Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
How to Decide Which Covid-19 Patient with Myocardial Infarction to Send to the Cath Lab? - A Case Series of Covid-19 Patients with Myocardial Infarction
*Corresponding author: Denis Nikolov, Heart and Brain Center of clinical Excellence Hospital, Pleven, Bulgaria.
Received: November 24, 2023; Published: December 04, 2023
DOI: 10.34297/AJBSR.2023.20.002753
Abstract
Introduction: The Coronavirus pandemic has hit the world with its vast contagiousness, high morbidity, and mortality. Apart from the direct damage to the lung tissue, the corona virus infection is able to predispose patients to thrombotic disease, thus causing cerebral or coronary incidents.
Aims: The aim of this study was to find a clinical or laboratory parameter, that would help in distinguishing between COVID-19 patients with Myocardial Infarction (MI), who have an Infarct-Related Artery (IRA) and therefore, require immediate revascularization, and those, who have no IRA. This is indeed necessary in order to be able to make faster and more correct decisions for those patients. Methods: This was a single-center, cohort observational study of 26 consecutive patients with COVID-19, who were admitted with confirmed MI.
Results: In our study group of 26 patients, the mean age was 68.35±10.92 years for the patients with IRA and 64.33±9.62 for the patients without IRA. All of the patients with IRA had arterial hypertension; After coronary angiography, we found that 17 patients (65.38%) had an IRA, and they underwent pPCI. The other 9 (34.62%) did not have an IRA, they did not require pPCI, and the diagnosis of Myocardial Infarction with No Obstructive Coronary Arteries (MINOCA) was made, most probably due to myocarditis or microvascular dysfunction. Comparing the patients with IRA to those without, we found that the subjects who finally required pPCI had significantly higher hsTRI values and exclusively had typical chest pain. We performed a binary logistic regression and we found that hsTrI values>2.63 was the only independent predictive factor for the presence of IRA and need for pPCI
Conclusion: Comparing the patients with IRA to those without we found that the subjects who required pPCI had significantly higher hsTRI values, and more often typical chest pain. We found that hsTrI values >2.63 was the only independent predictive factor for the presence of IRA and need for pPCI.
Introduction
The coronavirus pandemic has hit the world with its vast contagiousness, high morbidity, and mortality [1]. Apart from the direct damage to the lung tissue, the corona virus infection is associated with multiple organ damage, including the heart. Emerging evidence reveals a direct correlation between COVID-19 and cardiovascular complications, such as heart failure, myocarditis, arrhythmias, conduction abnormalities and acute coronary syndromes [2]. The SARS-CoV-2 infection can frequently induce coagulation abnormalities that are associated with cardiopulmonary deterioration and death as a possible complication in all patients, despite presence or absence of concomitant risk factors and diseases. In addition, many patients with severe COVID-19 undergo thromboembolic events, which seem to be related to this particular coagulopathy [3,4]. One of the most unpleasant and life-threatening types of thromboembolism is the one involving the coronary circulation, thus causing a heart attack. Many additional problems arise due to this condition e.g., access to a Cath lab, exposure of additional medical personnel, more complications and increased mortality for the patients.
Invasive angiography for COVID-19 patients is logistically challenging and, in some cases, there is no intervention target, since microcirculatory disease and thrombosis is common in this group. Therefore, we studied in detail the case series of 26 patients referred for primary percutaneous coronary intervention (pPCI) for MI in our catheterization laboratory during the course of COVID-19 infection [5]. And we set ourselves the purpose to evaluate if there are some factors or parameters that could predict the presence of an interventional target - infarct related artery (IRA), prior to catheterization, and to determine their sensitivity and specificity.
Materials and Methods
The COVID-19 department of Heart & Brain University Hospital, Pleven, Bulgaria, functions since 11.2020, with 64 beds, 24 of which are intensive with the option for mechanical ventilation. For the last two months of 2020, 214 patients were treated in our COVID-19 department. 26 of them were referred to the catheterization laboratory for selective coronary angiography with Myocardial Infarction (MI), defined by the fourth universal definition of MI [6]. Most of our patients were directed to our hospital with ACS as their diagnosis, while others developed ACS during their stay in the COVID department and were therefore brought to the Cath lab. During the procedure, appropriate Personal Protective Equipment (PPE) is worn by the medical personnel, including a sterile gown, gloves, goggles and a N95 mask. The patient is brought to the Cath lab trough a different one-way corridor, in order to reduce chances of infection. The angiography includes a standard set of diagnostic and guiding catheters, mainly EBU 3.5/6Fr for the left coronary artery, and JR3.5 for the right, coronary guidewires, drug eluting stents and balloons. The majority of the catheterization laboratories have either normal or positive ventilation systems and are not designed to contain an infectious environment. Therefore, catheterization laboratories will require a thorough disinfection following every procedure, leading to delays for the scheduled procedures.
Statistical Analyses
Statistical analyses were performed using SPSS statistical software for Windows version 19.0. The distribution of continuous variables was tested using the Kolmogorov-Smirnov test. Normally distributed data were presented as mean±Standard Deviation (SD), whereas non-normally distributed data - as median and Interquartile Range (IQR) (the difference between the 25th and 75th percentile). Categorical variables were presented in percentage terms. We compared differences between groups with Independent-Samples T-Test. Sensitivity, specificity, Positive and Negative Predictive Values (PPV and NPV) were calculated according to the True Positive (TP), False Positive (FP), True Negative (TN) and False Negative (FN) results, using the following formulas: Sensitivity = TP/ (TP+FN); Specificity = TN/(TN+FP); PPV = TP/(TP+FP); NPV = TN/ (TN+FN). A two-tailed p value < 0.05 was considered statistically significant.
For the data analysis we performed a binary logistic regression and for assessment of the diagnostic capabilities of the evaluated parameter, we performed ROC analysis.
Ethics
All patients signed an informed consent for coronary angiography and PCI, and for personal data analysis. The study protocol is in accordance with the Declaration of Helsinki and was approved by the Institutional Board Review (IRB). Also, the study was registered in clinicaltrials.gov.
Results
Mean age in our group was 66.9 years; 15 (57.6%) patients were male and respectively 11 (42.3%) - female. All patients but one had hypertension; 22 (84.6%) patients had dyslipidemia. On admission 19 (73.08%) had typical chest pain. Eleven of the patients with IRA (73.3%) had ST segment elevation, typical for ST elevation myocardial infarction (STEMI) against 5 (55.6%) in the group of patients without IRA.
After coronary angiography, we found that 17 patients (65.3%) had an infarct related artery / lesion (IRA) and they underwent pPCI. The other 9 (34.6%) did not have an IRA, pPCI was not performed, and the diagnosis of myocardial infarction with no obstructive coronary arteries (MINOCA) was made, most probably due to myocarditis.
Comparing the patients with IRA to those without, we found that the subjects who finally required pPCI had significantly higher hsTRI values and exclusively had typical chest pain. The other studied variables did not differ significantly between the groups with or without IRA - (Table 1).
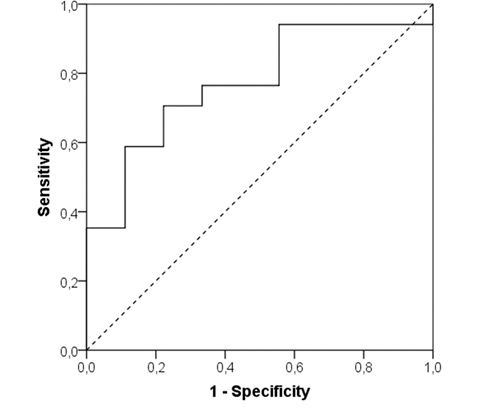
We performed a ROC analysis for hsTrI values and we found that hsTrI cut-off >2.63 showed sensitivity 70.6%, specificity 77.8%, positive predictive value (PPV) of 85.7% and negative predictive value (NPV) of 58.3% for detecting the presence of IRA and need for pPCI in ACS COVID-19 patients (Area under the curve - AUC 0.771; 95% confidence interval 0.59-0.96; p=0.025) - (Figure 1).
We performed a binary logistic regression and we found that hsTrI values >2.63 was the only independent predictive factor for the presence of IRA and need for pPCI (Odds ratio 8.4; 95% CI 1.27- 55-39, p=0.027).
Discussion
Myocardial infarction, defined by the fourth universal definition of MI, could complicate up to 5% of COVID-19 cases. In our study group, 34.6% of the patients with MI did not have an IRA and, consequently, did not need a coronary intervention. Patients with MI and IRA had significantly higher hsTrI values and exclusively typical chest pain, compared to patients with MI but without an IRA, whose hsTrI values were lower and chest pain was atypical or non-stenocardic. ECG changes had no statistical significance for distinguishing between MI patients with or without IRA. Our results suggest that using a higher cut-off value for hsTrI increases the specificity for diagnosing a MI and therefore - interventional treatment.
According to the fourth universal definition of myocardial infarction the diagnosis requires evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. These criteria require detection of a rise and/or fall in cardiac biomarker levels (preferably cardiac troponin) with at least one value above the 99th percentile upper reference limit, with at least one of the following: symptoms of myocardial ischemia, new or presumed new significant ST-segment T-wave changes or new left bundle branch block, development of pathological Q-waves on the ECG, imaging evidence of loss of viable myocardium or new regional wall motion abnormality or identification of intracoronary thrombus by angiography or autopsy.
This universal definition of MI, however, might not be the optimal guide to send a patient to the catheterization laboratory in the setting of procoagulation abnormalities in the course of acute or post-acute COVID-19. The range of clinical responses to COVID-19 is extremely broad. Endothelial injury is an underlying mechanism that links the inflammation and consequent thrombosis [7,8]. It is currently hypothesized that the ACE-2 receptor is the entry gate for the virus to invade and infect tissues. The vascular endothelium appears to be targeted directly by the virus as ACE-2 is expressed widely in the blood vessels and the heart. The result is exocytosis of endothelial granules containing VWF (Von Willebrand Factor), P-selectin, and other proinflammatory cytokines, which mediate platelets adhesion, aggregation, and leukocyte adherence to the vessel wall, with a final result of intravascular thrombosis [9].
Limitations
Even though our analysis is on a small number of patients, a similar incidence of arterial (coronary and cerebral) thrombosis (4%) has been described by other authors. In this study, however, the authors have not provided a guide to the right moment of interventional treatment. According to our published data search, we were not able to find another study, analyzing the predictors for the presence of IRA and the need for pPCI in COVID-19 MI patients.
Conclusion
In our analysis we confirm that a higher cut-off value for hsTrI helps distinguish between COVID patients with ACS, who have IRA and therefore, require immediate revascularization, compared to those, who have no IRA. However, we should also keep in mind that high troponin levels are not a pathognomonic finding and it is raised for many reasons.
Acknowledgement
None.
Conflict of Interest
None.
References
- Manuel Rattka, Jens Dreyhaupt, Claudia Winsauer, Lina Stuhler, Michael Baumhardt, et al. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. BMJ Journals 107(6).
- Arun Samidurai, Anindita Das (2020) Cardiovascular Complications Associated with COVID-19 and Potential Therapeutic Strategies. Int J Mol Sci 21(18): 6790.
- Hidesaku Asakura, Haruhiko Ogawa (2021) COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol 113(1): 45-57.
- Marco Schiavone, Cecilia Gobbi, Giuseppe Biondi Zoccai, Fabrizio D’Ascenzo, Alberto Palazzuoli, et al. (2020) Acute Coronary Syndromes and Covid-19: Exploring the Uncertainties. J Clin Med 9(6): 1683.
- Daniel Arthur Kasal, Andrea De Lorenzo, Eduardo Tibiriçá (2020) COVID-19 and Microvascular Disease: Pathophysiology of SARS-CoV-2 Infection With Focus on the Renin-Angiotensin System. Heart Lung Circ 29(11): 1596-1602.
- Thygesen K, Alpert JS, Jaffe AS (2012) Fourth universal definition of myocardial infarction. Eur Heart J 33: 2551-2567.
- Zsuzsanna Varga, Andreas J Flammer, Peter Steiger, Martina Haberecker, Rea Andermatt (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234): 1417-1418.
- Charles J Lowenstein, Scott D Solomon (2020) Severe COVID-19 Is a Microvascular Disease. Circulation 142(17): 1609-1611.
- James D McFadyen, Hannah Stevens, Karlheinz Petern (2020) The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res 127(4): 571-587.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.