Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Efficacy of Oral Administration of Ergothioneine (DR. ERGO™) on Skin Health Improvement: A Single-Center, Open-Label Clinical Study
*Corresponding author: Chunyue Zhang, Lithy Research Institute, Lithy One-Health Technology Group Co., Ltd., Shanghai, China.
Received: December 15, 2023; Published: December 20, 2023
DOI: 10.34297/AJBSR.2023.20.002779
Abstract
The prevalent global trend of “Beauty from within” underscores the understanding that the skin serves as an external reflection of internal physiological states, prompting a growing preference for natural and endogenous approaches to maintain skin youthfulness. This single-center, open-label clinical trial was designed to evaluate the dermatological effects of oral administration of ergothioneine (DR.ERGO™), a potent natural antioxidant. The study enrolled 19 healthy subjects, average age 34.1 years, presenting with mild dermatological concerns, including acne, freckles, and a dull complexion. Participants were administered a daily dosage of 25 mg ergothioneine (EGT) capsules over a four-week period. Dermatological assessment was performed using the VISIA photographic system, which provided quantitative data. Measurements were taken at baseline, two-week midpoint, and the four-week conclusion. No adverse effects were reported throughout the study duration, emphasizing the safety and tolerability of the supplement. The test results revealed significant improvements in several dermatological parameters, with notable enhancements observed in the appearance of pores, wrinkles, UV spots, brown spots, and porphyrins. Some parameters exhibited substantial improvement within just two weeks of EGT administration. The study demonstrates the clinical efficacy of DR.ERGO™ in improving skin health, reinforcing the potential of oral antioxidant supplementation in dermatological care.
Ultimately, the conversation transcends the field of education, revealing universal principles that resonate across industries such as an emphasis on a holistic approach, the power of emotional connection, arts and humanities as bridges, and the difficulties of balancing diverse elements. It serves as a wellspring of inspiration for educators and professionals who seek to fathom the profound psychology of holistic education, reaffirming the enduring importance of a comprehensive curriculum in the contemporary educational landscape.
Keywords: Ergothioneine, Oral beauty, Cosmetics, Skin health
Abbreviations: EGT: Ergothioneine
Introduction
According to projections by the World Health Organization, the proportion of the global population aged 60 and above is anticipated to nearly double, increasing from 12% to 22% by the year 2050. In addition to the heightened health concerns typically associated with advanced age, individuals over 40 express apprehension regarding aesthetic changes such as graying or thinning hair, wrinkles, and skin laxity. While conventional approaches historically relied on external skincare and medical interventions to maintain skin youthfulness, contemporary perspectives prioritize a natural approach to enhance overall bodily condition for a radiant complexion. The paradigm of “Beauty from within” has garnered increasing global attention in recent years.
Ergothioneine (EGT), a unique thio-histidine betaine amino acid, is distinguished by its potent antioxidant properties, contributing significantly to its role in biological systems [1]. Predominantly found in mushrooms, and also present in red and black beans, EGT levels in animals are dependent on their consumption of EGT-rich grasses [2]. Studies have indicated a correlation between higher blood levels of EGT and reduced risk of cardiovascular diseases and mortality related to heart conditions [3]. EGT also exhibits anti-inflammatory effects that provide cellular protection. Its neuroprotective benefits have been acknowledged, with ongoing research exploring its potential in ameliorating inflammation and damage in vital organs such as the lungs, liver, kidneys, and brain [4,5].
Owing to its remarkable antioxidant and skin-protective attributes, has found extensive application in the cosmetic industry. A distinctive feature of EGT is its transport to the mitochondria via the carnitine/organic cation transporter 1 transporter in skin cells, where it plays a crucial role in combating oxidative stress by neutralizing free radicals and absorbing harmful UV rays, thereby offering robust skin protection [6]. EGT demonstrates a multifaceted approach in skincare, encompassing antioxidant, anti-inflammatory, and anti-aging properties, along with the inhibition of melanin production [7, 8]. The potential of oral supplementation of EGT in enhancing skin health has recently garnered increasing attention. A clinical investigation revealed that consecutive daily intake of EGT for 28 days led to noticeable improvements in skin hydration, trans-epidermal water loss, skin elasticity, wrinkle reduction, and anti-carbonylation, all without any adverse effects [9]. Oral EGT supplementation could be a beneficial addition to skincare regimens, offering a systemic approach to skin health and beauty.
Despite the emerging evidence supporting the cosmetic benefits of EGT, research specifically focusing on the effects of pure oral EGT supplementation on skin health remains scarce. This gap in the literature underscores the novelty and importance of our study. Our research aims to analyze and discuss the impact of a daily oral intake of 25 mg of EGT (DR.ERGOTM) on the skin condition of participants. Over a period of four weeks, this study tracks the changes in the facial skin health of subjects, aiming to provide a comprehensive understanding of the “beauty from within” effects of oral EGT supplementation.
Materials and Methods
Test Supplement
The test supplement used in this study was a hard gelatin capsule DR.ERGO™. Each capsule was formulated with 25mg of pure L-(+)-EGT with a purity of 99.9%. The DR.ERGO™ used in this study was manufactured and supplied by Shanghai EGT SYNBIO Group Co., Ltd.
Intervention
This study was conducted as a single-center, open-label, pilot study involving 19 healthy subjects. The primary objective was to investigate the effects of oral supplementation of DR.ERGO™ on the improvement of skin conditions. During the 28-day intervention period, participants were instructed to adhere to their normal dietary habits, exercise routines, and overall lifestyle patterns, ensuring that any observed changes in skin condition could be attributed primarily to the test supplement. To maintain the study’s integrity, participants were advised against the consumption of any additional supplements or functional foods known to influence skin health, such as collagen peptides.
To monitor adherence to the study protocol, all subjects were required to report their daily compliance with the supplementation regimen. Moreover, any adverse events or unexpected reactions experienced during the intervention period were to be reported immediately to the professional medical team.
Efficacy measures, which included various dermatological assessments, were conducted at three key points: at the start of the intervention (0 day), midway through the intervention (14 day), and at the end of the intervention period (28 day).
Participants
In this study, we recruited 19 healthy subjects aged between 25 and 40, with an average age of 34.1 ± 5.46 years. Prior to the commencement of the study, all subjects were provided with detailed informed consent documents. These documents thoroughly explained the nature and purpose of the study, as well as any potential risks associated with participation. It was explicitly stated in the consent form that participants had the right to withdraw from the study at any time and for any reason, without any consequences. Adequate time was allotted for the subjects to review and contemplate the information contained in the consent forms. This ensured that all participants had a clear understanding of the study and its implications. The informed consent signatures were collected from all participants before the start of the study, confirming their voluntary participation and comprehension of the study’s parameters. This process was conducted in strict adherence to regulatory requirements, ensuring ethical conduct and the protection of the participants’ rights and wellbeing throughout the study.
Inclusion and Exclusion Criteria
Inclusion Criteria: Participants with skin conditions primarily characterized by mild acne, light to moderate pigmentation, and a generally dull skin tone were eligible. These specific conditions were considered suitable for evaluating the impact of the test supplement on various skin health parameters.
Exclusion Criteria:
1) Females who were pregnant or breastfeeding.
2) Individuals with obvious facial defects, such as sunburn, scars, or pigmented nevi, that could impair the assessment of the test supplement’s effects.
3) Presence of facial microbial infections.
4) History of chronic skin diseases, including but not limited to skin tumors, rosacea, eczema, lupus erythematosus, seborrheic dermatitis, psoriasis, or severe epidermal shedding.
5) History of immunosuppressive or immunodeficiency disorders (including HIV or AIDS) or current use of immunosuppressive drugs or undergoing radiotherapy.
6) Chronic or endocrine diseases like asthma, epilepsy, diabetes, hypertension, hyperthyroidism, or hypothyroidism.
7) Participation in any other clinical studies in the past three months.
8) Use of medications in the past six months or currently that could affect skin status or response, such as antihistami nes, antibiotics, insulin, anti-inflammatory drugs, vitamins A, steroids, aspirin, thyroid drugs.
9) Undergoing facial medical treatments like laser therapy, chemical peeling, or minimally invasive cosmetic procedures.
10) History of mental illness or inability to care for oneself.
11) Any other condition or situation that, in the opinion of the researchers, could interfere with the participant’s safety, study compliance, or the interpretation of study results.
Efficacy Measures
The efficacy of the oral supplementation with DR.ERGO™ in improving skin conditions was assessed using a standardized approach. Prior to the measurement, each participant was acclimatized in a controlled environment set at a temperature of 21 ± 1 ℃ and a relative humidity of 50 ± 10% for 30 minutes. This step was crucial to ensure that the skin conditions were measured under consistent and ideal environmental conditions.
After the acclimatization period, participants underwent detailed facial skin analysis using the VISIA photographic system. The parameters assessed with VISIA included pores, wrinkles, UV spots, brown spots and porphyrins. For each participant, facial images were captured from three different angles: the left side, the frontal view, and the right side of the face.
Statistical Analysis
The data collected in this study were statistically analyzed using IBM SPSS Statistics software. Measurements were expressed as mean ± standard deviation, and initially subjected to a normal distribution test to determine their distribution pattern. For data following a normal distribution, a t-test was employed to compare skin parameters before and after supplementation. Changes were considered statistically significant at a two-sided test level of α = 0.05.
Results
During the study, participants took a capsule containing of 25 mg of EGT everyday and underwent VISIA skin analysis on days 0, 14, and 28. This comprehensive assessment focused on five key skin health parameters: pores, wrinkles, UV spots, brown spots, and porphyrins. VISIA, an advanced skin analysis instrument, utilizes a combination of white light, ultraviolet light, and polarized light to scan both the superficial and deeper layers of the skin.
The quantitative analysis provided by VISIA is based on a database comparison, allowing for a relative ranking of an individual’s skin health against a demographic database of individuals from the same region, age group, and ethnicity. In this context, higher scores in the VISIA report are indicative of better skin health status, reflecting a more favorable ranking compared to peers within the same demographic criteria.
Effects on Pores
Pores are an indicator of the state of the sebaceous glands’ openings, with their size generally correlating with the amount of sebum secretion; more active sebaceous glands tend to result in larger pores. In VISIA imaging, denser purple spots signify more severe pore enlargement. During this trial, significant changes were observed in the pore size of participants following supplementation with EGT.
After two weeks of daily intake, there was a notable increase in the pore score for the right side of the face, with an improvement of 26.08% (p < 0.05). This positive trend continued and became more pronounced by the end of the fourth week. VISIA scores for the entire face showed significant enhancement across all areas – the left, front, and right sides of the face exhibited increases of 42.89%, 46.40%, and 54.84%, respectively (p < 0.05). These results suggest that oral intake of DR.ERGO™ capsules can effectively improve the condition of enlarged pores (Figure 1).
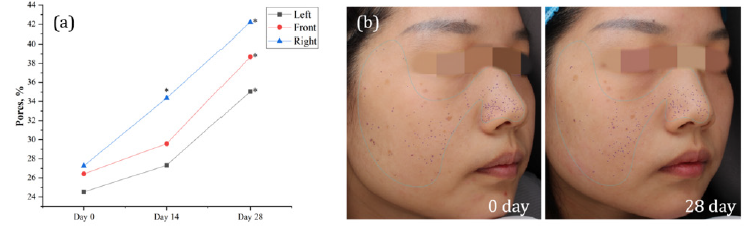
Figure 1: Effect of DR.ERGO™ on the pores. (a) VISIA values expressed in %, * indicates significant difference from 0 day, 0.01≤p<0.05. (b) VISIA images of pores, 0 day on the left, 28 day on the right, pores are signified by purple spots.
Effects on Wrinkles
DR.ERGO™ supplementation demonstrated a significant amelioration in facial wrinkles. Quantitative improvements were evident in the VISIA wrinkle scores, with a marked increase after two weeks, particularly on the left side of the face, which showed a 13.4% improvement (p < 0.01). This enhancement was more pronounced after four weeks, with a notable increase of 21.61% in the same area (p < 0.01). Corresponding improvements were also observed on the front and right sides of the face, with increases of 13.74% and 9.3% respectively (p < 0.05). These results underscore the efficacy of DR.ERGO™ capsules in significantly reducing the appearance of wrinkles, indicating its potential as a therapeutic agent in dermatological anti-aging interventions (Figure 2).
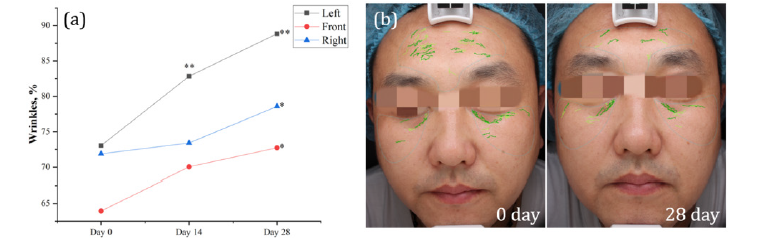
Figure 2: Effect of DR.ERGO™ on the wrinkles. (a) VISIA values expressed in %, * indicates significant difference from 0 day, 0.01≤p<0.05; ** indicates extremely significant difference from 0 day, 0.001≤p<0.01. (b) VISIA images of wrinkles, 0 day on the left, 28 day on the right, wrinkles are signified by green.
Effects on UV spots
UV spots are a manifestation of skin damage caused by sun exposure, resulting from the accumulation of melanin beneath the epidermis. These spots, typically invisible to the naked eye, require specialized equipment utilizing minute amounts of ultraviolet light for detection and observation. Generally, insufficient sun protection in daily life can lead to lower scores in this parameter, indicating more significant sun damage.
In this study, a significant improvement in the UV spots parameter was observed among the participants. After four weeks of daily intake of the DR.ERGO™ capsules, there was a notably high increase in the scores for UV spots on the front face, with an increase of 18.44% compared to the baseline (p < 0.01). This substantial improvement indicates a marked difference and suggests that oral supplementation with DR.ERGO™ can effectively mitigate the accumulation of facial melanin caused by UV exposure (Figure 3).
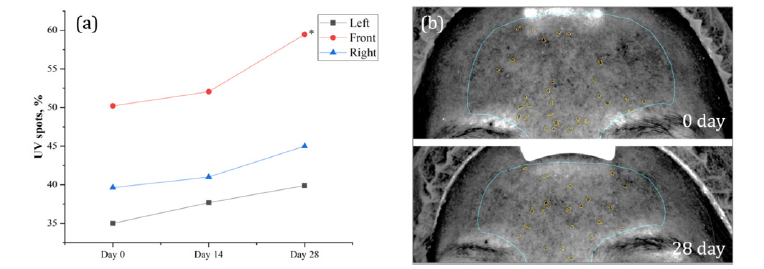
Figure 3: Effect of DR.ERGO™ on UV spots. (a) VISIA values expressed in %, ** indicates extremely significant difference from 0 day, 0.001≤p<0.01. (b) VISIA images of UV spots, 0 day above, 28 day below, UV spots are signified by yellow spots.
Effects on Brown Spots
Brown spots, like UV spots, are not visible to the naked eye. They represent deeper skin issues, lying beneath the dermis as latent pigmentation, such as freckles, melasma, and age spots. These spots can become more prominent and rise to the surface as one ages or if the skin barrier is compromised. In this study, a significant improvement in brown spots was observed among participants. After two weeks of daily supplementation with DR.ERGO™ capsules, there was a notable increase of 7.99% in the brown spot scores on the right side of the face (p < 0.05). This improvement was even more pronounced after four weeks of supplementation. The values for brown spots on the right side of the face showed a highly significant increase compared to the baseline, with an elevation of 16.57% (p < 0.01). These findings suggest that oral intake of DR.ERGO™ capsules can effectively alleviate deep-seated pigment issues in the skin (Figure 4).

Figure 4: Effect of DR.ERGO™ on brown spots. (a) VISIA values expressed in %, * indicates significant difference from 0 day, 0.01≤p<0.05; ** indicates extremely significant difference from 0 day, 0.001≤p<0.01. (b) VISIA images of pores, 0 day on the left, 28 day on the right, brown spots are signified by blue spots.
Effects on Porphyrins
Porphyrins, are metabolic by-products of certain bacteria residing in hair follicles and are indicative of the sebaceous gland secretion status. They are often associated with the development of acne, with lower values suggesting increased sebum secretion. In this study, an improvement in porphyrin levels was observed, indicating a positive impact on skin conditions related to sebum production and bacterial activity.
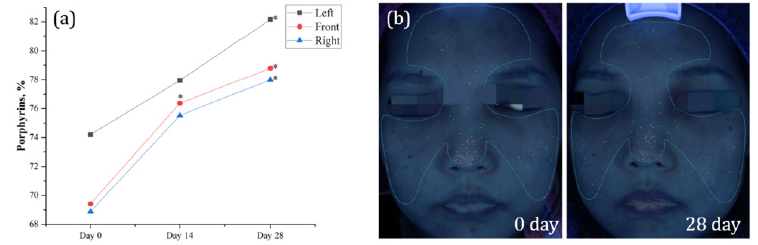
Throughout the trial, there was a consistent increase in the overall facial scores for porphyrins among the participants. After two weeks of daily supplementation with DR.ERGO™ capsules, there was a significant increase of 10.01% in the porphyrin scores on the front face (p < 0.05). This improvement was more pronounced after four weeks of intake. The VISIA scores for porphyrins across the entire face showed a significant enhancement (p < 0.05), with the left, front, and right sides of the face showing increases of 10.71%, 13.50%, and 13.22%, respectively. These results indicate that oral supplementation with DR.ERGO™ capsules can effectively improve the condition of the skin concerning bacterial metabolic by-products, primarily porphyrins (Figure 5).
Discussion
A novel finding of our study is the ability of EGT to balance skin sebum, evidenced by the reduction in pore size and porphyrins. Pores reflect the state of sebaceous gland openings, typically larger with increased sebum secretion. Porphyrins, metabolic by-products of bacteria in hair follicles, indicate sebaceous gland activity. The alleviation of both symptoms suggests EGT’s efficacy in balancing sebum. Skin oil-water balance involves several factors, including water retention, oil production, skin barrier function, moisturizers and transepidermal water loss (TEWL) [10]. EGT shows promise in preserving skin hydration, reducing TEWL, and maintaining the skin barrier [1,7-8], thereby promoting skin oil-water balance regulation.
The observed increase in porphyrin scores suggests a reduction in sebum secretion and possibly a decrease in the bacterial activity associated with acne formation. This outcome demonstrates the potential of DR.ERGO™ as a beneficial supplement for managing skin conditions related to sebum production and bacterial activity, contributing to overall skin health and appearance.
Building on previous research, our experiment similarly found that oral intake of EGT can improve wrinkles. This effect is primarily due to EGT’s antioxidant and anti-inflammatory properties, which protect the skin against UV damage, reduce inflammation, and inhibit signs of skin aging, including the appearance of wrinkles. A notable study on the effects of EGT on skin hydration, elasticity, and wrinkles showed significant improvements in wrinkle area after 28 days of supplementation [9]. Furthermore, EGT has been identified for its anti-aging activity in UVA-irradiated human dermal fibroblasts.
Various spots in the skin, such as UV spots and brown spots, are related to photoaging, glycation and melanin deposition respectively. EGT demonstrates significant anti-aging properties in combating photoaging, glycation, and melanin deposition through its unique molecular structure and biological mechanisms. The primary mechanism for EGT’s inhibition of melanin production is attributed to its sulfur-substituted imidazole ring structure [11]. Thiol-containing compounds, similar to EGT, are known melanogenesis inhibitors as they react with dopaquinone to form colorless conjugates [12]. EGT’s sulfur atom exists as a thione rather than a sulfhydryl group, differentiating its inhibition mechanism from other tyrosinase inhibitors. EGT acts as a reversible tyrosinase inhibitor, binding to the substrate site of the enzyme and inhibiting its activity more effectively than common inhibitors like kojic acid and arbutin [13,14].
In terms of combating oxidative stress, EGT activates the SIRTs and Nrf2 pathways to enhance antioxidant capacity, thereby mitigating oxidative damage [15-17]. EGT’s structure is instrumental in absorbing UV radiation and inhibiting melanin generation (Liu et al., 2023). Additionally, the suppression of inflammation and the activation of immune responses by EGT are potentially regulated through the ROS or dominated by the AP-1 and MAPK signaling pathways [18,19]. These pathways contribute to the overall protective mechanism of EGT against skin aging, although it’s suggested that other undiscovered mechanisms may also be involved.
Conclusion
This clinical study demonstrates that daily oral supplementation of EGT leads to notable improvements in various skin health parameters. These include a marked reduction in pore size and porphyrin levels, indicating its effectiveness in balancing sebum production and bacterial activity in the skin. Furthermore, EGT’s role in diminishing the appearance of wrinkles showcases its potential as an anti-aging agent. The supplement also proved effective in reducing UV and brown spots, suggesting its capability in mitigating photoaging effects and melanin deposition. The findings of this study emphasize the potential of DR. ERGOTM as a potent oral supplement with comprehensive skincare benefits. It presents solutions for the preservation of skin hydration, reduction of trans-epidermal water loss, and maintenance of the skin barrier. These outcomes collectively contribute to the enhancement of overall skin health and appearance. The results suggest that EGT holds promise as a key component in the pursuit of “beauty from within”.
Acknowledgements
The authors wish to acknowledge Shanghai EGT SYNBIO Group Co., Ltd., for providing the test supplement and Shangdong UGEL Bio-tech Co., Ltd., for the clinical experimentation.
Conflict of Interest
The authors declare no conflict of interest.
References
- Cheah IK, Halliwell B (2021) Ergothioneine, recent Redox Biology 42: 101868.
- Cheah IK, Halliwell B (2020) Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants (Basel) 9(7): 595.
- Wu LY, Cheah IK, Chong JR, Chai YL, Tan JY, et al (2021) Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in Free Radical Biology and 381 Medicine 177: 201-211.
- Servillo L, Castaldo D, Casale R, D’Onofrio N, Giovane A, et al (2015) An uncommon redox behavior sheds light on the cellular antioxidant properties of ergothioneine. Free Radical Biology and Medicine 79: 228-236.
- Song TY, Chen CL, Liao JW, Ou HC, Tsai MS (2010) Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in Food and Chemical 372 Toxicology 48(12): 3492-3499.
- Shimizu T, Masuo Y, Takahashi S, Nakamichi N, Kato Y (2015) Organic cation transporter Octn1-mediated uptake of food-derived antioxidant ergothioneine into infiltrating macrophages during intestinal inflammation in mice. Drug Metabolism and Pharmacokinetics 30(3): 231-239.
- Bazela K, Solyga Zurek A, Debowska R, Rogiewicz K, Bartnik E et al (2014) Ergothioneine Protects Skin Cells against UV-Induced Damage-A Preliminary Cosmetics 1(1): 51-60.
- Liu HM, Tang W, Wang XY, Jiang JJ, Zhang W et al. (2023) Safe and Effective Antioxidant: The Biological Mechanism and Potential Pathways of Ergothioneine in the Skin. Molecules 28(4): 1648.
- Cheng D, Fei WC, Zhang YX, Xu QY (2022). The Effects of L-ergothioneine on Skin Hydration, Elasticity, Brightening, Anti-carbonylation and Anti-glycation: A Single Center, Open Label Pilot Current Trends on Biotechnology & Microbiology, 3(2).
- Honari G, Maibach H (2014) Chapter 1 - Skin Structure and In H. Maibach & G. Honari (Eds.), Applied Dermatotoxicology 1-10.
- Liao WC, Wu WH, Tsai PC, Wang HF, Liu YH et al (2012) Kinetics of Ergothioneine Inhibition of Mushroom Applied Biochemistry and Biotechnology 166(2): 259-267.
- Tsuji Naito K, Hatani T, Okada T, Tehara T (2007) Modulating effects of a novel skin- lightening agent, α-lipoic acid derivative, on melanin production by the formation of DOPA conjugate products. Bioorganic & Medicinal Chemistry 15(5): 1967-1975.
- Chan CF, Lai ST, Guo YC, Chen MJ (2014) Inhibitory effects of novel synthetic methimazole derivatives on mushroom tyrosinase and Bioorganic & Medicinal Chemistry 22(9): 2809-2815.
- Luisi G, Stefanucci A, Zengin G, Dimmito MP, Mollica A (2019) Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Antioxidants 8(1): 7.
- D'Onofrio, N, Martino E, Balestrieri A, Mele L, Cautela D, et al (2022) Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Letters, 596(10): 1313-1329.
- Hseu YC, Lo HW, Korivi M, Tsai YC and Tang MJ, et al (2015). Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human Free Radical Biology and Medicine 86: 102-117.
- Hseu YC, Vudhya Gowrisankar Y, Chen XZ, Yang YC and Yang HL (2020). The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Oxidative Medicine and Cellular Longevity 2020, 2576823.
- Colognato R, Laurenza I, Fontana I, Coppede F and Siciliano G, et al (2006). Modulation of hydrogen peroxide-induced DNA damage, MAPKs activation and cell death in PC12 by Clinical Nutrition 25(1): 135-145.
- Hseu YC, Vudhya Gowrisankar Y, Chen XZ, Yang YC and Yang HL (2020). The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Oxid Med Cell Longev 2020: 2576823.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.