Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Comparative Cosmetical Trials Investigating the Effects of Hybrosome (Umbilical Cord Blood Exosome-Liposome Hybrid Vesicles) Containing Cream Formulation on Skin Wrinkle and Elasticity
*Corresponding author: Tunc Tiryaki, Department of Plastic and Reconstructive Surgery Cadogan Clinic 120 Sloane St London, UK.
Received: December 18, 2023; Published: December 22, 2023
DOI: 10.34297/AJBSR.2023.20.002781
Abstract
Skin aging is a natural process that is influenced by both intrinsic and extrinsic variables, such as genetic predisposition, hormone changes, UV radiation exposure, environmental toxins, and lifestyle. These elements contribute to the development of wrinkles, fine lines, an uneven skin tone, and other aging symptoms over time. There are several cosmetic products for the treatment of skin aging problems in the market.
Exosomes are small messenger vesicles that are essential for intercellular communication. Exosomes are thought to boost positive benefits for anti-aging effects and are found in skincare products. They might empower collagen synthesis, skin texture, and wrinkle reduction. Due to the regenerative potential, cord blood exosomes have attracted attention recently. The study aimed to assess the long-term efficacy and safety of a cream formulation that contained the cord blood exosome component “Hybrosome”. The study design was randomized, double-blind, and controlled, which is considered a robust method to minimize bias in scientific research.
Keywords: Exosome-like nanovesicles, Umbilical cord blood exosomes, Anti-aging, Anti-wrinkle, Skin regeneration
Introduction
Skin aging is a natural process influenced by intrinsic and extrinsic factors. Intrinsic factors include genetics, cellular metabolism, and hormonal changes, while extrinsic factors include UV radiation, pollution, smoking, and poor nutrition [1,2]. These factors can lead to various skin changes such as loss of elasticity, wrinkles, hyperpigmentation, and dryness [3]. To prevent or reduce skin aging, it’s important to protect the skin from the sun, avoid smoking and pollution, maintain a healthy diet, and use skin care products with scientifically backed ingredients [2]. Retinol, vitamin C, hyaluronic acid, and peptides are some ingredients that have anti-aging properties [4,5]. However, it’s important to note that not all cosmetic products may be effective, and it’s essential to choose products based on scientific research. Maintaining a healthy lifestyle with enough sleep, hydration, and stress management also plays a role in skin aging prevention [2,3].
Topical anti-aging technologies are becoming increasingly popular as the global population continues to age [4,5]. As people become more aware of the effects of aging on their appearance, they are seeking out innovative solutions to help them look and feel younger. To meet this demand, researchers are constantly working to develop new anti-aging topical treatments and technologies including everything from topical creams and serums to laser treatments and injectables. Among these new technologies, one of the most interesting subjects is exosomes [6].
Exosomes are small extracellular vesicles that play a crucial role in cell-to-cell communication in the body. They contain a variety of bioactive chemicals that can alter biological molecules, such as proteins, lipids, nucleic acids, and growth factors [7,8]. They are involved in a wide range of physiological processes, including immune response, tissue regeneration, and cancer progression [9]. Exosomes have gained attention in the cosmetic industry due to their unique properties, including their small size, biocompatibility, and ability to cross biological barriers, such as the blood-brain barrier [10]. The small size of exosomes allows them to penetrate the deeper layers of the skin, which can increase the bioavailability and effectiveness of the loaded cosmetic ingredients [11]. Some research suggests that exosomes may be more effective than other delivery methods, such as liposomes or nanoparticles, because they are more biocompatible and can penetrate deeper into the skin [12]. The delivery potential of exosomes can be particularly useful for developing novel cosmetic products with anti-aging affect to stimulate collagen production and promote skin regeneration and rejuvenation [11,13,14].
In this study, a stable dermatological formulation containing ‘Hybrosome’, the cord blood exosome as a main ingredient, was formulated and applied on human faces and compared with controlled formulation (without Hybrosome) and the results were investigated in first clinical cosmetic traial. Moreover, the same formula with key ingredient Hybrosome was compared with gold standard anti- aging ingredient retinol. Different parameters such as wrinkle reduction, trans epidermal water loss, melanin level and regeneration capacity related to skin-aging were also evaluated.
Material Methods
Materials
Base Cream: Sodium Hyaluronate (0,1%), Allantoin (1%), Niacinamide (%2), Panthenol (3%), Caprylic/Capric Triglyceride (1,5%), Cetearyl Alcohol (3%), Dimethicone (1%), Ethylhexyl Palmitate(4%), Glyceryl Stearate (4%), Euxyl Pe 9010 1% (Phenoxyethanol, Ethylhexylglycerin), Lactobacillus/Radish Root Ferment Filtrate (3%), Argireline 2% (Aqua, Acetyl Hexapeptide-8, Caprylyl Glycol), Sodium Pca (1%), X50 Antiaging Solution (Pf) 0,5% (Aqua, Xanthan Gum, Polyvinyl Alcohol, Glycolic Acid, Lactic Acid, Copper Palmitoyl Heptapeptide-14, Heptapeptide-15 Palmitate), Liposomal Gotu Kola 5% (Centella Asiatica Extract, Phosphatidylcholine, Tocopheryl Acetate, Aqua, Xanthan Gum, Citric Acid, Potassium Sorbate, Sodium Benzoate), Activespheres Vit Pmg 2% (Aqua (And) Butylene Glycol (And) Magnesium Ascorbyl Phosphate (And) Atelocollagen (And) Sodium Chondroitin Sulfate (And) Xanthan Gum (And) Polysorbate 20)
Active Materials: Hybrosome 0,75% (Phospoolipids, Serum Protein), and retinol (0,2%)
Thermodynamic Stability Tests
All Emulsion characterization and stability tests was done for base cream combination with Hybrosome active ingredient. The preliminary stability of the emulsions was evaluated by centrifugation and thermal stress tests within 24 hours. Stability was determined by macroscopic observation of the emulsions. The centrifuge test carried out for the purpose of accelerate possible stability issues, centrifugation at room temperature (25±1°C) and at 3500 rpm speed was applied to 10g emulsion for 30 minutes with a laboratory type centrifuge device (ThermoScientific, Waltham, MA, USA). 10g emulsion was exposed to different temperatures in thermostatic water bath test from 40°C to 80°C, with 5°C temperature increases in the thermal stress test. The formulations were kept for 30 minutes at each temperature point. Formulations in which any phase separation or creaming was not observed as a result of the two tests were continued to work with. At the later phase, characterizations of formulations which were found to be successful in the thermodynamic stability tests were performed.
pH Measurements: pH measurements were made at room temperature (25±2°C) and climate chamber (at 40±2°C and 75% relative humidity) under the determined conditions and time intervals. (n=3). Samples were diluted 1:10 with distilled water prior to the test.
Viscosity Measurements: Viscosity measurements of the formulations were determined using rotational-type viscometer (Brookfield DVII, Germany TA spindle, 25±1°C). Measurements were taken in 3 replications in 100 rpm (n:3). Viscosity values were recorded in centipoise (cP).
Physical Controls: Physical controls (color, odor, appearance, liquefaction, creaming, phase separation) of the formulations were carried out under the determined conditions and time intervals.
Microbiological Stability Test: Microbiological limit test of formulations (FS1, FS2, FS3) selected after studies of stability and characterization was carried out according to the procedures reported in the USP 42 edition during 1 month with the aim of evaluating the microbiological stability thereof.
Cosmetic Clinical Trials
Cosmetic and personal care products fall into the non-therapeutic category and do not require the same level of clinical testing as medicinal drugs and do not have to be published in public databases. Meanwhile, they still need to undergo some form of clinical testing to ensure their safety and effectiveness. Cosmetic clinical trials typically involve testing the product on a small group of human volunteers to assess its safety, efficacy, and any potential side effects.
Four cosmetic clinical studies with test numbers 10/05/21/A/39, 10/05/21/A/40, 10/05/21/A/41 and 10/05/21/A/42 were conducted to evaluate the active substance obtained with Hybrosome technology. The first study compared different formulations of cream, including the base formula, the base formula with 0.75% active substance, the base formula with 0.2% retinol, and the base formula with 1.5% active substance, and was conducted on 10 people. An extended study was then carried out with report number L16456/22/JSHR and L16457/22/JSHR based on the findings of the first study, comparing the base formula with 0.75% active substance and the base formula with 0.2% retinol on 54 volunteers. The third study with report number INF.1051.20.10 and INF.1051.42.10 confirmed the regenerative effect and wrinkle reduction effect of the 0.,75% Hybrosome product on 53 patients. The fourth study with report number 22-125 was a single-blinded study in which two different creams, one containing Hybrosome and the other containing 0.2% retinol, were applied on different sides of the face of the same patient. Measurements of various parameters such as skin moisture, Transepidermal Water Loss (TEWL), and wrinkles were analyzed using SPSS 12.0 software, employing paired samples t-test for comparing the two preparations and twoway ANOVA for analyzing variations over different time intervals, with a significance level of 5%.
Trial I: The study aimed to compare the effects of four different cream formulations on wrinkles and elasticity in a single-blinded design, where the participants were not aware of which cream formulation they were using, but the researchers knew the details of each cream formulation. The four cream formulations used in the study were: a base cream (control group), B cream with 0.75% Hybrosome combined by base cream, C cream with 0.2% retinol combined by base cream, and D cream with 1.5% Hybrosome combined by base cream. Ten female volunteers between the ages of 40-56 years with no skin or other diseases were selected for the study and provided with one cream each to apply once a day for 4 weeks. Skin parameters were measured at a laboratory using measuring devices. The study was conducted at home under the constant care of a dermatologist, and volunteers were informed about the study’s purpose, procedures, and possible side effects.
Trial II: A single-blinded controlled study was conducted to compare the effects of two different cream formulations on TEWL, melanin level in the skin, and wrinkles. The first group used a cream formulation containing a base cream and 0.75% Hybrosome concentration while the second group, which serves as the control group, used a cream formulation that contains the gold standard retinol with a concentration of 0.2%. Fifty-three male and female volunteers between the ages of 40 and 55 with no skin condition or other diseases participated in the study and applied the cream once a day for 56 weeks. Measurements were taken at 0, 28, and 56 days. The volunteers were not informed about the contents of the creams, and a dermatologist provided constant care throughout the study.
Instrumental tests were conducted to evaluate the effects of the creams on TEWL, melanin level, and wrinkles. TEWL was measured using Tewameter® TM 300, melanin level was measured using Mexameter® MX 18, and wrinkles were analyzed using Primos 3D Lite. The measurements were taken at the site of application before product application, after 28 days, and after 56 days of regular use, in controlled environmental conditions.
The efficacy of the tested product was confirmed based on positive results obtained in more than 50% of the subjects in each instrumental test. The study design and methodology followed established protocols for evaluating the effects of the cream formulations on TEWL, melanin level, and wrinkles in a controlled and blinded manner.
Trial III: A single-blinded study was conducted to evaluate the effects of a cream formulation containing 0.75% Hybrosome concentration on skin irritation or sensitization, wrinkles, and skin regeneration. The study included 52 volunteers between the ages of 40 and 55 with visible signs of wrinkles on crow’s feet, forehead, and nasolabial fold. The volunteers were not informed about the contents of the creams. Wrinkle analysis was conducted using a Structured-light 3D scanner, and measurements were taken before (D0), 28 days (D28), and 56 days (D56) time points after the first application. Dermatological surveillance was included in the study.
The effects of the cream formulation on skin regeneration were analyzed in the forearm of 52 volunteers for 10 days, and after 24 hours of previous exposure to 10% Dihydroxyacetone (DHA) occlusive patches. Melanin levels, which indicate skin color, were quantified using Mexameter® in two areas of the right forearm: one treated with the cream formulation and the other untreated (control) or treated with the base cream. Melanin levels were measured at various time points, including D0 (after DHA exposure and before starting treatment), D2 (after 2 days of topical treatment), D5 (after 5 days of topical treatment), and D10 (after 10 days of topical treatment). Basal melanin values before induced tanning were also recorded and stored. Use test and dermatological surveillance were included in the study.
Trial IV: A single-blinded study was conducted to analyze the effects of cream formulation. Two different creams were applied on different sides of the face of the same patient. One cream contained Hybrosome, while the other cream contained retinol at a concentration of 0.2%. This was done to compare the effects of the two creams on the skin, with the left side of the face serving as the area where the cream with Hybrosome was applied, and the right side of the face as the area where the cream with retinol was applied.
Twenty female-male volunteers with an age between 35 and 65 with no skin condition or other diseases were selected for the study and informed consent was obtained. Each volunteer applied the cream for the period of 28 weeks once a day. Every volunteer was instructed to come for measurement after 0 and 28 days. The volunteers were not informed about the contents of the creams. All volunteers selected for the study met the requirements for inclusion in the study and signed consent to voluntary participation in the study, and were informed about the purpose of the study, how it was conducted and about possible side effects. During the entire study, the volunteers were under the constant care of a dermatologist.
Ethics
The study protocol is in accordance with the Scientific Committee on Consumer Safety (SCCS) guidance. It meets all international standards for research studies involving human subjects, the Good Clinical Practices (ICH-GCP), and World Medical Association. It has been conducted pursuant to the Declaration of Helsinki (1964), with the amendments of Tokyo (1975), Venice (1983), Hong Kong (1989), and Seoul (2008).
Results
Color
Freshly prepared base and formulation were white and creamy. There was no change in color of any sample of base and for- mulation under different storage conditions up to end of the observation period of 56 days. This shows that both base and formulation were stable at different storage conditions up to 56 days.
Liquefaction
No liquefaction was observed in any of the samples kept at 8℃ and 25℃during the whole observation period of 56 days. A slight liquefaction was observed in the sample of base and for- mulation kept at 40℃and 40℃+ 75% relative humidity (RH) on the 48st and 56th day of observation. The rate of creaming is inversely pro- portional to the viscosity of the dispersion medium according to the Stokes’ law. So as creaming increases, the viscosity of the base and formulation gradually decreases with increasing temperature resulting in liquefaction [15].
Phase Separation
The samples of base and formulation were stable at 8℃, 25℃, but a slight phase separation in the sample of base occurred at 40℃ and 40℃ + 75% RH on 56th day of observation but the formulation was stable at 40℃ and 40℃+ 75% RH on 56th day of observation. This may be due to the conditions of storage. The emulsions can be more stable at lower temperature due to increased phase viscosity.
Trial I
This study was aim to evaluate the viscoelastic properties of the skin with the different formulations and concentrations, and to determine the effects of the base formula as well. Wrinkle analysis would involve assessing the presence and severity of wrinkles on the skin, which could be subjective or objective measures depending on the methods used in the study.
To compare the effects of four cream formulations on skin elasticity and wrinkle count, specifically: a base cream as the negative control, a base cream combined with 0.75% Hybrosome, a base cream combined with 1.5% Hybrosome, and the gold standard retinol formulation with 0.2% concentration were formulated in the study. The objective is to determine any differences in how different formulations affect the measured outcomes.
It is seen that there is a minimum difference in the percentage of wrinkle removal between the base cream alone (11.4%) and the retinol-base cream combined formulation (11.5%). This difference is not statistically significant, meaning that the observed variation is likely due to random chance rather than a true difference in efficacy between the two formulations. The results that when different doses of Hybrosome combinations were combined with base creams, there was no significant difference observed in wrinkle reduction and elasticity test results (Figure 1A,1B).
The Derma Lab Combo and ASW 300 instrument was used to measure the patients’ skin elasticity and wrinkle level before and after the application of four different cream formulations. Before and after images of the applications are in Figure 1B. Skin photos was made with the use of distributed light at x30 magnification.
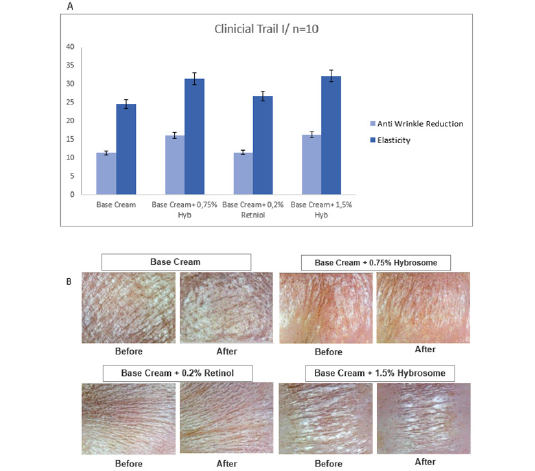
Figure 1: Graphical representation of the percent change in wrinkles and elasticity levels from the baseline value. (A) Before and after visuals of skins applied with 4 different cream formulations. (B).
Trial II
Percentage-based recovery rate of wrinkle volume difference between D28 vs D0 and D56 vs D0 was measured for two groups: one group using a cream containing Hybrosome with 0.75% concentration, and another group using a cream containing retinol as a control. The results indicate that the group using the cream with Hybrosome showed an average decrease of 12% in wrinkle volume difference between D28 and D0, while the group using the retinol cream showed an average decrease of 8% in wrinkle volume difference between D28 and D0. Formulation containing Hybrosome showed an increase in healing from 12% to 14% at Day 56, while the group using retinol alone remained at 8% healing over the same time- period (Figure 2A). Both groups used the same base cream.
The results of the study show that the cream containing 0.75% Hybrosome exhibited an improvement in melanin levels over time (Figure 2B). On Day 28, the melanin recovery rate increased by 5% compared to Day 0, indicating a mild improvement. However, by Day 56, the melanin recovery rate increased to 13%, indicating a more significant improvement in melanin levels with prolonged use of the cream. It’s important to note that this study only measured the melanin recovery rate and did not mention any comparison to a control group or other treatments.
Hybrosome cream group had a higher recovery rate in TEWL compared to the retinol group. The Hybrosome cream group showed an 8% recovery rate initially, and by Day 56, their recovery rate improved by 14%, indicating a positive response to the treatment. On the other hand, the retinol group had a 6% recovery rate initially, and their recovery rate improved to 12% by Day 56, which also indicates improvement but at a slightly lower rate compared to the Hybrosome group (Figure 2C).
It’s important to note that recovery rate is typically calculated as a percentage of improvement or decrease compared to the baseline value. In this case, the baseline value is the wrinkle volume difference between D56, D28 and D0, and the recovery rate is the percentage decrease in this difference.
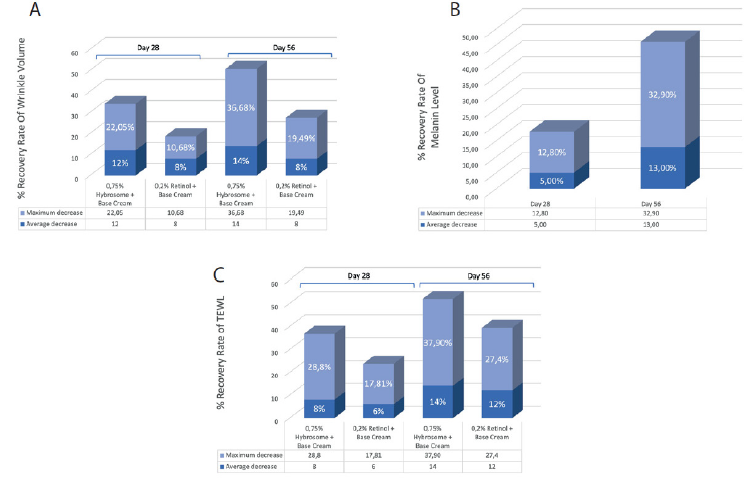
Figure 2: Results of Percentage-based recovery rate of wrinkle volume difference, melanin levels, and TEWL for creams Containing Hybrosome and retinol in Day 28 and Day 56. Wrinkle volume difference recovery rate cream containing Hybrosome (0.75% concentration) and retinol (0.2% concentration). (A) Melanin levels recovery rate cream containing Hybrosome (0.75% concentration). (B) TEWL recovery rate cream containing Hybrosome (0.75% concentration) and retinol (0.2% concentration). (C).
Trial III
The results of the HRIPT (Human Repeat Insult Patch Test) for the cream with 0.75% Hybrosome concentration showed no allergic reactions. Additionally, the cream combination demonstrated an average wrinkle reduction of 16.56% on Day 28 compared to Day 0, with the highest observed wrinkle reduction value being 67.70%. When Day 0 and Day 56 were compared, the average wrinkle reduction was 20.40%, with the best result showing a wrinkle reduction of 80.70% (Figure 3A). These findings suggest that the cream with 0.75% Hybrosome concentration may be effective in reducing wrinkles, and it did not cause any allergic reactions in the tested population according to the HRIPT results. Wrinkle analysis images visualized at time points of D0, D28 and 56 using a Structured- light 3D scanner are shown in (Figure 3B).
In regeneration test results showed that exposure to 10% DHA significantly increased skin melanin levels by 30.8±1.76 %, comparing each volunteer with its own basal control (before DHA exposure) as shown in (Figure 4A). In this context, when 0,75% Hybrosome cream were topically applied after DHA, results showed a statistically significant reduction of melanin levels upon treatment. A significant reduction by 4.42 ± 0.85, 10.18 ± 1.31 % and 15.53±1.63 % was observed after 2, 5 and 10 days, respectively (Figure 4B). Images of 0,75% Hybrosome + base cream treated and untreated areas at time points of D0, D2, D5 and D10 are included in (Figure 4C).
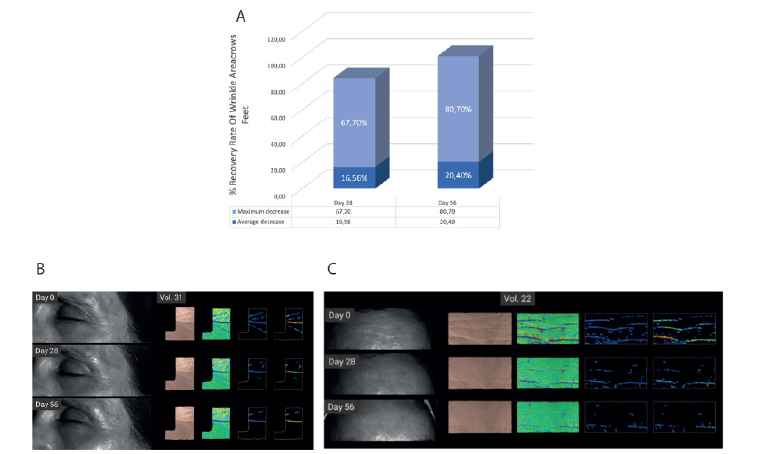
Figure 3: Graphical representation of the percent change of crow’s feet wrinkles area according to the comparison with before (D0) measurement and after 28 days and 56 days of treatment. (A) Wrinkle reduciton visual of crow’s feet and forehead at time points of D0, D28 and 56. (B).

Figure 4: Melanin levels comparing Basal and D0. (A) Bar graphs representing relative basal melanin levels without DHA treatment and after remove DHA occlusive patch (D0). Graphical representation of the normalized melanin levels before (D0) and after 2, 5 and 10 days with 0,75% Hybrosome cream treatment. (B) Visual representation of the normalized melanin levels before (D0) and after 2, 5 and 10 days with and without 0,75% Hybrosome cream treatment. (C) Asterisks indicate significant differences as follows: * p-value< 0.0001, ** p-value < 0.01, *** p-value< 0.001, **** p-value < 0.0001.
Trial IV
Based on the results of the randomized blind controlled trial, it appears that 0,75% Hybrosome cream may be more effective than retinol in reducing wrinkles, improving radiance and effective in homogenate effect. After 28 days of use, 0,75% Hybrosome cream showed a 13.4% reduction in wrinkles compared to the first day, while retinol showed a lower effect of 6.89% (Figure 5A). This suggests that 0,75% Hybrosome cream may be more effective in reducing wrinkles compared to retinol. To homogenate effect, 0,75% Hybrosome cream composition showed an effect of 13.16% after 28 days, while 0.2% retinol cream composition showed an effect of 4.88%. This indicates that Hybrosome cream is more effective in homogenate effect compared to retinol cream. In terms of radiance values, 0,75% Hybrosome cream was 10.75% effective, while 0.2% retinol cream was 6.25% effective after 28 days of use (Figure 5B). In (Figure 5C and 5D), there are D0 and D28 day images of two different patients who were applied 0.75% hybrosome cream formulation to the left side of their face and 0.2% retinol cream formulation to the right side. Within the scope of these results, it has been suggested that 0,75% Hybrosome cream is more effective in improving radiance compared to 0.2% retinol cream.
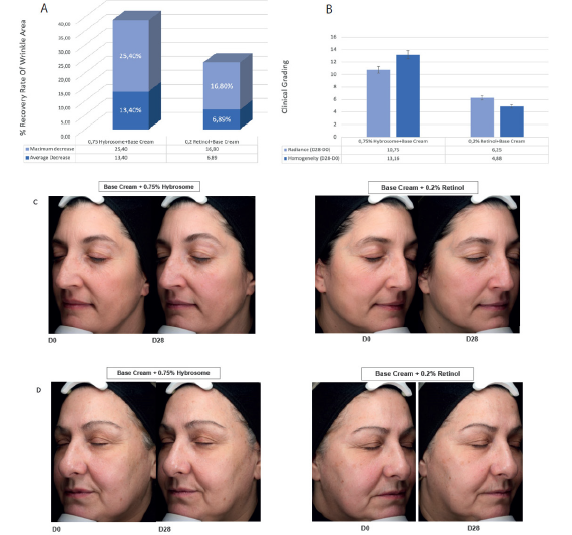
Figure 5: Comparison of Hybrosome cream and retinol in reducing wrinkles, improving radiance, and homogenate effect. Graphical representation of the percent change of crow’s feet wrinkles area according to the comparison with before (D0) measurement and after 28 days of treatment. (A) Graphical representation of the percent change of radiance and homogeneity according to the comparison with before (D0) measurement and after 28 days of treatment. (B) Wrinkle reduction visuals of crow’s feet at time points of D0 and D28 with 0,75% Hybrosome + base cream and 0,2% retinol + base cream. Left side of face was treated with 0,75% Hybrosome + base cream formula while right side of the same patient was treated with 0,2% retinol cream formula. (C-D).
Discussion
Cosmetic products focused on combatting dermal aging aim to make structural and functional enhancements to the skin emphasizing the need for these products to produce reliable and efficient results. While demonstrating that the product has beneficial physical properties and stability, it is important to prove its claimed cosmetic capability [16] Furthermore, regardless of product efficiency, customers prefer product that do not result in unpleasant cosmetic appearance, have poor absorption, are unstable, or have irritant or allergic effects on the skin.
In the creation of emulsion formulations, determining an acceptable shelf life is crucial [17]. Pharmaceutical and cosmetics businesses aim for a minimum two- to three -year shelf life for their goods [18]. A cosmetic cream needs to be able to maintain stability over the course of its shelf life without experiencing phase separation or altering in color, smell, or appearance [19,20]. A stability test conducted under typical storage circumstances is a reliable method of assessing the system’s robustness [21]. However, accelerated stability tests are performed to shorten the time and to get information about stability more quickly and accurately. In these tests, the product is exposed to various stress conditions. Measurement of the product’s physicochemical characteristics under accelerated conditions may provide insight into how well the product holds up during long-term storage [22,23].
Creaming and flocculation, two of the stability issues seen in emulsions, are slow-moving processes [24]. So, to accelerate the formulation studies in the first phase, centrifugation and accelerated heat stability experiments were carried out. Formulations that did not exhibit separation or creaming were selected for use and characterizations of these formulations were performed. When kept at 25℃, no liquefaction was observed for the entire observation period. Storage at 40℃+ 75% RH led to minor liquefaction on the 3rd and 6th month of observation. In addition, organoleptic properties were stable under 25±2°C and climate chamber at 40±2°C and 75% RH during the stability test. There was no change in color, odor, appearance, weight, pH of cream under different storage conditions up to end of the observation period of 6 months. It was decided that formulations containing Hybrosome active ingredient were susceptible to heat and humidity.
Consumer use and production processes can make cosmetic products subject to microbial contamination and degradation. Although these products are not required to be sterile, they must be sufficiently protected against both events [25]. Contamination resulting from production can be reasonably prevented while probability of contamination resulting from consumer use can only be mitigated through the addition of antimicrobial preservatives that protect against bacteria, yeast and mold growth during its shelf life [26-28]. The most widely used preservative, Phenoxyethanol [28] and Ethylhexylglycerin [28] have been combined in this study to avoid microbiological contaminations.
The chosen formulation was found to be acceptable in terms of microbiological quality as a result of the microbiological limit test. In addition, none of the formulations contained pathogens that are prohibited from being present in cosmetic items, such as Staphylococcus aureus and Pseudomonas aeruginos bacteria from the Enterobacteriaceae family [29]. Consumers and researchers in the field of cosmetics prefer non-invasive biophysical measurement techniques because they are more ethically acceptable and offer significant benefits like reproducibility. Without causing irritation, they guarantee the detection of undetectable changes in the skin or hair. They are also suitable for statistical analysis [21].
Skin has elastic and viscous characteristics and is a complex structure. Collagen and elastin fibers in the dermis are necessary for the skin’s viscoelastic properties [30]. Moreover, elasticity measurement in cosmetics typically refers to the assessment of stretch and rebound ability of skin [31]. Skin elasticity is an important parameter in the cosmetic industry as it is associated with youthful and healthy-looking skin [32]. In trial I, four different cream formulations prepared and applied to investigate the effect of different concentrations of active ingredients. Hybrosome and retinol were used in the formulations at varying concentrations as active ingredients. Retinol at 0.2% concentration is considered a proven negative control group, as supported by literature [33-35]. Formula A consisted of only base cream, which served as the control group to be compared with the other three groups. Formula B contained base cream with 0.75% active Hybrosome, Formula C contained base cream with 0.2% retinol, and Formula D contained base cream with 1.5% active Hybrosome. Figure 1A results indicate that, Hybrosome creams with different doses have been found to be approximately 30% more effective in reducing wrinkles and 22% more effective in elasticity when compared to base cream or base cream retinol used alone.
Skin elasticity is influenced by various factors, including the presence of collagen and elastin fibers in the dermis, which contribute to the viscoelastic characteristics of the skin [30]. Skin elasticity measurements shown in Figure 1B were conducted using Derma Lab Combo and ASW 300 device, which are commonly used for assessing skin elasticity in researches. The results suggest that different doses of Hybrosome to the base cream did not result in a statistically significant difference in wrinkle reduction or elasticity compared to the base cream alone or the base cream combined with retinol formulation. However, the Hybrosome creams with different doses were found to be more effective in reducing wrinkles and in improving elasticity compared to the base cream or base cream with retinol used alone. This suggests that Hybrosome may have some potential benefits in improving skin appearance.
Trial II results indicate that, the cream containing 0.75% Hybrosome showed better performance compared to the rRetinol cream in several aspects. As seen in Figure 2A, the Hybrosome cream showed a greater decrease in wrinkle volume difference between D28 and D0 compared to the retinol cream. The Hybrosome cream also exhibited an increase in healing from 12% to 14% at Day 56, while the retinol cream remained at 8% healing over the same time- period. Moreover, the Hybrosome cream showed an improvement in melanin levels over time, with a higher recovery rate at Day 56 compared to Day 28, indicating a more significant improvement with prolonged use (Figure 2B). Additionally, the Hybrosome cream showed a higher recovery rate in TEWL compared to the retinol cream as determined in Figure 2C.
The trial III results indicate that the cream with 0.75% Hybrosome concentration may be safe for use on the skin as it did not cause any allergic reactions in the tested population according to the HRIPT results. Additionally, as seen in Figure 3A, the cream showed statistically significant results in the wrinkle reduction. Moreover, as demonstrated in the regeneration test results in Figure 4A and 4B, the cream with 0.75% Hybrosome concentration demonstrated statistically significant reduction in melanin levels after topical application following exposure to 10% DHA. Images of 0,75% Hybrosome + base cream treated and untreated areas at time points of D0, D2, D5 and D10 are included in Figure 4C. These findings suggest the potential of the cream in reducing wrinkles and regulating melanin levels.
The results of the randomized blind fourth controlled trial suggest that Hybrosome cream is more effective than retinol in reducing wrinkles, improving radiance, and homogenate effect. Figure 5A and 5B showed that, after 28 days of use, Hybrosome cream demonstrated a higher reduction in wrinkles (13.4% compared to 6.89% for retinol), a higher homogenate effect (13.16% compared to 4.88% for retinol), and a higher improvement in radiance (10.75% compared to 6.25% for retinol). Comparing the D0 and D28 day images of two different patients with 0.75% Hybrosome cream formulation on the left side of the face and 0.2% retinol cream formulation on the right side in Figures 5C and 5D, a visible improvement in the area where 0.75% Hybrosome was used in crow’s feet, forehead and fine wrinkles is observed. These findings indicate that 0.75% Hybrosome cream formulation is a more effective option for addressing wrinkles, radiance, and homogenate effect compared to retinol.
Conclusion
As a result of studies, it has been seen that cream formulation with Hybrosome molecule, reduces the wrinkle area, increases the elasticity, as well as gives better results in wrinkle area and TEWL recovery against the gold standard retinol. The recovery of the forehead and crow’s feet wrinkle areas as well as the melanin level results of Hybrosome molecules improved in direct proportion to the usage, according to findings of 28 and 56-day blind tests. It is important to note that the study used non-invasive biophysical measurement techniques, which are considered more ethically acceptable and offer reproducibility, without causing irritation to the skin. The formulations were also tested for microbiological quality and found to be acceptable in terms of microbial contamination. The use of antimicrobial preservatives, such as Phenoxyethanol and Ethylhexylglycerin, was incorporated in the formulations to protect against bacterial, yeast, and mold growth during the shelf life of the product. Furthermore, stability tests were conducted to ensure that the formulations-maintained stability over the course of their shelf life, without experiencing phase separation or changes in color, smell, or appearance. Accelerated stability tests, such as centrifugation and accelerated heat stability experiments, were also performed to obtain information about stability more quickly and accurately. Overall, the formulation of cosmetic products against skin aging requires careful consideration of various factors, including product stability, microbiological quality, and efficacy as demonstrated through reliable and efficient testing methods.
Disclosures
The authors declare no conflicts of interests with respect to the authorship and/or publication of this manuscript. Dr. Cohen is a shareholder in Millennium Medical Technologies and in the Mage Group and receives royalties from Lipocube Nano. He is an investigator, trainer, and consultant for Apyx Medical, and an investigator and consultant for Musculoskeletal Transplant Foundation (MTF). He is part of the advisory board of, and investigator for, Paracrine and is key opinion leader of Cytrellis. He receives book royalties from Quality Medical Publishing (QMP)-Regenerative Facial Surgery, along with Tunc Tiryaki, Tonnard, and Verpaele. Dr. Tiryaki is an investigator for Mentor, receives book royalties from Springer, and is on the advisory board of, and holds equity in, Mage Group and Lipocube Ltd. Dr. Duncan is a plastic surgeon in private practice in Fort Collins, CO, USA. Dr. Schlaudraff is shareholder in Lipocube, Ltd. and a plastic surgeon in private practice, Geneva, Switzerland. Dr Ascher is a plastic surgeon in private practice, Paris, France, and Scientific Director of the IMCAS. Dr. Ghannam is a dermatologist in private practice in Kuwait City, Kuwait and Associate Professor of Dermatology & Venereology at the University of Alexandria, Egypt. Dr. Sterodimas is shareholder in Lipocube, Ltd. and Head of the Plastic Surgery Department of Metropolitan General Hospital, Athens, Greece. Mrs. Canikyan Turkay is Chief R&D officer at Lipocube Ltd. Dr. Koçak is Chief Executive officer at Cellestetix Biotechnology. Mrs. Kul is R&D engineer at Lipocube Ltd. and Master of Science Student at the Yıldız Technical University, Istanbul, Turkey. Mr. Tiryaki is a bachelor student at the Imperial College of Science, Technology and Medicine, London, UK.
References
- Makrantonaki E, Bekou V, Zouboulis CC (2012) Genetics and skin aging. Dermatoendocrinol 4(3): 280-284.
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC, et al. (2012) Skin anti-aging strategies. Dermatoendocrinol 4(3): 308-319.
- Farage MA, Miller KW, Elsner P, Maibach HI (2008) Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 30(2): 87-95.
- Michalak M, Pierzak M, Kręcisz B, Suliga E (2021) Bioactive Compounds for Skin Health: A Review. Nutrients 13(1): 203.
- Baumann L (2007) Skin ageing and its treatment. J Pathol 211(2): 241-251.
- Ge X, Lu L, Bai W, Wang M, Han C, et al. (2023) The novel roles of bovine milk-derived exosomes on skin anti-aging. bioRxiv: 2023-2103.
- Bu H, He D, He X, Wang K (2019) Exosomes: Isolation, Analysis, and Applications in Cancer Detection and Therapy. Chembiochem 20(4): 451-461.
- Kul Y, Erbaş O (2022) Exosomes: Classification, Isolation, and Therapeutic Applications in Various Diseases. Journal of Experimental and Basic Medical Sciences 3(1): 006-012.
- Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, et al. (2020) Isolation and characterization of exosomes for cancer research. J Hematol Oncol 13(1): 152.
- Szwedowicz U, Łapińska Z, Gajewska-Naryniecka A, Choromańska A (2022) Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules. 27(4): 1303.
- Kee LT, Ng CY, Al Masawa ME, Foo JB, How CW, et al. (2022) Extracellular Vesicles in Facial Aesthetics: A Review. Int J Mol Sci 23(12): 6742.
- Shafiei M, Ansari MNM, Razak SIA, Khan MUA (2021) A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers (Basel) 13(15): 2529.
- Lv J, Yang S, Lv M, Lv J, Sui Y, et al. (2022) Protective roles of mesenchymal stem cells on skin photoaging: A narrative review. Tissue Cell.
- Csekes E, Račková L (2021) Skin Aging, Cellular Senescence and Natural Polyphenols. Int J Mol Sci 22(23): 12641.
- Rasul A, Akhtar N, Khan BA, Mahmood T, Uz Zaman S, et al. (2012) Formulation development of a cream containing fennel extract: in vivo evaluation for anti-aging effects. Pharmazie 67(1): 54-58.
- Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit-Otmani Z, et al. (2018) Cosmetics Preservation: A Review on Present Strategies. Molecules 23(7): 1571.
- Costa C, Medronho B, Filipe A, Mira I, Lindman B, et al. (2019) Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers (Basel) 11(10): 1570.
- Walsh G (2006) Biopharmaceutical benchmarks 2006. Nat Biotechnol 24(7): 769-776.
- Akhtar N, Khan BA, Khan MS, Mahmood T, Khan HM, et al. (2011) Formulation development and moiturising effects of a topical cream of Aloe vera extract. World Academy of Science Engineering and Technology 51: 172-179.
- Venkataramani D, Tsulaia A, Amin S (2020) Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv Colloid Interface Sci 283: 102234.
- Altuntaş E, Yener G (2015) Anti-aging potential of a cream containing herbal oils and honey: Formulation and in vivo evaluation of effectiveness using non-invasive biophysical techniques. IOSR Journal of Pharmacy and Biological Sciences 10(6): 51-60.
- Bakshi M, Singh S (2002) Development of validated stability-indicating assay methods--critical review. J Pharm Biomed Anal 28(6): 1011-1040.
- Qiu F, Scrivens G (2018) editors. Accelerated predictive stability (APS): fundamentals and pharmaceutical industry practices. Academic Press.
- Lerche D, Sobisch T (2011) Direct and accelerated characterization of formulation stability. Journal of Dispersion Science and Technology 32(12): 1799-17811.
- Kim HW, Seok YS, Cho TJ, Rhee MS (2020) Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health. Sci Rep 10(1): 1561.
- Varvaresou A, Papageorgiou S, Tsirivas E, Protopapa E, Kintziou H, et al. (2009) Self-preserving cosmetics. Int J Cosmet Sci 31(3): 163-175.
- Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit Otmani Z, et al. (2018) Cosmetics Preservation: A Review on Present Strategies. Molecules 23(7): 1571.
- Stoffels KM (2012) Modern and safe antimicrobial stabilization of cosmetic products. H & PC Today 7: 18-21.
- Bashir A, Lambert P (2020) Microbiological study of used cosmetic products: highlighting possible impact on consumer health. J Appl Microbiol 128(2): 598-605.
- Silver FH, Freeman JW, DeVore D (2001) Viscoelastic properties of human skin and processed dermis. Skin Res Technol 7(1): 18-23.
- Lynch B, Pageon H, Le Blay H, Brizion S, Bastien P, et al. (2022) A mechanistic view on the aging human skin through ex vivo layer-by-layer analysis of mechanics and microstructure of facial and mammary dermis. Sci Rep 12(1): 849.
- Mukherjee PK, Maity N, Nema NK, Sarkar BK (2011) Bioactive compounds from natural resources against skin aging. Phytomedicine 19(1): 64-73.
- Milosheska D, Roškar R (2022) Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv Ther 39(12): 5351-5375.
- Duell EA, Kang S, Voorhees JJ (1997) Unoccluded retinol penetrates human skin in vivo more effectively than unoccluded retinyl palmitate or retinoic acid. J Invest Dermatol 109(3): 301-305.
- Babcock M, Mehta RC, Makino ET (2015) A randomized, double-blind, split-face study comparing the efficacy and tolerability of three retinol-based products vs. three tretinoin-based products in subjects with moderate to severe facial photodamage. J Drugs Dermatol 14(1): 24-30.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.