Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
CblC Deficency And Optic Atrophy: Wait and See. Case Report
*Corresponding author: Rigaudière Florence, Service de Physiologie Clinique, Exploration fonctionnelle, Hôpital Lariboisière, AP-HP, Paris, France.
Received: October 26, 2023; Published: October 30, 2023
DOI: 10.34297/AJBSR.2023.20.002721
Abstract
Background: Inborn errors of vitamin B12 (or cobalamin) metabolism are rare. However, cblC defect is the most commun genetic abnormality of intracellular cobalamin metabolism. Onset can be early (neonatal period) or late (>2 years). The clinical manifestations are heterogeneous (hematological and/or neurological symptoms, feeding difficulties, etc.). Ophthalmological complications in early onset forms are more frequent than in late ones, with nystagmus, limited visual acuity and maculopathy or, rarely, pigments onto fundi. Few cases of optic atrophy have been reported in literature appearing several years after maculopathy, with optic disc pallor. Six cases of bilateral optic atrophy visible onto fundi without maculopathy have been described in very young children with abnormal Visual Evoked Potentials (VEPs).
Case Report: We report the case of a girl suffering from cblC defect diagnosed in the neonatal period and confirmed by molecular analysis. Her retinas were normal until the age of three years 11 months.
Result: Her retina functionings were normal all over the described period (normal ERGs). VEPs recorded at 6 m were normal. Those recorded at 18 m, 2 years-9 m and 3 years-10 m were nearly not discernible from background noise with normal fundi. At the age of 3 years-11 m, the child’s fundi presented with bilateral maculopathy.
Comments: The abnormal VEPs recorded after an earlier normal one suggested the emergence of bilateral optic atrophies while the fundi were normal. The maculopathy visible a little later led us to interpret the VEP results as being more related to a subclinical macular dysfunction than to an early optic disc atrophy.
Keywords: CblC defect, Optic atrophy, normal ERG, abnomal VEPs, Fundi evolution, Maculopathy
Abbreviations: CblC: Cobalamin C; ERG: Electroretinogram; VEP: Visual Evoked Potential
Introduction
CblC defect (OMIM#277400) is a rare disease with an estimated prevalence of 1/100.000 birth [1]. However, it is the most frequent inborn error of intracellular cobalamin metabolism caused by mutations in the MMACHC gene (OMIM#609831) located on chromosome 1p34.1. It results in decreased intracellular production of adenosylcobalamin and methylcobalamin, cofactors for the enzymes methyl malonyl-coA mutase and methionine synthase respectively, and ultimately methylmalonic aciduria and hyper homocysteinemia with hypo methioninemia. Clinical manifestations are heterogeneous, and patients can be classified according to their age of presentation as early- or late-onset forms [2]. The early- onset forms usually present in the neonatal period with feeding difficulties, failure to thrive, neurological symptoms such as hypotonia, convulsions and even coma. Patients can have acidosis with hematological abnormalities ranging from megaloblastic anemia to pancytopenia. Other possible manifestations include cardiomyopathy, hemolytic uremic syndrome, renal failure, liver dysfunction and interstitial pneumopathy [2,3]. Late-onset forms can present with delayed development, gradual or acute onset of neuropsychiatric symptoms, thromboembolic events, subacute combined degeneration of the spinal cord, or leukoencephalopathy [2-4].
Early diagnosis and treatment are crucial. Early treatment allows correction of hematological abnormalities and feeding difficulties. However complete normalization of the metabolic parameters is rare [5] and the long-term neurological and ophthalmological outcome is poor [6]. Visual manifestations in early-onset patients include lack of eye-tracking or unstable fixation, low visual acuity when measurable and sometimes, reduced light perception [7]. Oculomotor abnormalities are very common with nystagmus and/ or strabismus usually seen at diagnosis [6]. Ophthalmological complications are frequent as the disease evolves. They include macular degeneration and/or clinically evident retinopathy which can lead to blindness [1, 8]. Very few cases of isolated optic atrophy have also been reported in cblC deficiency patients with no maculopathy [7, 9]. Some other optic atrophy cases were diagnosed after retinal evolution with maculopathy [10]. The aim of this case report was to describe the evolution of the visual function assessed by flash ERG and VEPs on a young girl suffering from early onset cblC deficiency. Normal flash ERG and normal fundi, associated with abnormal VEPs suggested the presence of a subclinical optic atrophy. However, later emergence of a maculopathy led us to reconsider this diagnosis.
Case Report
Clinical Assessment
The child (a girl) was hospitalized at 19 days of life due to feeding difficulties with poor weight gain since birth, hypotonia, hypothermia and drowsiness. Eye contact was difficult. The initial assessment found bicytopenia with thrombocytopenia and neutropenia, no anemia but macrocytosis. The association of these signs with hyper homocysteinemia, methylmalonic aciduria and a decrease in methionine, evoked a cblC defect, subsequently confirmed by a reported homozygous variant in the gene MMACHC (c.481c>T (p.R161X)/ c.481c>T (p.R161X), both parents being heterozygous for the same variant. Treatment was instituted [11] (hydroxocobalamin 1mg/day IM + folinic acid 5mg twice a day and carnitine 80mg 2/day and betaine citrate 150mg 4/day and L methionine 25mg 2/day) with a favorable evolution. Further treatment included hydroxocobalamin 1 mg twice a week SC. Evolution of her development showed a delay in acquisitions (motor skills, language, etc.) even if she progressed slowly from the age of 14 months. Brain MRI performed at the age of 6 months revealed diffuse T2 hypersignal in the white matter and myelination delay in T1 predominant in the posterior fossa, signs of intraventricular hemorrhage with ventricular enlargement.
Visual Assessment
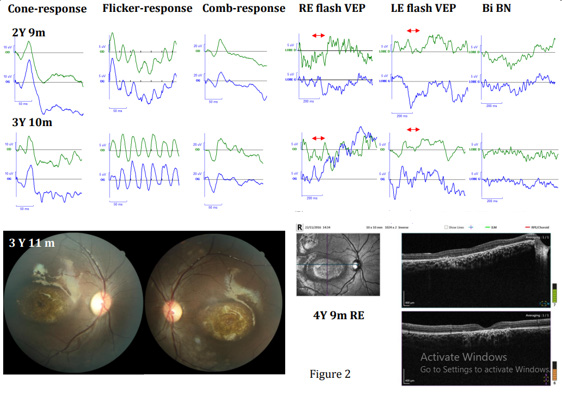
At the age of 4 months, eye contact improved, but the child had difficulty following objects. She had abnormal movements especially when looking laterally. At 9 months, visual development improved, with a nystagmus of high frequency and low amplitude, which was still present at 14 months, 16 months and 2 years-9 m, with progression of eye pursuit. The fundi were normal, even at the age of 2 years-9 m, although her mother felt that she did not see well from far. At the following visit, at 3 years 11 months,-which was preceded by flash ERG-VEP recordings one month earlier - the child had horizontal nystagmus which decreased slightly on the left but increased in extreme lateral gaze. There was no ametropia. Visual acuity with pediatric tests was around 1/10° (i.e., 20/200 Snellen) in binocular vision. The corneas and lenses were clear, the pupils remained in tight miosis even in the dark. After dilation, both fundi showed a large irregular atrophy corresponding to a large maculopathy. Given the frequency of maculopathy in cblC deficiencies, the child’s visual function was regularly assessed by flash ERG and VEPs from the age of 6 months.
Material and Methods
The electrophysiological device was a MonColor system (Métrovision, 59 Pérenchies, France).
Flash-ERG Recordings
The child was never sedated. Her pupils were not dilated given her physical condition. Child size skin electrodes were used (Comepa, Neurocom, 93 Bagnolet, France) [12,13]. Two active electrodes were applied on each inferior eyelid. The forehead was used as reference electrode site, and the two earlobes were connected to the ground. The protocol is not the standard one [14], adapted and reproduced to all pediatric population identically. After a short light adaptation to a white background (30 cd/m²), two light-adapted flash-ERG responses were recorded to standard flash (3 cd.s/ m²) (i.e., bright flash): cone-response and flicker-response (30 Hz). After 10 minutes of dark-adaptation, the combined-response was recorded to standard flash. This response is not equivalent to ISCEV standard mixed-response, but the recording session could not last longer for pediatric population. This combined-response is dominated by the response of the scotopic system [15-16]. They were recorded at the ages of 6 months, 18 months, 2 years-9 months, and 3 years-10 months.
VEPs Recordings
Cortical visual evoked responses (i.e. VEPs) were recorded with two active scalp electrodes placed on O2 and O1 according to the international 10/20 system [17] preceding flash ERG recordings. The number of averages was 50. Two averages were performed to verify the reproducibility of each VEP. Background noise was recorded with both eyes open (Bi Bn). Analysis time was over 750ms to visualize the response adequately.
Binocular Flash-VEPs
Binocular flash-VEPs were recorded when both opened eyes were stimulated together at the age of 6 months (BI flash VEP).
Monocular Flash-VEPs
Monocular flash-VEPs were recorded when each eye was stimulated separately (Right eye: RE flash VEP-Left eye: LE flash VEP) at the age of 18 m, 2 years-9m, and 3 years-10m. Therefore, evolution of her visual functioning (flash ERG and VEPs) was followed over a period of 3 years-5 months. OCT was recorded at the age of 4 years- 9m.
Results
Results were interpreted by two experienced physicians specialized in pediatric electrophysiology, in comparison with results from a normal population of comparable age. They were considered normal when between mean value +/-2 SD. Flash ERGs were normal for all four recordings (6m, 18m, 2 years-9m, and 3 years-10m). The Bi flash VEP was normal at 6 m but monocular flash VEPs were abnormal even nearly not discernible from background noise at 18m, 2 years-9m and 3 years-10m (figure 1 and 2). Maculopathy was evident at 3 years-11m (figure 2).
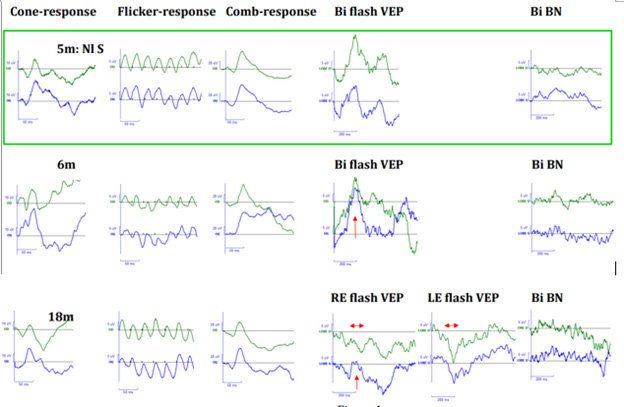
Figure 1: Abscissa = a-ordinate = o. Flash ERG responses : Upper trace : RE- lower trace : LE. Photopic responses : Cone-response (a: 50ms-o: 10μV) and flicker-response (a: 50ms-o: 5μV)-Scotopic response i.e Combined-response : Comb-response (a: 50ms-o: 20μV)-Flash VEP: Upper trace : O2- lower trace : O1 (a: 200ms-o: 5μV)-Binocular (BI flash VEP), Right eye (RE flash VEP), Left eye (LE flash VEP)-Background noise recorded binocularly :BI BN (a: 200ms-o: 5μV)-Normal subject (Nl S) at the age of 5months- Our patient’results at the age of 6 months (6m) : normal flash ERG and VEPs and at the age of 18 months (18m): normal flash ERG and abnormal VEPs.
Comments
CblC deficiency was diagnosed in the neonatal period and treated appropriately according to the clinical child’s condition. The treatment was followed continuously. For her first four years of life and follow-up, the child presented nystagmus. Her fundi were normal until the last examination (at 3years-11m) where bilateral maculopathies were evident. Therefore, these maculopathies appeared between the 3rd and 4th assessment, between the age of 2 years-9m and 3 years-10m. During the first 3 assessments, the fundi and the flash ERGs were normal, indicating that her retinas were normal. The girl had a bright optic disc, appearing pallor in the nasal sector than in the temporal one. However, as the patient is black, this aspect is due to light reflection on her pigmented fundi and differs from an optic disc pallor. Flash VEPs were normal at the first recording and nearly not discernible from background noise at the age of 18m and 2 years-9m. VEPs reflect macular functioning if visual pathways are normal or that of visual pathways if macular areas are normal. These two abnormal VEPs suggested a subclinical atrophy of the visual pathways, as Patton, et al., suggested, as well it in two of their young cblC defect patients with normal fundi, normal ERG and abnormal VEPs [8]. However, further fundus evolution in these two patients was not reported.
The other cases of optic atrophy published without maculopathy were based on the appearance of the fundi, presenting optic disc pallor in four patients described by Brooks, et al., [7]. In six cblC deficient patients published by Ku, et al., [10] three showed a maculopathy initially and an optic disc pallor later with signs of optic atrophy after few months of evolution. For these three patients, the signs of optic atrophies occurred after the maculopathy onsets. In our case, normal fundi and normal flash ERG associated with abnormal flash VEPs recorded at the age of 18m and at 2years-9m, is compatible with subclinical macular dysfunctions more than an early visual pathway dystrophy, even if this latter cannot be formally excluded.
In cblC deficiency cases, maculopathies are common. Isolated optic atrophies are very rare. In the case of normal fundi and normal ERG with abnormal VEPs, it is wise to consider clinical evolution before relating abnormal VEPs to an early optic atrophy. Our case was an illustration of the wise : « wait and see ».
Informed consent was obtained from the patient’s parents for submission of this case report to the journal.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the Welfare of Animals
This article does not contain any studies with animals performed by any of the authors.
Acknowledgements
Pr P Tiberghien.
Conflict of Interest
None.
References
- Weisfeld Adams JD, Emily A McCourt, George A Diaz, Scott C Oliver (2015) Ocular disease in the cobalamin C defect: a review of the literature and a suggested framework for clinical surveillance. Mol Genet Metab 114(4): 537-546.
- DS Rosenblatt, AL Aspler, MI Shevell, BA Pletcher, WA Fenton, et al. (1997) Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC). J Inherit Metab Dis 20(4): 528-538.
- Carrillo Carrasco N, RJ Chandler, CP Venditti (2012) Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis 35(1): 91-102.
- Silvia Kalantari, Brigida Brezzi, Valeria Bracciamà, Antonella Barreca, Paolo Nozza, et al. (2022) Adult-onset CblC deficiency: a challenging diagnosis involving different adult clinical specialists. Orphanet J Rare Dis 17(1): 33.
- Watkins D, DS Rosenblatt, Fowler B (2012) Disorders of cobalamin and folate transport and métabolism, in Inborn metabolic diseases :386-402.
- Sabine Fischer, Martina Huemer, Matthias Baumgartner, Federica Deodato, Diana Ballhausen, et al. (2014) Clinical presentation and outcome in a series of 88 patients with the cblC defect. J Inherit Metab Dis 37(5): 831-840.
- Brian P Brooks, Amy H Thompson, Jennifer L Sloan, Irini Manoli, Nuria Carrillo Carrasco, et al. (2016) Ophthalmic Manifestations and Long-Term Visual Outcomes in Patients with Cobalamin C Deficiency. Ophthalmology 123(3): 571-582.
- Gaillard MC, JM Matthieu, FX Borruat (2008) Retinal dysfunction in combined methylmalonic aciduria and homocystinuria (Cblc) disease: a spectrum of disorders. Klin Monbl Augenheilkd 225(5): 491-494.
- N Patton, S Beatty, IC Lloyd, JE Wraith (2000) Optic atrophy in association with cobalamin C (cblC) disease. Ophthalmic Genet 21(3): 151-154.
- Cristy A Ku, Jacqueline K Ng, Daniel J Karr, Leah Reznick, Cary O Harding, et al. (2016) Spectrum of ocular manifestations in cobalamin C and cobalamin A types of methylmalonic acidemia. Ophthalmic Genet 37(4): 404-414.
- Martina Huemer, Daria Diodato, Bernd Schwahn, Manuel Schiff, Anabela Bandeira, et al. (2017) Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis 40(1): 21-48.
- Fulton AB, EE Hartmann, RM Hansen (1989) Electrophysiologic testing techniques for children.Doc Ophthalmol 71(4): 341-354.
- Bradshaw K, R Hansen, A Fulton (2004) Comparison of ERGs recorded with skin and corneal- contact electrodes in normal children and adults. Doc Ophthalmol 109(1): 43-55.
- Anthony G Robson, Laura J Frishman, John Grigg, Ruth Hamilton, Brett G Jeffrey, et al. (2022) ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144(3): 165-177.
- Hamilton R, K Graham (2016) Effect of shorter dark adaptation on ISCEV standard DA 0.01 and DA 3 skin ERGs in healthy adults. Doc Ophthalmol 133(1): 11-9.
- Bach M, C Meroni, SP Heinrich (2020) ERG shrinks by 10% when reducing dark adaptation time to 10 min, but only for weak flashes. Doc Ophthalmol 141(1): 57-64.
- J Vernon Odom, Michael Bach, Mitchell Brigell, Graham E Holder, Daphne L McCulloch, et al. (2016) ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol 133(1): 1-9.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.