Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Insight into the Mechanisms Underlying the Tracheorelaxant Activities of Harungana madagascariensis Stem Barks Aqueous Extract
*Corresponding author: Esther Ngo Lemba Tom, Department of Biological Sciences, Higher Teacher’s Training College, University of Yaounde I, Cameroon, University of Yaoundé I, Yaoundé, Cameroon.
Received: September 26, 2023; Published: October 02, 2023
DOI: 10.34297/AJBSR.2023.20.002686
Abstract
Stem bark decoction of Harungana madagascariensis is traditionally used orally to treat asthma and cough. However, no study has been reported on its effect on the airway smooth muscle. This study assesses the impacts of an aqueous extract from Harungana madagascariensis (HMAE) stem bark on the tracheal smooth muscle and investigates the related mechanisms. The activity of different concentrations of HMAE was evaluated on resting tension of isolated trachea and tracheal rings precontracted with acetylcholine (Ach, 10μM) or potassium chloride (KCl, 60mM). In addition, the effects of the preincubation of the tracheal rings with HMAE on contraction induced by Ach or KCl were evaluated. Then, several pharmacological antagonists were used to identify the related mechanisms. HMAE (1.5mg/mL, 3mg/mL, and 4.5mg/mL) significantly decreases the basal tone of tracheal rings as compared to the tone of non-incubated rings. Moreover, the extract inhibited cholinergic and high K+- induced contractions in rat trachea. Cumulative concentrations of HMAE (1-5 mg/mL) induced relaxation respectively in tracheal rings precontracted with Ach (Emax = 67.5±2.9 %; EC50 =3.3±0.4 mg/mL) and KCl (Emax = 70.8±2.7 %; EC50 = 3.0±0.4mg/mL). The effect of HMAE was potentiated in the presence of glibenclamide (an ATP-dependent K+- channel blocker), whereas it was inhibited by Nω- nitro-L-arginine methyl ester (300μM) a Nitric Oxide (NO) synthase antagonist, and by mequitazine (an H1-histaminergic antagonist, 1μM). The overall data suggest the inhibition of voltage-dependent calcium channels and receptor-operated calcium channels, the stimulation of NO production, the inhibition of H1- histaminic receptors, and the opening of K+-ATP channels as mechanisms involved in the relaxant effect of HMAE on the tracheal smooth muscle.
Keywords: Harungana madagascariensis, Tracheorelaxation, K+-ATP channels, H1- receptor, Nitric oxide, Calcium channels
Abbreviations: Ach: Acetylcholine; ASM: Airway Smooth Muscle; ATP: Adenosine Triphosphate; cAMP: Cyclic Adenosine Monophosphate; cGMP: Cyclic Guanosine Monophosphate; COPD: Chronic Obstructive Pulmonary Disease; COX: Cyclooxygenase; CRDs: Chronic Respiratory Diseases; H1: Histamine 1; HMAE: Harungana madagascariensis Aqueous Extract ; K+-ATP: Potassium- Adenosine Triphosphate; L-NAME: Nω-nitro-L-arginine Methyl Ester; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; NO: Nitric oxide; NOS: Nitric Oxide Synthase; ROCCs: Receptor-Operated Calcium Channels
Introduction
Airways and other structures of the lungs can be affected by Chronic Respiratory Diseases (CRDs). CRDs are among the main causes of morbidity and mortality worldwide [1]. Asthma and Chronic Obstructive Pulmonary Disease (COPD) are two of the most prevalent chronic respiratory disorders [2]. Asthma affects about 300 million people worldwide and its prevalence rises by 50% every ten years [3], whereas COPD affects 328 million people [4]. 3.23 million people died from COPD in 2019, making it the third-leading cause of death globally. The majority of deaths among people under 70 (90%) occurred in low- and middle-income countries [5]. These respiratory diseases are both characterized by airway smooth muscle constriction and are therefore typically treated using bronchodilators [6]. Nevertheless, these treatment modalities can cause many side effects, including cardiovascular diseases, gastrointestinal disorders, and hyperglycemia [7]. In order to improve the treatment of respiratory disorders, it is crucial to hunt for novel molecules that can relax the smooth muscle of the airways. Because of their low toxicity and higher tolerance by the human body, medical plants, which are utilized by around 75 to 80 percent of the world’s population for primary health care, appear to be an interesting alternative treatment [8].
The only species of flowering plant in the family Hypericaceae that belongs to the genus Harungana is called Harungana madagascariensis (Lam. ex Poir.). It can be found in clearings in forest and savanna areas of tropical Africa [9]. This species, often known as the “Dragon’s blood tree,” is a tropical, heavily branching shrub or small tree that can grow up to 12 meters tall [10]. It has historically been employed as an antiseptic, an abortifacient, and a natural source of cosmetics and dermatological agents [11]. Additionally, it is used to treat a number of illnesses, such as anemia, jaundice, bleeding, gonorrhea, malaria, liver problems, diabetes, piles, gastrointestinal disorders, parasitic skin diseases, tuberculosis, and asthma [12-14]. H. madagascariensis is very useful therapeutically. It has been reported to possess antispasmodic, antibacterial, antifungal, antiviral, and antihelminthic effects [15-19], antidiabetic, antioxidant, hypotensive and cardioprotective properties [20-23], anti-inflammatory and analgesic effects [24]. Additionally, we previously investigated the H. madagascariensis aqueous extract’s acute and subacute toxicity and concluded that this extract did not show a significant toxicity effect in rodents [25].
According to published research, H. madagascariensis includes a variety of bioactive substances, including tannins, alkaloids, flavonoids, saponins, and terpenes [20]. A prenylated 1, 4-anthraquinone that was extracted from the stem bark of H. madagascariensis and has alpha-glucosidase inhibition and antioxidant activity is one of the chemicals that have been identified from this species [26].
Previous studies have demonstrated the relaxant effect of H. madagascariensis on the ileum smooth muscle [27] and on the vascular smooth muscle [28]. Stem bark decoction of Harungana madagascariensis is traditionally used orally to treat asthma and cough [29,30]; however, as far as we are aware, no study on its effect on the airway smooth muscle has been reported. As a result, the current work sought to explore the potential mechanisms underlying the relaxant effects of the aqueous extract of H. madagascariensis on tracheal smooth muscle.
Material and Methods
Extraction of Plant Material
In June 2019, fresh stem barks from H. madagascariensis were gathered in Essazok, Mbalmayo (Center Region, Cameroon). The Cameroon National Herbarium is where the plant was identified. A voucher sample was deposited there under registration number 4224HNC. Bark fragments were air-dried at room temperature and then ground into a fine powder. 500g of powder was added to 4L of distilled water, and the mixture was allowed to boil for 20 minutes. The resultant decoction was further lyophilized after being filtered using Whatman paper No. 3. With a yield of 9.89%, a crude brown extract powder (98.90g) was produced.
Qualitative Analysis of Phytochemicals
The presence of phenolic compounds, saponins, tannins, anthocyanins, and anthraquinones was screened for in the crude aqueous extract of the stem bark of H. madagascariensis. Standard procedures were followed as described by Harbone [31].
Detection of Alkaloids
The extract (3mL) was mixed with 1 mL of 1% HCl and a few drops of Mayer’s reagent (potassium mercuric iodide solution). Alkaloids are present when a precipitate that is cream-white forms.
Flavonoids Analysis
5 ml of 95% v/v ethanol, 5 drops of hydrochloric acid, and 0.5g of magnesium turnings were added to the extract (3mL). Flavonoids can be detected by their brick red or violet effervescence appearance.
Test For Steroids and Triterpenoids
In a test tube, the extract (5mL) and chloroform (2mL) were mixed. Four drops of acetic anhydride were then added, and the test tube was heated in a water bath and quickly chilled in ice water. In the test tube was put 2 mL of concentrated H2SO4. Steroids are shown by the creation of a green hue, whereas triterpenoids are shown by the formation of a purplish-red tint.
Test for Phenolic Compounds
Three drops of a 10% aqueous ferric chloride solution were applied to 5 mL of the aqueous extract sample. When phenolic compounds are present, a blue or green hue will appear.
Saponins Test
In a test tube, distilled water (2.5mL) and plant extract (5mL) were mixed. The presence of saponins is indicated by the development of persistent foaming.
Tannins Test
A few drops of 3% ferric chloride were added to the extract (5mL). The presence of tannins was indicated by a filthy blue or dark green hue.
Test for Coumarins
A tube containing 8 mg of the plant extract was covered with filter paper soaked in a solution of sodium hydroxide (10%), then exposed to UV for 1 to 2 minutes. The formation of yellow-green, fluorescent spots was an indication of the presence of coumarins.
Test for Anthocyanins and Anthraquinones
15 mL of distilled water and 12 mL of ether were added to 1mL of aqueous extract and stirred. The formation of 2 phases (aqueous with red-brown color and ethyl with yellow color) and a brown agar at the bottom of the tube was an indication that anthraquinones and anthocyanins were present.
Animals and Chemicals
Twenty-six male albino Wistar rats weighing between 220 and 250g were obtained from the University of Yaounde I, Higher Teachers’ Training College’s Animal House (Cameroon). They were kept in plastic cages with free access to tap water and regular food while under normal lighting conditions (12-hour day/night cycles). The study was carried out in accordance with the internationally accepted principles for laboratory animal use and care as found in the US guidelines (NIH publication #85-23, revised in 1985) for the use and care of laboratory animals in research. The Cameroon National Ethical Committee authorized this study (approval N° FWIRB00001954).
Sigma-Aldrich provided the following chemicals: potassium chloride (KCl), acetylcholine chloride (Ach), Nw-nitro-L-arginine methyl ester (L-NAME), glibenclamide, propranolol, indomethacin, and atropine (Germany). All additional compounds were of the analytical variety and were dissolved in distilled water.
Tissues Preparation
The rats were killed by being struck in the neck, the chest was opened, and extra connective tissue and fat were removed in order to access the trachea. Five to six cartilage rings from a piece of the trachea were isolated, and isometric contractile responses were then assessed by putting the rings in an organ bath (20 mL) containing Krebs-Henseleit solution (37°C, pH 7.4) that contained the following ingredients (in mM): NaCl 118, KCl 4.65, CaCl2 2.52, MgSO4 1.64, KH2PO4 1.18, NaHCO3 24.9, glucose 12 and aerated with oxygen. Tracheal rings were hung between two stainless steel hooks in a horizontal position. The chamber wall was connected to one of the hooks, and an isometric force transducer was linked to the other hook (it50, EMKA Technologies, France). The bath solution was changed every 15 minutes while the tissues were kept under 1.5g initial strain and allowed to equilibrate for 1 hour [32]. 60mM KCl was originally injected throughout each experiment to test the vitality of the tracheal tissue and only responsive tracheal rings were used in the investigation.
Effect of H. madagascariensis Extract on the Basal Tone Trachea
To evaluate the effect of H. madagascariensis extract on the basal tone, tracheal rings were incubated with H. madagascariensis aqueous extract at the concentration of 1.5, 3, or 4.5mg/mL for 30 minutes after the 1h equilibration period. The changes in the basal tone were recorded and compared to non-incubated tissues.
Effects of H. madagascariensis Aqueous Extract on Tracheal Smooth Muscle Contraction Elicited by Acetylcholine or Potassium Chloride
The tracheal rings from rats were contracted in two different tests using acetylcholine (10-5 M, Ach) or potassium chloride (60mM, KCl) to examine the relaxant effects of H. madagascariensis on the airway smooth muscle. The extract was cumulatively added to the organ bath at the corresponding concentrations of 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5mg/mL for the contraction generated by KCl and Ach, respectively, when the contraction curves for the rings reached the plateau phase of maximum tension. Additionally, for the control group, distilled water was introduced in the organ bath for tracheal rings contracted with either Ach or KCl. The tensions were measured, and a percentage of the trachea rings’ relaxation in response to the contractive agents was used to calculate the relaxant effect of H. madagascariensis.
Effect of H. madagascariensis Aqueous Extract Pre-Incubation on Acetylcholine and Potassium Chloride-Induced Contraction in Tracheal Rings
Tracheal rings obtained from rats were contracted with increasing doses of acetylcholine to investigate the impact of pre-incubation with H. madagascariensis Aqueous Extract (HMAE) on the contraction elicited by acetylcholine. (Ach, 0.1-100μM) then rinsed 3 times with Krebs-Henseleit solution and the tension was stabilized to 1.5g. These tracheal rings were then incubated with one of the H. madagascariensis concentrations (1.5, 3, or 4.5 mg/mL) for 10 minutes and contracted with increasing concentrations of Ach (0.1-100μM). The same protocol was repeated to access the pre-incubation effect of HMAE on the tracheal rings’ contraction induced by potassium chloride with the only difference being that Ach was replaced by potassium chloride (KCl, 15-60mM).
Studies on the Mechanisms Underlying the Tracheal Smooth Muscle-Relaxing Effects of H. madagascariensis
To identify the underlying mechanisms, some tracheal rings were pre-incubated for 10 minutes with propranolol (1μM), Nω-nitro- L-arginine methyl ester (L-NAME, 300μM), and glibenclamide (1μM), then contracted with Ach (10-5M) while other tracheal rings were pre-incubated for 10minutes with mequitazine (1μM), atropine (1μM), and indomethacin (1μM), and then KCl (60mM) was added. The HMAE was then cumulatively added and concentration- response curves for the inhibitory responses were created in the presence of Ach or KCl.
Statistical Analysis
The analyses and representations were carried out with the software GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, United States). Values are represented as mean±standard error of the mean (SEM), the median effective concentrations (EC50), and the Area Under the Curve (AUC). For comparisons with the corresponding controls, the student’s t-test or Repeated Measures ANOVA was used, followed by the Bonferroni post-test. At P<0.05, differences were considered significant.
Results
Qualitative Evaluation of Phytoconstituents
A variety of secondary metabolites, including flavonoids, phenolic compounds, saponins, tannins, anthocyanins, and anthraquinones, were identified through phytochemical screening of the chemical compounds present in the crude aqueous extract of the bark sample of H. madagascariensis, as shown in (Table 1).
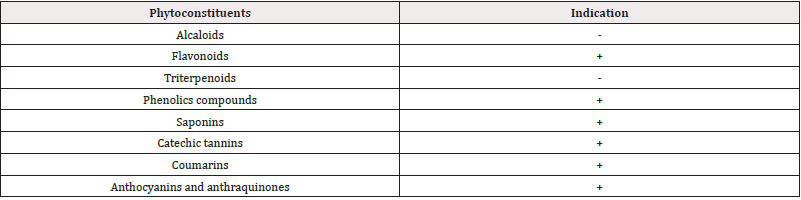
Table 1: Qualitative phytochemical composition of the aqueous extract of Harungana madagascariensis stem bark.
Note*: + =presence, - = absence.
Harungana madagascariensis Extract Alone Decreases the Basal Tone of the Trachea
In the initial series of tests, we evaluated the potential impact of the H. madagascariensis Aqueous Extract (HMAE) on the tracheal basal tone following a 1-hour equilibration period under 1.5g of resting tension. As shown in Figure 1 below, the incubation of rings for 30 minutes with the HMAE significantly decreased the tracheal basal tone in a concentration-dependent manner. The percentages of decrease recorded for the extract concentrations of 1.5, 3 and 4.5mg/mL were respectively 5.9±0.5 % (P<0.001), 9.0±1.0 % (P<0.001) and 11.6±1.6 % (P<0.001) as compared to the non-incubated rings.

Figure 1: Effect of the H. madagascariensis aqueous extract (HMAE) on the basal tone of the tracheal smooth muscle. Values shown mean ± SEM of 6-8 rings by curve from 6-8 rats. ***P<0.001 versus non-incubated tissues.
The Tracheorelaxant Effects of H. madagascariensis Aqueous Extract after Cholinergic and High K+-Mediated Contractions.
Pre-contacted tracheal preparations with Ach (10μM) and KCl (60 mM) were concentration-dependently relaxed by HMAE (1-5 mg/mL) with the maximum effect (Emax) values of 67.5±2.9 % and 70.8±2.7 % at the concentration of 5 mg/mL respectively for Ach and KCl precontracted rings (Figures 2A and 2B). The concentration producing 50% of the maximum effect (EC50) was 3.3±0.4 mg/ mL in the Ach pre-contracted rings and 3.0±0.4 mg/mL in the KCl pre-contracted rings. Since there was not a significant difference in the Emax obtained under Ach and KCl pre-contracted rings, HMAE showed a more effective response under Ach-mediated contraction.

Figure 2: Concentration-response curves of the relaxant effect of H. madagascariensis Aqueous Extract (HMAE) on acetylcholine (10μM, A) and potassium chloride (60mM, C) contracted rat tracheal rings and the related area under curves (B and D). Data and error bars represent the mean±SEM (n=6 rings by curve from 6 rats). **P<0.01, ***P<0.001 vs. control.
Antagonist-Like Effects of H. madagascariensis Against Contractions Brought By Acetylcholine and Potassium Chloride

Figure 3: Effect of the pre-incubation of tracheal rings with H. madagascariensis Aqueous Extract (HMAE) on contraction induced by Ach (A) or KCl (B). Values are expressed as mean±SEM (n=6-11 rings by curve from 6-11 rats). **P<0.01, *** P<0.001 ± vs. non incubated rings.
The pre-incubation of tracheal rings with Harungana madagascariensis extract led to an inhibitory effect of the contraction induced by cumulative concentrations of Ach (0.1-100μM) or KCl (15- 60mM). This inhibitory effect was significant (P<0.01 to P<0.001) for the three concentrations of H. madagascariensis used (1.5, 3, or 4.5 mg/mL) both in the case of Ach and KCl contraction (Figures 3A and 3B). The maximal inhibitory effect was observed at the concentration of 3mg/mL on Ach contracted rings with a percentage of inhibition of 124.2± 6.9% (P<0.001) and at the concentration of 4.5mg/mL on KCl with a percentage of inhibition of 125.1±12.5 % (P<0.001).
Role of NO, β-Adrenergic and ATP-Dependent K+ Channel-Mediated Signaling in The H. madagascariensis Tracheorelaxant Effect After Ach-Induced Contraction

Figure 4: Concentration-response curves of H. madagascariensis in the absence of antagonist (non-incubated) and the presence of L-NAME (300μM), propranolol (1μM), and glibenclamide (1μM) in isolated rat tracheal preparations precontracted with acetylcholine. Values are expressed as mean±SEM (n=6-8 rings by curve from 6-8 rats). *p<0.05, **P<0.01, ***P<0.001 compared to non-incubated tissues.
L-NAME (300μM, a non-selective NOS inhibitor) significantly decreased the HMAE-induced relaxation. The Emax was 39.6 ± 4.2% in the presence of L-NAME vs. 67.5±2.9 % in the non-incubated tracheal rings (P<0.001) (Figure 4A). After incubation with glibenclamide (1μM, an ATP-dependent K+- channel blocker), the relaxing effect of HMAE on Ach-contracted tracheal rings was further potentiated (P<0.01) as the Area Under the Curve shows (Figure 4B). Whereas propranolol (1μM, a non-selective β-adrenergic blocker) significantly (P<0.05 to P<0.01) reduced the initial relaxing action of H. madagascariensis extract at the two first cumulative concentrations (1 and 1.5 mg/mL) (Figure 4A). However, no significant changes were observed globally as compared to non-incubated rings (Figure 4B).
Implication of the H1-Receptors Pathway on the Tracheorelaxing Action of H. madagascariensis on High K+-Contracted Rings

Figure 5: Relaxant effects of cumulative concentrations of H. madagascariensis on rat tracheal smooth muscle contractions induced by KCl (60mM) in non-incubated and incubated tissues with (A) mequitazine (1μM) (B) atropine (1μM) and (C) indomethacin (1μM), (n=6-8 rings by curve from 6-8 rats) *: P<0.05 **: P<0.01, compared to non-incubated tissues.
Pre-treatment of tracheal rings with mequitazine (1μM, an H1 receptor antagonist) significantly reduced the tracheorelaxing effect of H. madagascariensis (Figures 5A and 5B). Indeed, in the presence of mequitazine, the Emax was 52.9±3.4 % vs. 67.5±2.9 % (P<0.01) in the non-incubated tracheal rings. However, neither atropine (1μM, a muscarinic receptor antagonist) nor indomethacin (1μM, a cyclooxygenase pathway inhibitor) altered the tracheorelaxing action of H. madagascariensis.
Discussion
Airway Smooth Muscle (ASM) is a crucial component implicated in asthma physiopathology because it helps to generate airway hyperresponsiveness and airflow blockages [33]. Therefore, ASM is the focus of both current and possible future therapeutics for asthma. In the present research, the effects of H. madagascariensis stem bark aqueous extract (HMAE) on the trachea were determined to assess the possibility of its use as a bronchodilator medication. The first finding of this study revealed that HMAE significantly decreases the tracheal basal tone. Asthma patients have more ASMs, which are known to present a substantial and sustained basal tone [34]. Thus, it has been proposed that the tonic activation of ASM by the airway basal tone mainly contributes to its capacity to contract [35,36]. When at rest, the intracytoplasmic concentrations of Ca2+ and Na+ are continuously adjusted to maintain the smooth muscle’s basal tone [37]. It has been postulated that among other things, airway wall expansion brought on by structural alterations can be responsible for variations in airway basal tone and oxidative stress. In contrast to oxidative chemicals, which are linked to raised airway tone and airway hyperresponsiveness, reduced glutathione, an antioxidant, lowers the airway tone [38]. On this basis, we can hypothesize that the antioxidant capacity of HMAE could contribute to the decreased effect observed on tracheal basal tone [22,39,40]. Interestingly, HMAE induced relaxant effects in tracheal rings precontracted with acetylcholine (Ach) or high K+ (KCl). Likewise, the plant extract reduced the contraction induced by the two contractive agents. The fundamental factor that causes smooth muscle to contract is an increase in intracellular calcium concentration ([Ca2+] i). Through the sarcoplasmic reticulum, [Ca2+]i can come from intracellular storage and extracellular space. While acetylcholine links to a G-protein coupled receptor and increases the Ca2+ sensitivity, Ca2+ entrance by activating the voltage-dependent Ca2+ channels and receptor-operated calcium channels (ROCCs), KCl causes muscle contraction by allowing the influx of Ca2+ (across voltage-dependent Ca2+ channels) from the extracellular space into the cytosol via the cell membrane [41,42]. The release of Ca2+ from the sarcoplasmic reticulum (SC) is caused by the activation of the ROCCs, which are connected to muscarinic receptors [43]. The fact that HMAE inhibits both Ach and high K+-contraction suggests that non-specific tracheorelaxant function via calcium channel blockade mechanisms is involved. HMAE may reduce Ca2+ entry and/or block the ROCCs, both of which have an impact on the release of Ca2+ from the SC.
The relaxant activity of HMAE was evaluated on tracheal smooth muscle incubated with Nω nitro-L-arginine methyl ester (L-NAME), a NO Synthase (NOS) inhibitor, to investigate the function of Nitrite Oxide (NO) generation on the tracheorelaxant action of the plant. The NOS isoforms catalyze a reaction that uses L-arginine, molecular oxygen, and NADPH as substrates to create NO and L-citrulline, which is an endogenous tracheorelaxant mediator [44,45]. NO afterward activates soluble Guanylyl Cyclase (GC), which produces intracellular cGMP and relaxes the smooth muscle of the airway [46]. We observed that treatment with L-NAME significantly decreases the tracheorelaxant activity of HMAE. This finding suggests that the NO signaling pathway at least partially mediates the tracheorelaxing effects of HMAE. Indeed, the H. madagascariensis aqueous extract’s capacity to release NO contributes significantly to its vasodilating activity as previously demonstrated [28]. In another set of experiments, the tracheal smooth muscle was incubated with propranolol (a non-selective β antagonist) and contracted with acetylcholine to investigate if the β-adrenoceptor stimulatory activity is involved in the plant relaxant effect. It is generally established that treatments for asthma, such as β2-adrenoceptor agonists, cause bronchodilation by boosting cAMP synthesis or slowing down cyclic nucleotide breakdown (cAMP and cGMP) [47]. On tracheal smooth muscle, HMAE demonstrated significant relaxing effects, which were not statistically different from those of the extract in non-incubated tissues. Therefore, these results suggest that the tracheorelaxant effect of HMAE is not due to its β-adrenoceptor stimulatory property and indicates that the extract elicits relaxation independently of cyclic nucleotides. The extract was evaluated on acetylcholine-contracted rat tracheal rings in the presence of glibenclamide, which inhibits the ATP-sensitive potassium channels, to determine if the extract’s bronchorelaxant process is related to these channels. In fact, K+ channel activation generated membrane hyperpolarization, which led to dilation, while K+ channel inhibition resulted in membrane depolarization, which led to constriction [48,49]. The relaxing impact of HMAE was stronger in the presence of glibenclamide than it was in its absence. This finding implies that, despite the presence of glibenclamide, the extract may elicit relaxation through a variety of routes, including the reduction of [Ca2+] i, the blocking of ROCCs, as well as the opening of K+-ATP channels. Besides, this effect is observed when two drugs sometimes cause receptor potentiation rather than inhibition. One possible explanation is that: the two drugs are antagonists (with different actions) and they bind to the receptors (heteromeric receptors) that consist of different subunits, where each drug acts on only a part of the subunits [50]. Therefore, as regards the observed data we suggest that K+-ATP channels could be involved at least in part in the relaxation induced by the plant extract. Our result is similar to the finding of other researchers with the hydroalcoholic extract of Waltherica indica [51].
Tracheal smooth muscle was incubated with mequitazine, an antagonist of the H1-specific receptor (antihistaminic), then contracted with KCl in order to study the impact of histamine (H1) receptors in the extract’s relaxing effect. H1 and H2 receptors are two distinct types of receptors that histamine has been demonstrated to stimulate. While the H2 receptor mediates the activity of histamine in promoting stomach acid secretion, the H1 receptor mediates the action of histamine in inducing smooth muscle contraction of the small intestine and bronchi [52]. It has previously been observed that histamine (H1) receptor-blocking medications have a tracheorelaxing activity [53]. Mequitazine preincubation significantly attenuated the tracheorelaxant effect of H. madagascariensis extract indicating that the extract had an inhibitory impact on the tracheal smooth muscle’s H1- receptor. To investigate the muscarinic receptors’ function in the relaxing action of H. madagascariensis extract, the extract’s relaxing effects were also examined in tissues that were contracted with KCl and incubated with atropine, a muscarinic antagonist. Muscarinic inhibitors, a type of bronchodilator, have been known to have relaxing effects [54]. In tissues that had not been incubated but had been contracted with KCl, the results revealed a significant relaxant effect that was not statistically different from that of the plant extract. This study suggests that the relaxing action on tracheal smooth muscle may not be caused by the blockage of muscarinic receptors. The relaxing impact of H. madagascariensis was investigated on tracheal smooth muscle incubated with indomethacin, a non-selective COX inhibitor, to assess the influence of HMAE on cyclooxygenase (COX) pathways leading to the synthesis of prostaglandins. In this series of tests, we determined if the plant’s anti-inflammatory properties contributed to its relaxing effects. Previous studies have revealed that H. madagascariensis has anti-inflammatory properties [24]. It is suggested that the prostaglandins pathway is not involved in the H. madagascariensis extract-induced tracheorelaxant effect since the response in non-incubated tissues was not statistically different from that observed in in incubated tissues. This outcome also showed that H. madagascariensis’s ability to reduce inflammation is unaffected by its ability to relax the trachea.
Conclusion
In conclusion, this study revealed that H. madagascacriensis extract induced a relaxant effect on the basal tone of isolated rat trachea and the tracheal ring precontracted with Ach or KCl. These results indicate a potent tracheorelaxing effect of H. madagacasacriensis which may justify its popular use in respiratory diseases such as asthma. The inhibition of voltage-dependent and receptor- operated calcium channels and the stimulation of K+-ATP sensitive channels may contribute to the tracheorelaxant effect of H. madagascariensis extract on contraction induced by KCl or Ach. Moreover, the relaxant effect of H. madagascariensis could be explained through the stimulation of NO production and the histamine receptors -H1 inhibition. Further investigations need to be undertaken to determine the anti-asthmatic properties of this plant in asthmatic animal models and to elucidate the signaling mechanisms.
Acknowledgements
The authors are sincerely thankful to all individuals who were involved in this research. This work was supported by the International Foundation for Science grant (Ref. IFS Grant No. I-1-F-5882-2) awarded to Esther Ngo Lemba Tom.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- (2022) The global impact of respiratory disease. Third Edition. European Respiratory Society.
- Labaki, WW, Han MK (2020) Chronic respiratory diseases: a global view. Lancet Respir Med. 8(6): 531-533.
- Braman SS (2006) The global burden of asthma. Chest 130(Suppl 1): 4S-12S.
- Alwan A (2010) Global Status Report on Non-Communicable Diseases. World Health Organization 302.
- (2023) Chronic obstructive pulmonary disease (COPD). World Health Organization.
- Dekhuijzen PN, Bjermer L, Lavorini F, Ninane V, Molimard M, et al., (2014) Guidance on handheld inhalers in asthma and COPD guidelines. Respir Med 108(5): 694-700.
- Williams DM, Bruce KR (2018) Clinical pharmacology of bronchodilator medications. Respir Care 63(6): 641-654.
- Tabassum N, Ahmad F (2011) Role of natural herbs in the treatment of hypertension. Pharmacogn Rev 5(9): 30-40.
- Hutchinson J, Dalziel MD, Hepper FN (1954) Flora of West Tropical Africa. 2nd edition Crown Agents, London.
- Burkill HM (1985) The useful plants of west tropical Africa. Families AD Royal Botanic Gardens, Kew, UK, 960.
- Kamanzi AK, Schmid C, Brun R, Kone MW, Traore D (2004) Antitrypanosomal and antiplasmodial activity of medicinal plants from Cote d’Ivoire. J Ethnopharmacol 90(2-3): 221-227.
- Maikere Faniyo R, Puyvelde LV, Mutwewingabo A, Habiyaremye F (1989) Study of Rwandese medicinal plants used in the treatment of diarrhea I. J Ethnopharmacol. 26(2): 101-109.
- Adeneye AA, Olagunju JA, Elias SO, Olatunbosun DO, Mustafa AO, et al., (2008) Harungana madagascariensis in acute and repeated acetaminophen hepatotoxic rats. Int J Appl Res Nat Prod 1: 29-42.
- Nicolas J (2012) Plantes médicinales du nord de Madagascar ethnobotanique antakarana et informations scientifiques. Jardins du Monde 295 : 134-135.
- Koné MW, Kamanzi, AK (2006) Inventaire ethnomédical et évaluation de l'activité anthelminthique des plantes médicinales utilisées en Côte d'Ivoire contre les helminthiases intestinales. Pharmacopée et Médecine Traditionnelle Afr 14 : 55-72.
- Moulari B, Pellequer Y, Lboutounne H, Girard C, Chaumont JP, et al., (2006) Isolation and in vitro antibacterial activity of astilbin, the bioactive flavanone from the leaves of Harungana madagascariensis Lam. ex Poir. (Hypericaceae). J Ethnopharmacol 106(2) : 272-278.
- Moulari B, Lboutounne H, Chaumont JP, Guillaume Y, Millet J, et al., (2006) Potentiation of the bactericidal activity of Harungana madagascariensis Lam. ex Poir. (Hypericaceae) leaf extract against oral bacteria using poly (D, L-lactide-co-glycolide) nanoparticles: in vitro study. Acta Odontol Scand 64(3): 153-158.
- Lenta BN, Ngouela S, Bovom FF, Tantangmo F, Jiri Gut, et al., (2007) Antiplasmodial activity of some constituents of the root bark of H. madagascariensis Lam. (Hypericaceae). Chem Pharm Bull (Tokyo) 55(3) : 464-467.
- Mangambu M, KF Mushagalusa, NJ Kadima (2014) Contribution à l’étude phytochimique de quelques plantes médicinales antidiabétiques de la ville de Bukavu et ses environs (Sud-Kivu, R.D. Congo). J Appl Biosci 75(2014): 6211-6220.
- Antia BS, Ita BN, Udo UE (2015) Nutrient composition and in vitro antioxidant properties of Harungana madagascariensis stem bark extracts. J Med Food18(5): 609-614.
- Tom ENL, Nankia FD, Mezui C, Nyunaï N, Bekono YF, et al., (2018) Mechanisms of the hypotensive action of Harungana madagascariensis (Hypericaceae) stem bark aqueous extract in rats. Int J Curr Adv Res 7: 10580-10584.
- Tom ENL, Nankia FD, Nyunaї N, Thernier CG, Demougeot C, et al., (2018) Myocardial potency of an aqueous extract of Harungana madagascariensis stem bark against isoproterenol-induced myocardial damage in rats. Univ J Pharm Res 3(1): 17-24.
- Olugbenga IE, Bamigboye JT, Oluwatoyin MD, Gbade EA, Isaac OE, et al., (2008) Effects of Harungana madagascariensis Stem Bark Extract on the Antioxidant Markers in Alloxan Induced Diabetic and Carrageenan Induced Inflammatory Disorders in Rats. Journal of Complementary & Integrative Medicine. 5: 1-20.
- Nwodo OFC (1989) Antibiotic and anti-inflammatory analgesic activities of Harungana madagascariensis stem bark. Int J Crude Drug Res 27(3): 137-140.
- Tom ENL, Nyunaї N, Djaouro KG, Medou FM, Nankia FD, et al., (2018) Acute and Subacute Toxicity Evaluation of the Stem Bark Aqueous Extract of Harungana madagascariensis in Rodents. J Adv Pharm. Sci Tech 1(4): 1-12.
- Kouam SF, Ngadjui BT, Krohn K, Wafo P, Ajaz A, et al., (2006) Prenylated anthronoid antioxidants from the stem bark of Harungana madagascariensis. Braz J Med Biol Res 38: 1087-1094.
- Cimanga KR, Gatera GS, Tona LG, Kambu KO, Vlietinck AJ, et al., (2018) Spasmolytic activity of flavonoid extracts from some medicinal plants used as antidiarrheal agents in traditional medicine in kinshasa-drcongo. World J Pharm Pharm Sci 7(7): 170-182.
- Tom ENL, Billong JRM, Nyunaї N, Bekono YF, Longo F, et al., (2018) Vasodilatory Effects of Aqueous Extract from Harungana madagascariensis Stem Bark in Isolated Rat Aorta: The Roles of Endothelium and K+ Channels. Am J Ethnomed 5: 1-8.
- Moronkola DO, Yeboah SO, Majinda RRT, Sichilongo (2015) Compositions of Harungana madagascariensis Lam. ex Poiret leaf and stem essential oils. J Chem Pharm Res 7(5): 959-964.
- Fatokun OT, Wojuola TE, Esievo KB, Kunle OF (2016) Medicinal plants used in the management of asthma: a review. Eur J Pharm Med Res 3: 82-92.
- Harbone JB (1976) Phytochemical Methods. A Guide to Modern Techniques of Plant Analyses. 1-150.
- Arefani S, Mehran MSM, Moladoust H, Norasfard MR, Ghorbani A, et al., (2018) Effects of standardized extracts of Lamium album and Urtica dioica on rat tracheal smooth muscle contraction. J Pharmacopunture 21(2): 70-75.
- An SS, Bai TR, Bates JH, Black JL, Brown RH, et al., (2007) Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 29(5): 834-860.
- Ellis JL, Undem BJ (1994) Role of cysteinyl-leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. Am J Respir Crit Care Med 149(1): 118-122.
- Bergner A, Kellner J, Kemp Silva A, Fischer R, Gamarra F, et al., (2006) Bronchial hyperreactivity is correlated with increased baseline airway tone. Eur. J. Med. Res.11(2): 77-84.
- Molfino NA, Slutsky AS, Julià Serdà G, Hoffstein V, Szalai JP, et al., (2012) Assessment of airway tone in asthma: comparison between double lung transplant patients and healthy subjects. Am Rev Respir Dis 148(5): 1238-1243.
- Sommer B, Flores Soto E, Gonzalez Avila G (2017) Cellular Na+ handling mechanisms involved in airway smooth muscle contraction (Review). Int J Mol Med 40(1): 3-9.
- Kloek J, Mortaz E, Van Ark I, Lilly CM, Nijkamp FP, et al., (2010) Glutathione prevents the early asthmatic reaction and airway hyperresponsiveness in guinea pigs. J Physiol Pharmacol 61(1): 67-72.
- Iwalewa EO, Omisore NO, Adewunmi CO, Gbolade AA, Ademowo, et al., (2008) Anti-protozoan activities of Harungana madagascariensis stem bark extract on trichomonads and malaria. J Ethnopharmacol 117: 507-511.
- Oboh G, Akomolafe TL, Adefegha SA, Adetuyi AO (2010) Antioxidant and modulatory effect of ethanolic extract of Madagascar Harungana (Harungana madagascariensis) bark on cyclophosphamide induced neurotoxicity in rats. J Food Drug Analysis 18(3): 2.
- McFadzean I, Gibson A (2002) The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol 135(1): 1-13.
- Berridge MJ (2008) Smooth muscle cell calcium activation mechanisms. J Physiol 586(21): 5047-5061.
- Sakihara C, Jones KA, Lorenz RR, Perkins WJ, Warner DO (2002) Effects of primary alcohols on airway smooth muscle. Anesthesiology 96(2): 428-437.
- Moncada S, Palmer RM, Higgs EA (1989) Biosynthesis of nitric oxide from L-arginine. a pathway for the regulation of cell function and communication. Biochem Pharmacol 38(11): 1709-1715.
- Trischitta F, Pidala P, Faggio C (2007) Nitric oxide modulates ionic transport in the isolated intestine of the eel, Anguilla anguilla. Comp Biochem Physiol A Mol Integr Physiol 148(2): 368-373.
- Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43(2): 109-142.
- Nelson HS (1995) Beta-adrenergic bronchodilators. New Engl J Med 333: 499-507.
- Buckle DR, Arch JRS, Bowring NE, Foster KA, Taylor JF, et al., (1993) Relaxant effect of the potassium channel activators BRL 38227 and Pinacidil on Guinea-pig and Human airway smooth muscle, and blockage of their effects by Glibenclamide and BRL 31660. Pulm Pharmacol 6(1): 77-86.
- Doğan M, Yildiz O, Arslan S, Ulusoy G (2019) Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol 33(5): 504-523.
- Pollok S, Reiner A (2020) Subunit-selective iGluR antagonists can potentiate heteromeric receptor responses by blocking desensitization. Proc Natl Acad Sci U S A 117(41): 25851-25858.
- Ash ASF, Schild HQ (1966) Receptors mediating some actions of histamine. Br J Pharmacol 27: 427-439.
- Popa VT (1977) Bronchodilating activity of an H1 blocker, chlorpheniramine. J Allergy Clin Immunol 59(1): 54-63.
- Lronards B, Rampart M, Herman A (1992) Selective M3 muscarinic receptor inhibit smooth muscle contraction in rabbit trachea without increasing the release of acetylcholine. J Pharmacol Exp Ther 263(2): 773-779.
- Spina D (2014) Current and novel bronchodilators in respiratory disease. Curr Opin Pulm Med 20(1): 73-86.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.