Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Before the Study Begins: Infertility Considerations in Wistar Rat Models
*Corresponding author: Akunna GG, Department of Anatomy, Benue State University, Nigeria.
Received: October 16, 2023; Published: November 03, 2023
DOI: 10.34297/AJBSR.2023.20.002722
Abstract
Many researchers often assume that their research models are well-controlled, leading to the possibility of obtaining flawed results even before their studies commence. Animal models, like the Wistar rat, have proven valuable for studying the pathophysiological mechanisms underlying human illnesses, particularly those related to reproductive health. This paper explores the use of the Wistar rat model in reproductive studies and highlights the factors that can influence the outcomes of such research.
The Wistar rat model offers translational relevance to human fertility and infertility research, owing to similarities in reproductive anatomy, hormonal regulation, and physiological responses to human. However, various factors can impact the reproductive health of Wistar rats, including genetic background, the development of tumors, diet, and nutrition, housing conditions, exposure to environmental factors, age-related changes, and reproductive seasonality. Understanding and controlling these variables are crucial for accurate and meaningful research outcomes in the context of Wistar rat-based reproductive studies. By addressing these factors and conducting rigorous research, the Wistar rat model can continue to be a valuable tool for advancing our understanding of reproductive health and pathophysiological mechanisms, ultimately benefitting both human and animal populations.
Keywords: Wistar rat, Infertility, Reproductive health, Genetic background, Housing conditions, diet, Environmental factors, Translational research
Introduction
The lack of normal tissue from women of reproductive age who have not been exposed to high doses of hormones used in vivo for assisted reproductive techniques makes it difficult to do ovarian research on people [1]. Animal models that may be altered or utilised to get certain cells or tissues are regarded as useful research tools for studying the pathophysiological mechanisms underlying human illnesses. Some pertinent questions cannot be resolved by directly investigating the afflicted human patients due to the clear logistical and ethical restrictions on human testing [2]. Additionally, using animal models can aid in the development of novel medications or treatments and help us better understand the pathophysiology of human illnesses.
The lack of normal tissue from women of reproductive age who have not been exposed to high doses of hormones used in vivo for assisted reproductive techniques makes it difficult to do ovarian research on people [1]. Animal models that may be altered or utilised to get certain cells or tissues are regarded as useful research tools for studying the pathophysiological mechanisms underlying human illnesses.
Some pertinent questions cannot be resolved by directly investigating the afflicted human patients due to the clear logistical and ethical restrictions on human testing (Abedal-Majed and Cupp, 2009). Additionally, using animal models can aid in the development of novel medications or treatments and help us better understand the pathophysiology of human illnesses.
One rat historian has called attention to the significant differences between wild and laboratory rats using a poignant, though rather exaggerated, parallel: “the laboratory rat is the Hippocrates of ratdom; laboratory rats are to wild rats as Gandhi is to Hitler - they are a separate rat race of Koches, or Pasteurs, or Salks, or Madame Curies [3]. Many researchers in numerous locations in the early years of this century worked to transform the rat from the pestilential harbinger of doom to the hero of contemporary medicine. The Wistar family gave rise to the moniker “Wistar,” whose most notable member was the namesake and descendant of the early colonial glassmaker, Caspar Wistar, M.D., who held the chair of anatomy at the University of Pennsylvania from 1808 until his passing in 1818 [3].
The term “Wistar rats” refers to a group of rat sub-strains that have developed over the course of more than 50 years from a single lineage. The Wistar Kyoto and Wistar Furth rats are inbred strains, whereas the Wistar rat, Wistar Hannover (Wistar HAN), and Wistar Unilever (WU) rats are outbred strains [4]. The breeding efforts of Helen Dean King, the management and husbandry practises of Milton Greenman and Louise Duhring, and the supporting data supplied by Henry Donaldson are all responsible for the development and upkeep of the Wistar Rats as standardised animals [3].
However, Milton Greenman’s inventiveness, which recognized in the Wistar Rats a means for a modest institution to serve research, is what led to their widespread usage. In his Director’s Reports, which he wrote every year from 1905 until his death in 1937, Greenman’s language is preserved, demonstrating his extraordinary sensitivity to his times and the economics of science and society. He recognized that the rat had the potential to be a living analogue to the pure substances that gave experimental science legitimacy at the time biology was being established.
He took the principles of product consistency, quality standards, and production efficiency from management literature and applied them to scientific research to create an animal model that is still used as standard equipment in labs all over the globe today. Both in human and animal populations, infertility continues to be a complicated and pervasive issue. Wistar rats in particular have shown to be very useful in reproductive studies since they provide insights into the complex systems influencing fertility and infertility.
The Wistar rat model offers translational relevance to human infertility research. Similarities in reproductive anatomy, hormonal regulation, and physiological responses enable researchers to extrapolate findings from Wistar rat studies to human reproductive health.
Breeding and Reproduction of Rats
Rats mature sexually between the ages of 6 and 10weeks for males and 8 to 12 weeks for females. Pregnancy can occasionally be identified at around two weeks by touching the belly, seeing weight increase, or observing mammary (breast) growth. The usual gestation period is 21 to 23days. Females who are pregnant will build a nest, so provide them with the supplies they need to do so. Tissue paper is a great nesting material. There are typically 8 to 18 puppies every litter.
Baby rats are born deaf and blind. The cage should be kept in a quiet place and the litter should not be disturbed for at least 7days after birth, especially if this is the female’s first litter. Weaning occurs about 21days after birth. Female rats can quickly become pregnant again after giving birth; however, it is not healthy for a female rat to be both pregnant and nursing a litter. It is recommended that the female be given a rest period of at least 2months between pregnancies and litter rearing to restore her body to full strength.
Some Challenges in the Use of Rodents in General
When investigating reproductive processes such as folliculogenesis, the rodent is not as effective a model as other species in translating to human pathologies [5]. For example, rodents are considered poly-ovulatory while women are considered mono-ovulatory. Additionally, the onset and development of folliculogenesis is very different between rodents and women [6]. The rodent reproductive cycle is also very short (i.e., 4 d) when compared with 28 d in women. Finally, rodents have a gestation length of 21 d compared with 9 mo in women and it is very difficult to collect sufficient quantities of blood samples from rodents to obtain dynamic patterns of endocrine, metabolic, or steroid hormones for comparison to women [7].
Infertility in Wistar Rat
It is crucial to comprehend the causes of infertility in Wistar rats in order to properly evaluate the results of experiments and make relevant comparisons to the health of human reproductive systems. There are several factors that can lead to reproductive maladies in Wistar rats.
Genetic Background of Wistar Rat
The genetic background of Wistar rats can significantly impact their reproductive performance. Strain-specific differences may manifest in various aspects of fertility, including litter size, gestation period, and embryonic development. Researchers must consider these genetic variations when designing and interpreting studies involving Wistar rats. Rats are very susceptible to the development of tumors that may grow and spread to other locations in the body. For instance Rats most frequently develop mammary fibroadenomas. Rats’ widely dispersed mammary (breast) tissue makes it possible to discover tumours beneath the skin anywhere on the belly side of the body, from the chin to the tail. Tumours can form in both male and female rats Katherine and Kenneth, et al., (2022). These growths are often soft, spherical, or a little flat tumours that can be pushed with strong pressure. The majority of these tumours do not progress to cancerous malignancy.
Rats, particularly female rats, are more likely to develop tumours of the pituitary gland, a brain-connected organ that regulates the release of hormones. Consuming a high-calorie diet accelerates the growth of these tumours. Affected rats may abruptly pass away and exhibit head tilt and sadness. In rats, benign testicular tumours predominate Katherine and Kenneth, et al., (2022). These factors can affect fertility studies in Wistar rats without the researcher knowing the true cause.
Diet and Nutrition
Nutrition is a fundamental determinant of reproductive outcomes in Wistar rats. Dietary composition, caloric intake, and the presence of specific nutrients can profoundly affect fertility. Nutritional infertility is due to inhibition of both gonadotropin-releasing hormone secretion and copulatory behaviors. Recent work has focused on the nature of the metabolic signals to the brain, detection of these signals, and the neural circuitry involved. It was once erroneously believed that female mammals had to maintain a particular body fat content to remain fertile [8]. We now know that the primary metabolic factor is short-term availability of glucose and fatty acids for oxidation.
Metabolic fuel availability is detected in the caudal hindbrain and possibly elsewhere. This information is relayed to the forebrain via projections containing catecholamines and neuropeptide- Y, where they activate corticotropin-releasing hormone neurons. Acting as a neurotransmitter, this hormone inhibits gonadotropin- releasing hormone secretion and estrous behavior [9]. Conversely, corticotropin-releasing hormone antagonists reverse the effects of food deprivation on both measures, indicating that corticotropin- releasing hormone is vital in the nutritional suppression of reproduction. Leptin may modulate reproductive responses to changes in short-term fuel availability [10,11]. Researchers must carefully control and monitor the dietary aspects of their studies to ensure accurate results.
Housing Conditions and Environmental Factors
The housing environment plays a pivotal role in the reproductive health of Wistar rats. Factors such as cage density, temperature, humidity, and lighting conditions can influence mating behaviors, estrous cycling, and overall reproductive success. Maintaining optimal housing conditions is crucial to minimize stress-induced infertility. Exposure to environmental factors, including pollutants and endocrine-disrupting chemicals, can compromise reproductive function in Wistar rats [12]. Investigating the impact of these factors on infertility provides essential insights into the mechanisms of fertility disruption [13,14] (Figure 1).

Figure 1: The current regulatory standard of cage height for rats of 17.8 to 18 cm (7 to 7.5 inches) [15].
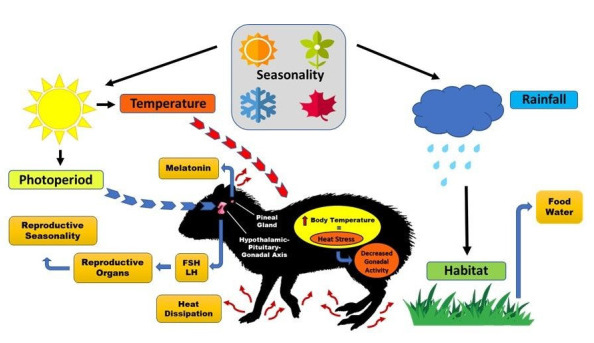
Figure 2: Diagram showing the key environmental factors that affect rodent reproduction. In general, the larger the seasonal changes in temperature and precipitation, which correspond to a region’s distance from the equator (Source:11).
Current laws emphasize that animals can participate in species- appropriate postural changes without contacting the cage walls thanks to sound husbandry practices. According to [15], all rats should have cages that are at least 7 inches (17.8 cm) high. Depending on weight, groups of rats should have a minimum of 17 to 70 square inches (109.6 to 451.5 cm) of floor area each. A female rat would require at least 124 square inches (800 cm) of floor area for her young. Rats kept alone or in small groups may require more floor area than what is advised. If there are several breeding arrangements with numerous adults, multiple litters, or litters of different sizes and ages, the dimension statistics rise. Juvenile rodents may need larger cage spaces than indicated by their size or weight in anticipation of their full growth and adult size. Larger groups can thrive in slightly higher-density housing, but not below the minimum recommended dimensions. Juvenile rodents typically require a larger cage space than adults due to their higher activity levels (Figure 2).
Rodent reproduction is influenced by a variety of environmental factors, including photoperiod [16], rainfall [17-19], temperature [18,20,21] and temperature (Sarli e In order to identify the physiological and behavioural responses of these animals and develop mitigation strategies for the negative effects on reproductive activity, whether brought on by climate change or intentionally harmful human actions to ecosystems, it is crucial to understand how these environmental factors affect rodents.
Age
Fecundity decline is a feature of male reproductive ageing. Similar to humans, older male mice have been shown to have diminished fertility, which is brought on by a range of endocrine, spermatogenic, and environmental variables [22]. According to [23], the lack of episodic luteinizing hormone production from the pituitary is associated with this reproductive failure. Additionally, older rats had lower fertility [24] and higher occurrences of defective sperm [25].
Age-related seminiferous epithelium degradation and aberrant spermatogenesis were discovered by histological analysis of elderly rat testes [26,27]. The spermatogonial stem cells can be maintained for much longer than a mouse’s typical lifespan, demonstrating that the stem cell niche plays a crucial role in male reproductive ageing [22,28]. By the age of 12 months, the fertility of ROSA26 mice was significantly reduced. It’s important to notice that rats’ testis weight is reduced with advancing age, which is important information for the intended audience.
Reproductive Seasonality
One important factor to consider at a time of research is season. According to [29,30], reproductive seasonality is the phenomenon through which certain species reduce or stop sexual activity during a specific time of the year. This phenomenon is typically brought on by environmental variables, particularly temperature, rainfall, or photoperiod. According to [31], the impacts of seasonality can alter the morphophysiology and biochemistry of sexual gonads throughout the reproductive cycle [32].
In order to direct parturition to the most advantageous time for reproduction, female rodents may display one or more series of estrous cycles during the reproductive season [29,32-34]. The female black agoutis (Dasyprocta fuliginosa), which has a non-seasonal polyestrous cycle and may reproduce all year long, is an example of a species that may exhibit non-seasonal reproduction [35].
Male rats have the ability to enlarge the size of their testicles and seminiferous tubules, which can boost the efficacy of spermatogenesis during reproductive seasons [32]. This variation in gonad activity in response to different seasonal periods raises the possibility of an evolutionary strategy for enhanced opportunistic breeding, which focuses on the accessibility of resources like food, water, and favourable environmental conditions [34].
Evaluating Reproductive Competence Pre-Reproductive Research
Understanding the effects of a medication or genetic alteration on the reproductive axis, also known as the hypothalamic-pituitary- gonadal axis, requires evaluation of reproductive competence. The reproductive axis plays a crucial role in integrating internal and external input to adjust fertility to optimal reproductive circumstances. Sexual maturity is assessed in mice and rats before beginning fertility research to rule out the possibility that the observed reproductive characteristics are brought on by delayed or missing pubertal onset.
The assessment of pubertal onset may be done non-invasively in both males and females, with males using the measurement of preputial separation and females using the measurement of vaginal opening and first estrus [36] Fertility research can begin after proof that puberty has ended and sexual maturity has been reached. Without these methods, the researcher will undoubtedly fail [37,38] (Figures 3-5).
Conclusion
Research on infertility using Wistar rats offers valuable insights into reproductive biology and pathology. Researchers should be mindful of genetic, environmental, and housing factors that can influence study outcomes. By addressing these considerations, infertility studies involving Wistar rats can provide meaningful insights into rodent reproductive health and their relevance to human fertility research.
Acknowledgement
None.
Conflict of Interest
None.
References
- Campbell BK, C Souza, J Gong, R Webb, N Kendall, et al. (2003) Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl 61: 429-443.
- Mohamed A Abedal Majed, Andrea S Cupp (2019) Livestock animals to study infertility in women. Anim Front 9(3): 28-33.
- Bonnie Clause (1993) The Wistar rat as a right choice: Establishing mammalian standards and the ideal of a standardized mammal. Article in Journal of the History of Biology 26(2): 329-349.
- McCormick DL (2017) Preclinical Evaluation of Carcinogenicity Using Standard-Bred and Genetically Engineered Rodent Models. A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition): 273-292.
- Paixão L, RB Ramos, A Lavarda, DM Morsh, PM Spritzer, et al. (2017) Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol 15(1): 12.
- Adams GP, J Singh, AR Baerwald (2012) Large animal models for the study of ovarian follicular dynamics in women. Theriogenology78(8): 1733-1748.
- Soncin F, D Natale, MM Parast (2015) Signaling pathways in mouse and human trophoblast differentiation: a comparative review. Cell Mol Life sci 72: 1291-1302.
- Furman M, Wade GN (2007) Animal models in the study of nutritional infertility. Curr Opin Endocrinol Diabetes Obes 14(6): 475-481.
- Wade GN, Jones JE (2004) Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol 287(6): R1277-1296.
- Schneider JE, Zhou D, Blum RM (2000 ) Leptin and metabolic control of reproduction. Horm Behav 37(4): 306-326.
- Barb CR, Hausman GJ, Czaja K (2005) Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol 29(1):186-192.
- Saalu LC, Osinubi AA (2009) Environmental Endocrine Disruptors Of Testicular Function. African Journal of Endocrinology and Metabolism 8(1): 15-25.
- Akunna GG, Saalu LC, Ogunlade B, Enye LA (2014) Spermatotoxicity in Animal Models Exposed to Fragrance Components. J of Med Sci 14(1): 46-50.
- Akunna GG, Saalu LC, Ogunlade B, Akingbade AM, Anderson LE, et al. (2015) Histo-Morphometric Evidences For Testicular Derangement In Animal Models Submitted To Chronic and Sub-Chronic Inhalation of Fragrance. American Journal of Research Communication 3(1).
- Wheeler RR, MP Swan, DL Hickman (2014) Effect of multilevel laboratory rat caging system on the well-being of the singly-housed Sprague Dawley rat. Laboratory Animals.
- Dantas MRT, Souza Junior JBF, Castelo TS, Lago AEA, Silva AR (2021) Understanding how environmental factors influence reproductive aspects of wild myomorphic and hystricomorphic rodents. Anim Reprod 18(1): e20200213.
- Dubost G, Henry O (2005) Comizzoli P Seasonality of reproduction in the three largest terrestrial rodents of French Guiana forest. Mamm Biol 70(2): 93-109.
- Sarli J, Lutermann H, Alagaili AN, Mohammed OB, Bennett NC, et al. (2016) Seasonal reproduction in the Arabian spiny mouse, Acomys dimidiatus (Rodentia: Muridae) from Saudi Arabia: The role of rainfall and temperature. J Arid Environ 124: 352-359.
- El Bizri HF, Fa JE, Bowler M, Valsecchi J, Bodmer R, et al. (2018) Breeding seasonality in the lowland paca (Cuniculus paca) in Amazonia: interactions with rainfall, fruiting, and sustainable hunting. J Mammal 99(5): 1101-1111.
- Salman SA, Shahid S, Ismail T, Chung ES, Al Abadi AM, et al. (2017) Long-term trends in daily temperature extremes in Iraq. Atmos Res 198(6): 97-107.
- Fabio Braga AP (2018) Klein W Temperature and circadian effects on metabolic rate of South American echimyid rodents, Trinomys setosus and Clyomys bishopi (Rodentia: echimyidae) Zoologia (Curitiba) 35: e24572.
- Schmidt JA, Oatley JM, Brinster RL (2009) Female mice delay reproductive aging in males. Biol Reprod 80(5): 1009-1014.
- Bronson FH, Desjardins C (1977) Reproductive failure in aged CBF1 male mice: interrelationships between pituitary gonadotropic hormones, testicular function, and mating success. Endocrinology 101(3): 939-945.
- Parkening TA (1989) Fertilizing ability of spermatozoa from aged C57BL/6NNia mice. J Reprod Fertil 87(2): 727-733.
- Parkening TA, Collins TJ, Au WW (1988) Paternal age and its effects on reproduction in C57BL/6NNia mice. J Gerontol 43(3): B79-B84.
- Humphreys PN (1977) The histology of the testis in aging and senile rats. Exp Gerontol 12(1-2): 27-37.
- Wang C, Leung A, Sinha Hikim P (1993) Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology 133: 2773-2781.
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL (2006) Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24(6): 1505-1511.
- Henry O, Dubost G (2012) Breeding periods of Gerbillus cheesmani (Rodentia, Muridae) in Saudi Arabia. Mammalia 76(4): 383-387.
- Maia KM Silva AR (2016) Influence of seasonality on mammals reproduction. Research & Reviews: Zool Sci 4(1): 43-50.
- Aguilera Merlo C, Fogal T, Sator T, Dominguez S, Sosa M, et al. (2009) Ultrastructural and biochemical seasonal changes in epididymal corpus and cauda of viscacha (Lagostomus maximus maximus). J Morphol 270(7): 805-814.
- Dantas MRT, Souza Junior JBF, Castelo TS, Lago AEA, Silva AR (2021) Understanding how environmental factors influence reproductive aspects of wild myomorphic and hystricomorphic rodents. Anim Reprod 18(1): e20200213.
- Bronson FH, Heideman PD (1994) Seasonal regulation of reproduction in mammals. In: Knobil E, Neil JD, editors. The physiology of reproduction. New York: Raven Press Ltd. 541-573.
- Muteka SP, Chimimba CT, Bennett NC (2006) Reproductive seasonality in the Tete veld rat (Aethomys ineptus) (Rodentia: Muridae) from southern Africa. J Zool 270(2): 314-322.
- Mayor P, Bodmer RE, Lopez Bejar M (2011) Functional anatomy of the female genital organs of the wild black agouti (Dasyprocta fuliginosa) female in the Peruvian Amazon. Anim Reprod Sci 123(3-4): 249-257.
- Hoffmann HM (2018) Determination of Reproductive Competence by Confirming Pubertal Onset and Performing a Fertility Assay in Mice and Rats. J Vis Exp (140): 58352.
- Tavolaro FM, Thomson LM, Ross AW, Morgan PJG (2015) Photoperiodic effects on seasonal physiology, reproductive status and hypothalamic gene expression in young male F344 rats. J Neuroendocrinol 27(2): 79-87.
- Trillmich F, Mueller B, Kaiser S, Krause J (2009) Puberty in female cavies (Cavia aperea) is affected by photoperiod and social conditions. Physiol Behav 96(3): 476-480.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.