Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Immunoreactivity of Monoaminoxidase Type B, Neun, Neuroglobin, and ATP Synthase in Rat Brain Histaminergic Neurons in the Dynamics of Postnatal Ontogenesis
*Corresponding author: Ekaterina M Phedina, Department of Histology, Cytology and Embryology, Grodno State Medical University, Grodno, Bolshaja Troickaja str, 4, 230023, Republic of Belarus.
Received: August 25, 2023 Published: September 05, 2023
DOI: 10.34297/AJBSR.2023.20.002663
Abstract
Objective: The purpose of the study is evaluation of monoaminoxidase type B, NeuN, neuroglobin, and ATP synthase immunoreactivity in E2 nucleus histaminergic neurons of rat hypothalamus in the postnatal ontogenesis dynamics.
Material and Methods: The scientific work was performed on the offspring of outbred white rats. The decapitation of rats was carried out on the 5th, 20th and 45th days after birth. We used immunohistochemical, cytophotometric and statistical research methods in the work. The obtained data were processed by nonparametric statistics.
Results: In developing histaminergic neurons of rat brain, from 5th to 90th day after birth, the immunoreactivity of monoaminoxidase type B, NeuN, neuroglobin, and ATP synthase simultaneously increases.
Conclusion: During the postnatal development of histaminergic neurons in parallel with their structural and metabolic formation, a simultaneous increase in the immunoreactivity of monoaminoxidase type B, NeuN, neuroglobin, and ATP synthase occurs.
Keywords: Histaminergic neurons, Monoaminoxidase type B, NeuN, Neuroglobin, ATP synthase, Postnatal development
Abbreviations: LQ: Upper Limit of the Lower Quartile; MAO-B: Monoaminoxidase type B; ME: Median; UQ: Lower Limit of the Upper Quartile; NGB: Neuroglobin
Introduction
The problem of brain formation in ontogenesis is one of the priorities in medicine and biology. A large number of clinical observations and experimental studies on animals indicate that the effects of adverse environmental factors during certain periods of brain development (the so-called critical periods) leave a long mark and create the basis for the development of various CNS pa thologies [1,2]. With the advent of new methods in the arsenal of neuromorphologists, the possibilities of studying the brain have expanded significantly. In recent decades, to study the development of neurons in postnatal ontogenesis, molecular markers have been actively used, which allow not only to determine the morphological features of cell maturation, but also to obtain information about their differentiation and functional state.
Thus, the marker of mature neurons, the NeuN (neuronal nuclei) protein, is localized in the nuclei and perinuclear cytoplasm of mammalian CNS neurons. This protein is actively used in immunohistochemical studies as a universal neurospecific marker in the study of neuronal differentiation [3]. Neuroglobin (Ngb) is an iron-containing protein that is typically localized in the vertebrate nervous system, predominantly in the perinuclear regions of nerve cells [4]. It serves to store and transport oxygen to the mitochondria of neurons, thereby contributing to the maintenance of brain oxygen homeostasis [5], and also affects several metabolic pathways, including the maintenance of ion homeostasis, energy metabolism, and cell signaling [6]. ATP synthase is an integral protein of the inner membrane of mitochondria. It is located in close proximity to the respiratory chain and is designated as complex V, which carries out the reaction of ATP synthesis from ADP [7].
One of the most important neurotransmitter systems in the brain is the histaminergic system. The bodies of histaminergic neurons in the brain of adult vertebrates are limited to the tuberomammillary region of the posterior hypothalamus, where they are located in separate groups-nuclei (E1-E5) [8,9]. The E2 nucleus is the largest and contains more than half of histaminergic neurons [9]. Like most other aminergic systems, the histaminergic system is arranged according to the “tree” principle: a small number of neurons (in the rat brain-only 3-4 thousand, in the human brain-64 thousand) innervate billions of cells of the cortex and subcortical structures and, thus, participate in the regulation of many CNS functions [10-12]. Monoamine Oxidase Type B (MAO-B) acts as a marker of histaminergic neurons. The protein is a key enzyme of histamine metabolism in the brain, since it is responsible for the oxidative deamination of this biogenic amine [9].
It is of considerable interest to study the immunoreactivity of the molecular markers listed above in developing histaminergic neurons, since there are no data on such studies in world literature. The purpose of the study is evaluation of monoaminoxidase type B, NeuN, neuroglobin, and ATP synthase immunoreactivity in E2 nucleus histaminergic neurons of rat hypothalamus in the postnatal ontogenesis dynamics.
Material and Methods
Animals and Experimental Design
The study was performed on the offspring of outbred white rats (15 pups), in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes [13]. The animals were on a standard vivarium diet. Rats that reached the required age were taken out of the experiment by decapitation and the hypothalamus was taken. Decapitation of rat pups was carried out on the 5th, 10th, 20th, 45th and 90th days after birth (for a better assessment of the dynamics of development, one rat pup was taken from each litter for each period). Identification of brain structures was carried out according to the stereotaxic atlas schemes [14].
Immunohistochemistry
Samples of the hypothalamus were fixed in zinc-ethanol-formaldehyde [15] at +4°C (overnight), and then embedded in paraffin. Paraffin sections 5 μm thick were prepared using a microtome Leica RM 2125 RTS (Leica Microsystems GmbH, Germany) and mounted on glass slides. The preparations were processed according to the protocol of the immunocytochemical reaction for light microscopy, excluding the procedure of thermal unmasking of antigens [16].
For immunohistochemical detection of MAO-B, a key enzyme of histamine metabolism in the brain and a marker of the hypothalamus histaminergic neurons [9], primary polyclonal rabbit antibodies against MAO-B (cat.No. EPP15673, Elabscience, China) were used at a dilution of 1:100, at +4ºС, 20 hours, in a humid chamber. Bound primary antibodies were detected using the detection kit (cat.No. E-IR-R213, Elabscience, China) [17]. To assess the maturation of histaminergic neurons, neuronal nuclear protein NeuN (a marker of mature neurons [3]) was determined in the hypothalamus. For immunohistochemical detection of NeuN, primary polyclonal rabbit antibodies (ab.128886, Abcam, UK) were used (at a dilution of 1:400, at +4°C, 20 hours, in a humid chamber). To detect bound primary antibodies, the EXPOSE Rabbit specific HRP/ DAB detection IHC kit (ab.80437, Abcam, UK) was used.
For immunohistochemical detection of neuroglobin, a protein involved in maintaining cell gas homeostasis [4,5], primary monoclonal mouse Anti-Ngb antibodies (ab. 14748, Abcam, UK) were used at a dilution of 1:600 at +4°C, exposure 20 hours, in a humid chamber. The EXPOSE Mouse and Rabbit specific HRP/DAB detection IHC kit (ab. 80436, Abcam, UK) was used to detect bound primary antibodies.
To determine the immunoreactivity of the molecular marker of mitochondria ATP synthase (complex V, which forms ATP from ADP [7]), primary monoclonal mouse Anti-ATP5A antibodies (ab. 14748, Abcam, UK) were used at a dilution of 1:2400 at +4ºС, exposure 20 hours, in a humid chamber. The EXPOSE Mouse and Rabbit specific HRP/DAB detection IHC kit (ab. 80436, Abcam, UK) was used to detect bound primary antibodies.
Histological preparations were studied, photographed and analyzed with help microscope Axioskop 2 plus (Zeiss, Germany), built-in digital video camera Leica DFC 320 (Leica Microsystems GmbH, Germany) as well as computer image analysis programs ImageWarp (BitFlow, USA).
Statistics
The primary data obtained were treated by nonparametric statistics using the Statistica 10.0 program (Stat Soft, Inc., USA). Quantitative results were presented as “Me (LQ; UQ)”, where Me is the median, LQ is the upper limit of the lower quartile, and UQ is the lower limit of the upper quartile. Comparison of groups on one basis was carried out using the Mann-Whitney U-test for independent samples. Differences between groups were considered statistically significant if the probability of an erroneous estimate did not exceed 5% (p<0.05, where p is the critical value of the significance level).
Results
The results of our immunohistochemical study showed that from the 5th to the 90th day of postnatal development in rats, the immunoreactivity of all studied molecular markers in histaminergic neurons increases significantly (Figure 1).
It was found that on the 5th day after birth, MAO-B was not detected in histaminergic neurons, and from the 10th to the 90th day it increased by 3.7 times (p<0.001). From the 10th to the 20th day, this indicator increases by 2 times, from the 20th to the 45th day-by 1.4 times, and from the 45th to the 90th day-by 1.3 times (Table 1) (Figure 1A, 1B).
The nuclear protein NeuN immunoreactivity in the hypothalamus histaminergic neurons increases by 1.4 times from the 5th to the 90th day of postnatal development (p<0.001). Thus, in the intervals from the 5th to the 10th day, from the 10th to the 20th and from the 20th to the 45th day, the immunoreactivity of this marker increases by 1.1 times, and from the 45th to the 90th day it does not undergo significant changes (Table 1) (Figure 1C, 1D).
The expression of Ngb from the 5th to the 90th day of postnatal development in the histaminergic neurons of the rat brain increases by 1.6 times (p<0.001). At the same time, from the 5th to the 10th day, the immunoreactivity of this marker increases by 1.2 times, from the 10th to the 20th day-by 1.1 times, from the 20th to the 45th day-by 1, 2 times, and does not change significantly from the 45th to the 90th day (Table 1) (Figure 1E,1F).
Expression of ATP synthase (a marker of mitochondria inner membrane) in the rat hypothalamus histaminergic neurons from the 5th to the 90th day of postnatal development increases by 1.5 times (p<0.001). At the same time, in the intervals from the 5th to the 10th, from the 20th to the 45th and from the 45th to the 90th day, the immunoreactivity of this protein increases by 1.1 times, and from the 10th to the 20th day it does not undergo significant changes (Table 1) (Figure 1G, 1H).
(Figure 1 & Table 1)
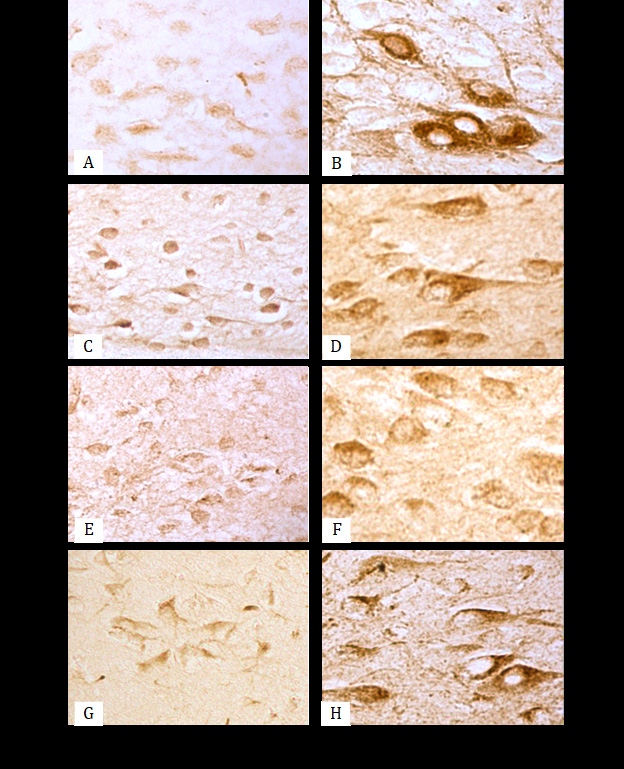
Figure 1: Immunoreactivity of MAO-B (A, B), NeuN (C, D), Ngb (E, F) and ATP synthase (G, H) in E2 nucleus histaminergic neurons of rat hypothalamus. 5th day (C, E, G), 10th day (A), 90th day (B, D, F, H). One can clearly see an increase in the size of histaminergic neurons and an increase in the immunoreactivity of the studied molecular markers in their cytoplasm in postnatal ontogenesis. The molecular markers are located in the form of fine grains mainly in the cytoplasm of perikarya, are not detected in the nuclei of neurons, and they are few in the neuropil, between the bodies of neurocytes. Immunocytochemical reaction. Digital micrograph. ×800.
Discussion
The transition of histaminergic neurons from a poorly differentiated to a mature state in postnatal ontogenesis is accompanied by certain changes in their structure. From the 5th to the 90th day after birth, in the E2 nucleus of the hypothalamus of rats, a significant increase in the size of histaminergic neurons is observed (especially from the 5th to the 10th day), while the distance between their perikarya increases (mainly during synaptogenesis, from days 5 to 20), which reflects the accelerated growth of the neuropil and leads to a significant decrease in the number of neuronal bodies per unit area of the E2 histaminergic nucleus. In the process of postnatal development in histaminergic neurons, the nuclear apparatus is reorganized, as a result of which the number of nucleoli and the number of ribosome subunits accumulating between the nucleoli and the karyolemma decrease in the nuclei, the transition from compact nucleoli observed in 5-day-old animals to larger nucleoli of the classical reticular type, while the number of all organelles in the cytoplasm increases. Thus, there is a regular development of the functional apparatuses of the cell: energy, synthetic, as well as digestion and protection [18,19].
It should be noted that by the time of birth and in the first two weeks of animal life, histaminergic neurons do not fully perform the function of histamine production [20]. The absence of activity of monoaminoxidase type B (a marker enzyme of histaminergic neurons [9]) revealed by us on the 5th day of postnatal development indicates a low oxidative deamination of histamine in the studied neurons. At this time, mast cells are responsible for the formation of a significant part of the total pool of this biogenic amine in the brain [20,21]. However, at the age of approximately two weeks of postnatal development, the total content of histamine produced by mast cells gradually decreases to the level characteristic of adults [20], while MAO-B activity in the cytoplasm of histaminergic neurons progressively increases. According to some scientists, the decrease in histamine synthesis in mast cells is due to the functional formation of histaminergic neurons in the tuberomammillary region, which also begin to produce it [20]. Thus, an increase in the immunoreactivity of the enzyme of oxidative deamination of histamine, MAO-B, in the cytoplasm of histaminergic neurons indicates the formation of a specific neurotransmitter metabolism of these neurons in postnatal ontogenesis.
Thus, after the birth of animals, the differentiation of histaminergic neurons actively continues. This is accompanied by a gradually increasing immunoreactivity of the NeuN protein (a marker of differentiating nerve cells [3]) up to the 45th day of postnatal ontogenesis. The high level of NeuN expression remains in the neurocytes studied by us on the 90th day. According to the literature, NeuN expression is detected throughout the life of a neuron [22]. It should be noted that the presence of this protein exclusively in neurons, its predominantly intranuclear localization, and the ability to bind to RNA allowed scientists to suggest that NeuN is involved in neuron-specific splicing of pre-mRNA. Indirect confirmation of its functioning as a splicing regulator is the concentration of this protein in the composition of nuclear speckles (nuclear bodies, consisting of interchromatin granules), which are the places of storage and modification of splicing factors. NeuN is supposed to be involved in the processing of primary microRNA [3]. The presence of NeuN throughout the life of a neuron, in turn, indicates the role of this protein as a constant regulator of the general manifestations of the neuronal phenotype, i.e., specific features of neurons [22].
In a growing and developing cell, there is an enhanced synthesis of plastic substances, accompanied by the activation of oxidative processes and, accordingly, an increase in the oxygen demand of cells. Since neuroglobin serves to store and transport oxygen to the mitochondria of neuronsin order to ensure the functioning of the oxidative phosphorylation system [5,23], its increasing immunoreactivity in histaminergic neurons from the 5th to the 90th day of postnatal development of rats is quite natural. It is assumed that the proteinneuroglobin should be concentrated in the cytoplasmic compartments, where oxidative processes directly occur [24]. Therefore, its localization in mitochondria or near these organelles is most probable. The biochemical studies carried out by Lechauve and co-authors indicate the presence of neuroglobin in the composition of the mitochondrial fraction [25]. However, data on the co-localization of this protein and mitochondrial markers have not yet been obtained; therefore, it can be assumed that neuroglobin is localized only in part of the mitochondria of nerve cells.
Actively differentiating histaminergic neurons need a significant amount of energy stored in macroergic bonds of ATP, the synthesis of which is one of the main functions of mitochondria. Mitochondrial membrane ATP synthase produces ATP from ADP via a transmembrane proton gradient generated by electron transport complexes in the respiratory chain. The energy of the transmembrane gradient is used for ATP synthesis and for the active transport of essential substrates across the inner mitochondrial membrane. The combination of these reactions provides an efficient exchange of ATP-ADP between the mitochondria and the cytosol, which makes it possible to maintain a high level of energy supply in the cell [7,26]. During cell differentiation, ATP synthase also promotes the formation of mitochondrial cristae [27-29]. Our earlier electron microscopic study showed that the number of mitochondria in the cytoplasm of histaminergic neurons increases dynamically as they develop, and their cristae develops [19]. Therefore, the increase in the immunoreactivity of ATP synthase from 5th to 90th day of postnatal development of the rat hypothalamus histaminergic neurons, which we demonstrated, is in full agreement with our electron microscopic data.
In the cytoplasm of histaminergic neurons stained for ATP synthase immunoreactivity, a predominantly uniform distribution of immunopositive granules and clumps is observed. In some of the studied neurons, the accumulation of these aggregates is visualized in the perinuclear region. Since ATP synthase is localized on the inner membrane of mitochondria, the location of these granules and clumps in the cytoplasm of histaminergic neurons apparently corresponds to the distribution of these organelles in them. This arrangement of mitochondria is indeed characteristic of histaminergic neurons in the hypothalamus of rats [19]. During the differentiation of histaminergic neurons, contact of mitochondria with the nuclear membrane is observed [19], which corresponds to the accumulation of immunopositive aggregates in the perinuclear region of the described neurocytes. It indicates a high level of metabolic processes with significant energy costs in this zone.
The results of our immunohistochemical study demonstrate that in the developing histaminergic neurons of the hypothalamus, from the 5th to the 90th day after birth, the immunoreactivity of MAO-B, NeuN, Ngb, and ATP synthase synchronously increases. This corresponds to the literature data, according to which a positive correlation in the brain has been established between the expression of Ngb and the marker of mature neurons NeuN, as well as neuroglobin and ATP synthase [30].
Conclusion
During the postnatal development period of E2 nucleus rat hypothalamus histaminergic neurons, from the 5th to the 90th day after birth, in parallel with the structural and metabolic formation of these neurons, a synchronous increase in the immunoreactivity of MAO B, NeuN, neuroglobin and ATP synthase occurs in their cytoplasm.
Author Contributions
All authors made the same contribution to this study and the preparation of the article.
Conformity with the Principles of Ethics
The study was performed in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes.
Acknowledgement
This work was supported by the Belarusian Republican Foundation for Basic Research (M20M-089).
Conflict of Interest
Authors declare that they have no financial or personal conflicts of interest that could inappropriately influence the conduct of this research.
References
- Charil A, Laplante DP, Vaillancourt C, King S (2010) Prenatal Stress and Brain Development. Brain Res Rev 65(1): 56-79.
- De Asis Cruz J, Andescavage N, Limperopoulos C (2021) Adverse Prenatal Exposures and Fetal Brain Development: Insights from Advanced Fetal Magnetic Resonance Imaging. Biol Psychiatry Cogn Neurosci Neuroimaging 7(5): 480-490.
- Duan W, Zhang YP, Hou Z, Huang Ch, Zhu H, et al. (2016) Novel Insights into NeuN: from Neuronal Marker to Splicing Regulator. Mol Neurobiol 53(3): 1637-1647.
- Fiocchetti M, Cipolletti M, Brandi V, Polticelli F, Ascenzi P (2017) Neuroglobin and Friends. J Mol Recognit 30(12): 1-12.
- Hankeln T, Ebner B, Fuchs C, Gerlach F, Haberkamp M, et al. (2005) Neuroglobin and Cytoglobin in Search of Their Role in The Vertebrate Globin Family. J Inorg Biochem 99(1): 110-119.
- Xie LK, Yang SH (2016) Brain Globins in Physiology and Pathology. Med Gas Res 6(3): 154-163.
- Lapuente Brun E, Moreno Loshuertos R, Acín Perez R, Latorre Pellicer A, Colas C, et al. (2013) Supercomplex Assembly Determines Electron Flux in The Mitochondrial Electron Transport Chain. Science 340(6140): 1567-1570.
- Haas H, Sergeeva O, Selbach O (2008) Histamine in The Nervous System. Physiol Rev 88(3): 1183-1241.
- Zimatkin SM, Kuznetsova VB, Strik ON (2006) Spatial Organization and Morphometric Characteristics of Histaminergic Neurons in The Rat Brain. Neurosci Behav Physiol 36(5): 467-471.
- Inagaki N, Yamatodani A, Ando Yamamoto M, Tohyama M, Watanabe T, et al. (1988) Organization of Histaminergic Fibers in The Rat Brain. J Comp Neurol 273(3): 283-300.
- Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, et al. (1991) Histamine Neurons in Human Hypothalamus: Anatomy in Normal and Alzheimer Diseased Brains. Neuroscience 44(2): 465-481.
- Panula P, Yang YY, Costa E (1984) Histamine-Containing Neurons in The Rat Hypothalamus. Proc Natl Acad Sci USA 81(8): 2572-2576.
- (2010) Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes: text with EEA relevance 20.10.2010. Strasbourg: Official Journal of the European Union, 46.
- Paxinos G, Watson C (2014) The Rat Brain in Stereotaxic Coordinates. 7th, London: Academic Press 480.
- Korzhevskii DE, Sukhorukova EG, Gilerovich EG, Petrova ES, Kirik OV, et al. (2014) Advantages and Disadvantages of Zinc-Ethanol-Formaldehyde as A Fixative for Immunocytochemical Studies and Confocal Laser Microscopy. Neurosci Behav Physiol 44(5): 542-545.
- Nguyen T (2022) Immunohistochemistry: A Technical Guide to Current Practices. Cambridge; New York: Cambridge University Press, 282.
- Zimatkin SM, Zaerko AV (2020) A method for Detecting Histaminergic Neurons in The Hypothalamus. Neurosci Behav Physiol 50(5): 655-657.
- Zimatkin SM, Zaerko AV, Fedina EM (2021) Nucleoli in Developing Histaminergic Neurons in The Rat Brain. Neurosci Behav Physiol 51(4): 535-540.
- Zimatkin SM, Zaerko AV, Phedina EM (2022) Postnatal Organellogenesis in Histaminergic Neurons of The Rat Brain. Am J Biomed Sci Res 16(2): 256-264.
- Panula P, Sundvik M, Karlstedt K (2014) Developmental Roles of Brain Histamine. Trends Neurosci 37(3): 159-168.
- Panula P, Nuutinen S (2013) The Histaminergic Network in The Brain: Basic Organization and Role in Disease. Nat Rev Neurosci 14(7): 472-487.
- Guselnikova VV, Korzhevskiy DE (2015) NeuN as a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Naturae 7(2): 42-47.
- Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T (2010) Neuroglobin Protects Nerve Cells from Apoptosis by Inhibiting the Intrinsic Pathway of Cell Death. Apoptosis 15(4): 401-411.
- Korzhevsky DE, Grigorev IP, Kirik OV, Alekseeva OS (2015) Neuroglobin Distribution in The Rat Cerebellar Purkinje Cells. J Evol Biochem Physiol 51(6): 517-519.
- Lechauve C, Augustin S, Cwerman Thibault H, Bouaita A, Forster V, et al. (2012) Neuroglobin Involvement in Respiratory Chain Function and Retinalganglion Cell Integrity. Biochim Biophys Acta 1823(12): 2261-2273.
- Capaldi RA, Aggeler R (2002) Mechanism of the F1F0-type ATP Synthase, a Biological Rotary Motor. Trends Biochem Sci 27(3): 154-160.
- Davies KM, Anselmi C, Wittig I, Faraldo Gomez JD, Kuhlbrandt W (2012) Structure of The Yeast F1f0-Atp Synthase Dimer and Its Role in Shaping the Mitochondrial Cristae. Proc Natl Acad Sci U S A 109(34): 13602-13607.
- Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, et al. (2002) The ATP Synthase Is Involved in Generating Mitochondrial Cristae Morphology. EMBO J 21(3): 221-230.
- Blum TB, Hahn A, Meier T, Davies KM, Kuhlbrandt W (2019) Dimer of Mitochondrial ATP Synthase Induce Membrane Curvature and Self-Assemble into Rows. Proc Natl Acad Sci U S A 116(10): 4250-4255.
- Hua S, Antao ST, Corbett A, Witting PK (2010) The Significance of Neuroglobin in The Brain. Curr Med Chem 17(2): 160-172.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.