledge-based approach (to deeper understanding and treatment of definite diseases) discussed in the manuscript is considered as a support for medical professionals in planning and application of definite treatment strategies. In this case, methods are considered within the frames of a systemic treatment strategy based on the plausible and systemic knowledge about a definite class of cancer diseases, and about the sources and causes of these diseases.
The author’s findings have contributed to the knowledge about the following types of epidemics, which may potentially be provoked by HPVs: (i) epidemics possible in connection with circulation of HPV genotypes in a definite site; (ii) epidemics possible in connection with co-circulation of various HPV genotypes in the same site; (iii) epidemics bound up with external factors (specific epidemics possible in society or else in the natural environment).
3. It is known that immunity potentials of many human organisms prevent various diseases and, in this connection, probably also epidemics [3,41].
Already in 2003, an epidemiological classification of HPV genotypes bound up with cervical cancer was constructed [19]. In 2006, a description of the epidemiology of genital HPV infection was given by H Trottier, et al., [20]. In 2007, EM Sturgis, et al., discussed an emerging epidemic of HPV-induced cancers [13]. Reviews of the current knowledge of epidemiology, pathogenesis, and, in this connection, prevention of human papillomavirus infection can be found in [14,23].
In 2018, multi-patch and multi-group epidemic models were constructed [5], and results of epidemiological investigations of the process of high-risk HPV infecting of the people characterized by abnormal cytology were published by W You, et al., [29].
HPV epidemiology of cancer diseases was discussed in [32]. Epidemic models with varying infectivity were proposed in [6,7]. M Song, et al., (2023) published their results of epidemiological investigations of HPV-induced cancers [60]. So, there is no doubt that HPV-induced cancer diseases and the character of carcinogenic impact of HPVs upon people have a definite epidemiological character.
4. It is very important to orient further investigations directed to prevention of infecting people with HPVs causing cancer cases to deeper understanding of the mechanisms of cancer acquisition and, in this connection, to (i) application of the systemic knowledge-based approach, (ii) constructing not only social barriers against infecting, but also, possibly, development of some natural environmental barriers against papillomaviruses. It is expedient to reveal an extended spectrum of important environmental factors (to be taken into account and assessed), consider these factors as biomarkers, and already now (iii) search for the keys to deeper understanding of possible epidemics.
Funding
The author declares that no funds, grants, or other support were received by him during the preparation of this manuscript.
Conflict of Interest Statement
The author has not any relevant financial or non-financial interests to disclose. The author has no competing interests or conflicts of interest.
Author Contributions
Formulation and conceptualization of the basic ideas, data analysis, interpretation and systematization, preparation of the figure and the tables, and writing the paper - Michael Yu. Chernyshov.
References
- Cooke KL (1976) An epidemic equation with immigration. Mathematical Biosciences 29(1-2): 135-158.
- Ball F, Clancy D (1992) The final outcome of a generalized stochastic multi-type of epidemic model. Res Rept: 92-94.
- Becker NG, Hall R (1996) Immunization levels for preventing epidemics in a community of households made up of individuals of various types. Math Biosci 132(2): 205-216.
- Narvskaya OV (2011) Virus of human papilloma. Epidemiology, laboratory diagnostics and prevention of papilloma viral infection. Russian Journal of Infection and Immunity 1(1): 15-22.
- Bichara D, Iggidr A (2018) Multi-patch and multi-group epidemic models: a new framework. J Math Biol 77(1): 107-134.
- Forien R, Pang G, Pardoux E (2021) Epidemic models with varying infectivity. SIAM J Appl Math 81(5): 1893-1930.
- Forien R, Pang G, Pardoux E (2022) Multi-patch multi-group epidemic model with varying infectivity. Probability Uncertainty and Quantitative Risk 7(4): 333-364.
- Kreimer RA, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev 14(2): 467-475.
- Prétet JL, Charlot JF, Mougin C (2007) Virological and carcinogenic aspects of HPV. Bull Acad Natl Med 191(3): 611-623.
- Petca A, Borislavschi A, Zvanca ME, Petca RC, Sandru F, et al. (2020) Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp Ther Med 20(6): 186.
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, et al. (2023) ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World.
- Van Doorslaer K (2013) Evolution of the papillomaviridae. Virology 445(1-2): 11-20.
- Sturgis EM, Cinciripini PM (2007) Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer 110(7): 1429-1435.
- Azevedo J, Pista A, Lisboa C, I Santo, L Azevedo, et al. (2017) Epidemiology of human papillomavirus on anogenital warts in Portugal - the HERCOLES study. J Eur Acad Dermatol Venereol 31(8): 1342-1348.
- Zhou Q, Hu X, Zhou J, Zhao M, Zhu X, et al. (2019) Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res 11: 3643-3654.
- Hu X, Zhou Q, Yu J, Wang J, Tu Q, et al. (2021) Prevalence of HPV infections in surgical smoke exposed gynecologists. Int Arch Occup Environ 94(1): 107-115.
- Riethmuller D, Schaal JP, Mougin C (2002) Épidémiologie et histoire naturelle de l'infection génitale à papillomavirus humain. Gynécol Obstétr Fertil 30(2): 139-146.
- Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, et al. (2020) Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog 16(3): e1008376.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348(6): 518-527.
- Trottier H, Franco EL (2006) The epidemiology of genital human papillomavirus infection. Vaccine 24(Suppl 1): S1-15.
- De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13(6): 607-615.
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet Tieulent J, et al. (2012) Global burden of human papillomavirus and related diseases. Vaccine 30(Suppli 5): F12-F23.
- Asiaf A, Ahmad ST, Mohammad SO, Zargar MA (2014) Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prevent 23(3): 206-224.
- De Martel C, Plummer M, Vignat J, Franceschi S (2017) Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 141(4): 664-670.
- Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K (2012) Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstetr 285(3): 771-777.
- Bienkowska Haba M, Patel HD, Sapp M (2009) Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathog 5(7): e1000524.
- Zhang W, Kazakov T, Popa A, DiMaio D (2014) Vesicular trafficking of incoming human papillomavirus 16 to the Golgi apparatus and endoplasmic reticulum requires γ-secretase activity. mBio 5(5): e01777-e01814.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, et al. (2019) ICO/IARC information centre on HPV and cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World.
- You W, Li S, Du R, Zheng J, Shen A (2018) Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med 15: 412-418.
- Aljunid S, Zafar A, Saperi S, Amrizal M (2010) Burden of disease associated with cervical cancer in Malaysia and potential costs and consequences of HPV vaccination. Asian Pac J Cancer Prev 11(6):1551-1559.
- De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, et al. (2013) The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine 31(Suppl 5): F32-46.
- Kombe AJK, Li B, Zahid A, Mengist HM, Bounda GA, (2021) Epidemiology and burden of human papillomavirus and related diseases molecular pathogenesis and vaccine evaluation. Front Public Health 8: 552028.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-E386.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Buchanan TR, Graybill WS, Pierce JY (2016) Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum Vaccines Immunother 12(6): 1352-1356.
- Lei JY, Ploner A, Elfström KM, Wang JR, Roth A, et al. (2020) HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 383(14): 1340-1348.
- Pett M, Coleman N (2007) Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol 212(4): 356-367.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE (2014) The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Trans Dis 41(11): 660-664.
- Senkomago V, Backes DM, Hudgens MG, Poole C, Agot K, et al. (2015) Acquisition and persistence of human papillomavirus 16 (HPV-16) and HPV-18 among men with high-HPV viral load infections in a circumcision trial in Kisumu, Kenya. J Infect Dis 211(5): 811-820.
- Stolbikov AS, Salyaev RK, Nurminsky VN, Chernyshov MY (2022) Investigation of the presence of DNA of highly pathogenic human papillomaviruses in water bodies of the Lake Baikal natural territory. Food Environ Virol 14(3):258-266.
- Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N (2016) Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis 213(9): 1444-1454.
- Motoyoshi Nagai, Ryolato Noguchi, Daisuke Takahashi, Takayuki Morikawa, Kouhei Koshida, et al. (2019) Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178(5): 1072-1087.e14.
- Zhang YR, Wang Y, Liu L, Fan YZ, Liu ZH, et al. (2016) Awareness and knowledge about human papillomavirus vaccination and its acceptance in China: a meta-analysis of 58 observational studies. BMC Public Health 16(1): 216.
- La Rosa G (2016) Papillomavirus. In: Meschke JS, Girones R (eds). Part 3 Viruses. In: Rose JB, Jiménez-Cisneros B (eds). Global Water Pathogen Project.
- Arbyn M, Castellsagué X, De Sanjosé S, Bruni L, Saraiya M, et al. (2011) Worldwide burden of cervical cancer in 2008. Ann Oncol 22(12): 2675-2686.
- Oeffinger KC, Fontham ETH, Etzioni R, Herzig A, Michaelson JS, et al. (2015) Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314(15): 1599-1614.
- Siegel RL, deSantis CE, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64(2): 104-117.
- DeSantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, et al. (2016) Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 66(1): 31-42.
- Teras LR, deSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR (2016) 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 66(6): 443-459.
- Torre LA, Trabet B, DeSantis CE, Miller KD, Samimi G, et al. (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68(4): 284-296.
- DeSantis CE, Jemal A (2018) Black-while breast cancer incidence trends: effects of ethnicity. J Natl Cancer Inst 110(11): 1270-1272.
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, et al. (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6): 438-451.
- DeSantis CE, Ma J, Sauer AG, Newman LA, Jemal A (2017) Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 67(6): 439-448.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram IA, et al. (2021) Global Cancer Statistics 2021: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Juul FE, Cross AJ, Schoen RE, Carlo Senore, Paul Pinsky, et al. (2022) 15-year benefits of sigmoidoscopy screening on colorectal cancer incidence and mortality: a pooled analysis of randomized trials. Ann Intern Med 175(11): 1525-1533.
- Wong J, Layton D, Wheatley AK, Kent SJ (2019) Improving immunological insights into the ferret model of human viral infectious disease. Influenza Other Respi Viruses 13(6): 535-546.
- Wang LH (2022) Accelerating cervical cancer prevention and control in China to achieve cervical cancer elimination strategy objectives. China CDC Weekly 4(48): 1067-1069.
- Song M, Bretthauer M (2023) Interpreting epidemiologic studies of colonoscopy screening for colorectal cancer prevention: understanding the mechanisms of action as key. Eur J Epidemiol 38(9): 929-931.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein D, et al. (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64(4): 252-271.
- Zhang FZ, Li MM, Li XX, Bai H, Gao JL, et al. (2022) Knowledge of cervical cancer prevention and treatment, and willingness to receive HPV vaccination among college students in China. BMC Public Health 22(1): 2269.
- (2020) World Health Organization. Human papillomavirus and cervical cancer.
- De Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, et al. (2007) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 7(7): 453-459.
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, et al. (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 202(12): 1789-1799.
- Venceslau EM, Bezerra MM, Lopes AC, Souza ÉV, Onofre AS, et al. (2014) HPV detection using primers MY09/MY11 and GP5+/GP6+ in patients with cytologic and/or colposcopic changes. Jornal Brasileiro de Patologia e Medicina Laboratorial 50(4): 280-285.
- Van de Wijgert J, Jespers V (2017) The global health impact of vaginal dysbiosis. Res Microbiol 168(9-10): 859-864.
- Wolday D, Derese M, Gebressellassie S, Tsegaye B, Ergete W, et al. (2018) HPV genotype distribution among women with normal and abnormal cervical cytology presenting in a tertiary gynecology referral clinic in Ethiopia. Infect Agent Cancer 13: 28.
- Brusselaers N, Shrestha S, Van de Wijgert J, Verstraelen H (2019) Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol 221: 9-18.e8.
- Gilham C, Sargent A, Kitchener HC, Peto J (2019) HPV testing compared with routine cytology in cervical screening: long-term follow-up of ARTISTIC RCT. Health Technol Assess 23(28): 1-44.
- Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, et al. (2020) The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127(2): 171-80.
- Awua AK, Adanu RMK, Wiredu EK, Afari EA, Zubuch V A et al. (2017) Unique LCR variations among lineages of HPV16, 18 and 45 isolates from women with normal cervical cytology in Ghana. Virol J 14(1): 85.
- Longworth MS, Laimins LA (2004) Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev 68(2): 362-372.
- Malhone C, Longatto Filho A, Filassi JR (2018) Is human papilloma virus associated with breast cancer? A review of the molecular evidence. Acta Cytol 62(3): 166-177.
- Ho L, Chan SY, Burk RD, Das BC, Fujinaga K, et al. (1993) The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J Virol 67(11): 6413-6423.
- Burk RD, Harari A, Chen Z (2013) Human papillomavirus genome variants. Virology 445(1-2): 232-43.
- Cornet I, Gheit T, Iannacone MR, Vignat J, Sylla BS, et al. (2013) HPV16 genetic variation and the development of cervical cancer worldwide. Br J Cancer 108(1): 240-244.
- Chen AA, Heideman DAM, Boon D, Chen Z, Burk RD, et al. (2014) Human papillomavirus 33 worldwide genetic variation and associated risk of cervical cancer. Virology 448: 356-362.
- Chen AA, Heideman DAM, Boon D, Gheit T, Snijders PJF, et al. (2014) Human papillomavirus 45 genetic variation and cervical cancer risk worldwide. J Virol 88: 4514-4521.
- Chen AA, Gheit T, Franceschi S, Tommasino M, Clifford GM (2015) Human papillomavirus 18 genetic variation and cervical cancer risk worldwide. J Virol 89: 10680-10687.
- King AJ, Sonsma JA, Vriend HJ, Van der Sande MA, Feltkamp MC, et al. (2016) Genetic diversity in the major capsid L1 protein of HPV-16 and HPV-18 in the Netherlands. PLoS ONE 11: e0152782.
- Graham SV (2017) The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci 131: 2201-2221.
- LeConte BA, Szaniszlo P, Fennewald SM, Lou D, Qiu S, et al. (2018) Differences in the viral genome between HPV-positive cervical and oropharyngeal cancer. PLoS ONE 13(8): e0203403.
- Tan G, Duan M, Li Y, Zhang N, Zhang W, et al. (2019) Distribution of HPV 16 E6 gene variants in screening women and its associations with cervical lesions progression. Virus Res 273: 197740.
- Deschuyteneer M, Elouahabi A, Plainchamp D, Plisnier M, Soete D, et al. (2010) Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in CervarixTM, the AS04-adjuvanted HPV-16 and-18 cervical cancer vaccine. Hum Vaccin 6(5): 407-419.
- Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC (2000) Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell 5(3): 557-567.
- Zhao Q, Li S, Yu H, Xia N, Modis Y (2013) Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol 31(11): 654-663.
- Sakakibara N, Chen D, McBride AA (2013) Papillomaviruses use recombination-dependent replication to vegetatively amplify their genomes in differentiated cells. PLoS Pathog 9(7): e1003321.
- Zeng ZM, Luo FF, Zou LX, He RQ (2016) Human papillomavirus as a potential risk factor for gastric cancer: A meta-analysis of 1,917 cases. Onco Targets Ther 9: 7105-7114.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.