Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Progress In the Study of the Role of Exosomal MiRNAs in Renal Interstitial Fibrosis
*Corresponding author: Jian Guo Xie, Institute (College) of Integrative Medicine, Dalian Medical University, No. 9, South Road of Lv shun, Dalian 116044, China. Department of Nephrology, The First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Xigang District, Dalian, Liaoning, China and Da Peng Wang, Department of Nephrology, The First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Xigang District, Dalian, Liaoning, China.
Received: November 25, 2023; Published: November 28, 2023
DOI: 10.34297/AJBSR.2023.20.002748
Abstract
Chronic Kidney Disease (CKD) has now become a global public health problem. Renal Interstitial Fibrosis (RIF) is a common pathological feature of various chronic kidney diseases, and there is no effective treatment for RIF due to the complex pathogenesis. Recent studies have found that exosomal miRNAs play an important role in the diagnosis and treatment of RIF. This review summarises the current research on the role of exosomal miRNAs in RIF and their therapeutic applications and looks forward to the future development direction and prospect of exosomal research, which will provide new ideas for exosomal therapeutic research in RIF.
Keywords: Exosomes, miRNAs, Renal interstitial fibrosis, Diagnosis and Therapy
Introduction
Epidemiological studies have shown that the increasing incidence of Chronic Kidney Disease (CKD) has become a serious public health problem worldwide[1], and Renal Interstitial Fibrosis (RIF) is the main pathological change in almost all CKD progressing to end-stage renal disease, and its formation can be divided into a reversible stage (including the inflammatory response stage and the pre-fibrotic formation stage) and an irreversible stage (scarring stage), and its main pathological The main pathological features include inflammatory cell infiltration, renal tubular atrophy, peritubular capillary detachment, myofibroblast proliferation and excessive deposition of Extracellular Matrix (ECM) [2]. miRNAs are a new class of non-coding RNAs with a special ring structure and resistant to degradation by nucleic acid exonucleases, with a length of about 22 nucleotides, which can be encapsulated in Exosomes by Vesicles (EVs) to constitute exosomal miRNAs [3]. Exosomal miRNAs are widely and stably present in humoral circulation and can be transported to target cells in vesicular form via blood circulation, thus regulating the pathophysiological processes in recipient cells. It has been demonstrated that after injury to renal proximal tubular epithelial cells, released EVs can specifically activate renal mesenchymal fibroblasts by carrying TGF-β1 mRNA and upregulate the expression of α-SMA, F-actin and collagen type I, which leads to RIF [4]. The aim of this paper is to summarise the function of exosomal miRNAs and their mechanism of action on renal interstitial fibrosis, and to provide a basis for the early diagnosis and treatment of renal interstitial fibrosis by exosomal miRNAs.
Production and Release of Exosomes and Exosomal MiRNAs
Exosomes are tiny vesicles with a phospholipid bilayer structure released from a variety of cells actively into extracellular and body fluids, with a diameter of about 40~160 nm[5], which are widely present in blood, tears, urine, saliva, breast milk, ascites, and other body fluids, and can act on the target cells to change their gene phenotypes and modify the microenvironment in which they are located, and play a role in regulating the physiological activity of the target cells, and is one of the important means of intercellular communication [6]. miRNAs are a class of short-stranded non-coding RNAs with a length of about 18-24 nucleotides, which are highly conserved, can regulate gene expression at the transcriptional or translational level, and are involved in the regulation of inflammatory responses, oxidative stress, and other physiological and pathological processes [3]. miRNAs, as the regulatory genes of mRNAs, can regulate fibrotic diseases, and they can be encapsulated in the exosomes by EVs, constituting the exosomal miRNAs. miRNAs, exosomal lipid bilayer wrapped miRNAs can be protected from degradation by RNA enzymes in body fluids. The formation and release process of exosomes and exosomal miRNAs can be divided into three phases: the initiation endocytosis phase, the Multivesicular Bodies (MVB) formation phase and the exosomal secretion phase [7]. Under different pathological states, miRNAs encapsulated and transported by exosomes stably exist in the humoral circulation and can mediate fibroblast proliferation and differentiation, mesenchymal cell transformation and apoptosis through multiple signalling pathways, and thus play a regulatory role in fibrotic diseases [1], therefore, exosomal miRNAs are expected to be important markers for the diagnosis of fibrotic diseases and an important target for therapy.
Role of Exosomal miRNAs in the Pathogenesis of Renal Interstitial Fibrosis
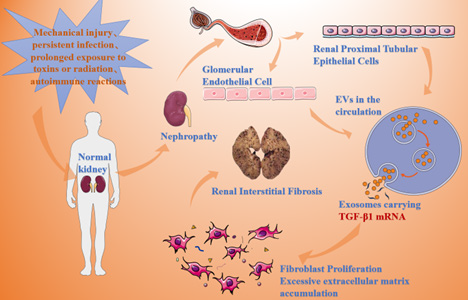
Kidneys stimulated by various causes (e.g. mechanical injury, persistent infection, long-term exposure to toxins or radiation, autoimmune response, etc.) can ultimately result in abnormal degradation and synthesis of extracellular matrix proteins in the renal parenchyma, which causes a large amount of extracellular matrix aggregation, resulting in severe disruption of the tissue structure, and ultimately leading to RIF (Figure 1) [2].
After receiving various stimuli, the normal kidney causes cellular abnormalities, with renal proximal tubular epithelial cells and glomerular endothelial cells releasing exosomes carrying TGF-β1 mRNA, respectively, which leads to excessive accumulation of extracellular matrix, resulting in renal interstitial fibrosis. In the development of RIF, exosomal miRNAs released by different types of cells play their regulatory roles through different pathways. For example, after renal proximal tubular epithelial cell injury, exosomes are released, and exosomes carrying TGF-β1 mRNA can activate renal mesenchymal fibroblasts, and TGF-β1 rapidly activates repair or (and) regenerative responses, promotes the proliferation of fibroblasts, and up-regulates the expression of α-SMA, F-actin, and collagen type I, which ultimately leads to fibrosis of the kidney[ 4]. The exosomes released from glomerular endothelial cells after glomerular endothelial cell injury carry a large amount of TGF-β1mRNA, which promotes the expression of α-SMA in glomerular thylakoid cells through the TGF-β/Smad3 signalling pathway, resulting in the over-accumulation of renal extracellular matrix and the formation of renal fibrosis [8]. According to Delic, et al., [9]. both telmisartan and riglitazidin normalized urinary exosomal miR-29c levels, a miRNA that exerts an antifibrotic effect in nephrectomised rat models. The study by He, et al., [9]. and others also found that exosomal miRNAs are key regulators in the progression of diabetic nephropathy that have a regulatory effect on RIF. Therefore, exosomal miRNAs can be involved in the occurrence and development of RIF by mediating multiple pathological factors.
Exosomal miRNAs and the Diagnosis of Renal Interstitial Fibrosis
Renal puncture biopsy is currently the golden index to understand the process of renal fibrosis, but this is an invasive operation, which is traumatic to organ tissues and difficult to take material, difficult to repeat in the short term, and difficult to understand the process of renal fibrosis in real time and dynamically due to the limitation of examination conditions. Therefore, finding a real- time, dynamic and easy to promote the application of renal fibrosis detection method is an urgent need for clinical diagnosis. The exosomes in body fluids have the most diagnostic potential, which carry a variety of substances such as miRNAs, rRNAs, proteins and other substances from intracellular sources and can be stably expressed. Human experiments [10] also found that miR-29c in exosomes extracted from urine was negatively correlated with Smad3 and MMP2, and the expression level of miR-29c was also negatively correlated with the renal chronicity index (i.e., scores of glomerulosclerosis, fibrous crescent, tubular atrophy, and mesenchymal fibrosis, with higher scores being more severe fibrosis), suggesting that miR-29c in exosomes can be used as a new biomarker for the diagnosis of renal fibrosis.
At this stage, the technology of isolating exosomes is very mature, which can perform real-time quantitative analysis of miRNAs and proteins. Lv, et al., [11]. collected urine samples from 32 CKD patients and 7 control patients, and the results of the study showed that the levels of exosomal renal fibrosis-related miRNAs (miR-29 and miR-200) in the urine of CKD patients were significantly decreased compared with those in normal controls, suggesting that the urinary levels of miR-29c in exosomes can be used to reflect the degree of renal function and histological fibrosis, and is a new, non-invasive marker of renal fibrosis. The universally applicable microRNA quantification and stability of exosomal miRNAs further emphasise their unique potential as novel, non-invasive biomarkers of CKD. In addition, one study [12] analysed the mRNA levels of CD2-associated protein (CD2⁃accosiatedprotein, CD2AP) and Synaptopodin (SYNPO) in urinary exosomes of patients with kidney disease and found that the level of CD2AP mRNA in urinary exosomes of patients with kidney disease decreased with the increase of urinary protein, and was negatively correlated with the renal fibrosis degree was negatively correlated with renal fibrosis, therefore, urinary exosomal source of CD2APmRNA can also be used as a marker of renal function and RIF in patients with kidney disease.
The study of SHI, et al., [3]. also found that the expression of urinary exosomal miRNAs in patients with IgA nephropathy was positively correlated with the severity of renal fibrosis, and the working characteristic curves of the subjects suggested that the expression of urinary exosomal miRNAs could differentiate between patients with IgA nephropathy with different degrees of fibrosis, and it was better than the traditional clinical biochemical indexes such as blood creatinine. It suggests that urinary exosomal miRNAs can be used as a novel, non-invasive biomarker for diagnosing renal fibrosis SONG, et al., [4]. collected renal puncture samples from 32 diabetic nephropathy patients with high glucose stimulation of renal tubular epithelial exosomal miRNAs for systematic analysis and found that as the disease severity of patients with early-stage diabetic nephropathy increased, the higher the mRNA expression levels of TGF-β1 and CTGF in the patients’ urinary exosomes. This suggests that fibrogenic genes TGF-β1 and CTGF in the urinary exosomes of diabetic nephropathy patients may be important auxiliary biological markers for clinical diagnosis of early diabetic nephropathy and its progression.
Exosomal miRNAs and the Treatment of Renal Interstitial Fibrosis
Exosomes play a bidirectional regulatory role in the development of fibrosis, and in the progression of CKD, exosomes released from its damaged renal cells promote renal fibrosis, while exosomes released from stem cells inhibit fibrosis, which provides a theoretical basis that exosomes can be used as a therapeutic target for fibrosis. For example, Wang, et al., [13]. found that bone marrow mesenchymal stem cell-derived exosomes with high expression of miR-Let7c and low expression of type IV collagen, matrix metalloproteinase 9, and transforming growth factor β1 in animal experiments of unilateral ureteral obstruction in mice had anti-fibrotic effects with a significant reduction in renal injury. This effect was antagonised if the exosome inhibitor GW4869 was used. Another study [14] confirmed that intramuscular injection of exosomes expressing miR-29 could effectively alleviate renal fibrosis and reduce the expression of transforming growth factor β, α-smooth muscle actin, and collagen in renal tissues. Jing, et al., [15-20]. explored the role of Human Umbilical Cord Mesenchymal Stem Cell Exosomes (huMSC-Ex ) in regulating renal fibrosis through the Unilateral Ureteral Obstruction (UUO) model of rat. Yes-Associated Protein (YAP) in renal injury repair. The results showed that HucMSC-Ex could inhibit RIF by promoting YAP ubiquitination and degradation through the delivery of CK1δ and β-TRCP, providing a novel approach for RIF treatment. On this basis, Yu, et al., [5]. injected huc-MSCs-derived exosomes containing adenovirus-TGF-β1 shRNA into UUO rats by tail vein and found that the exosomes were able to specifically target fibrotic renal tissues and repair renal interstitial fibrosis by inhibiting the TGF-β1/Smad 3 pathway. Of course, standardization and quality control issues should be addressed before exosomes are truly applied to clinical treatment. At present, there is still a lack of effective methods for exosome therapy for RIF, and many clinical trials are needed for validation.
Conclusion and Future Outlook
RIF, as a widespread pathological mechanism in renal diseases, has been the focus of medical workers’ research, and how to slow down the process of RIF and improve the prognosis is an urgent challenge. Among the various proposed potential treatments for renal fibrosis, exosomal miRNAs have gradually come into the limelight as a cell-free treatment. With the deepening of research, the mechanism of exosomal miRNAs involved in the development of RIF has been gradually revealed. With the continuous improvement of exosome detection technology, body fluid exosome biopsy method is expected to become a simple and non-invasive new technology to confirm the diagnosis of fibrosis. Meanwhile, the obvious differential expression of exosomal miRNAs plays an indispensable role in the development of organ fibrosis diseases. Although the research and development of exosomal miRNAs are also facing many challenges, exosomal miRNAs provide a new breakthrough point for exploring the mechanism of RIF and a new target for the future treatment of RIF. It is believed that with the development of science and technology and in-depth research, exosomal miRNAs will become an important tool for designing a new generation of diagnostic and therapeutic methods for RIF.
Acknowledgments
None.
Conflicts of Interest
None.
References
- Meike Ying, Xue Shao, Hongli Qin, Pei Yin, Yushi Lin, et al. (2023) Disease burden and epidemiological trends of chronic kidney disease at the global, regional, national levels from 1990 to 2019. Nephron.
- Wei Zhong Ying, Xingsheng Li, Sunil Rangarajan, Wenguang Feng, Lisa M Curtis, et al. (2019) Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J clin invest 129(7): 2792-2806.
- Liang Chen, Liisa Heikkinen, Changliang Wang, Yang Yang, Huiyan Sun, et al. (2019) Trends in the development of miRNA bioinformatics tools. Brief Bioinform 20(5): 1836-1852.
- Fernanda T Borges, Sonia A Melo, Berna C Özdemir, Noritoshi Kato, Ignacio Revuelta, et al. (2013) TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24(3): 385-392.
- Raghu Kalluri, Valerie S LeBleu (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478): eaau6977.
- Liangdi Jiang, Yongwei Gu, Yue Du, Jiyong Liu (2019) Exosomes: Diagnostic Biomarkers and Therapeutic Delivery Vehicles for Cancer. Mol pharm 16(8): 3333-3349.
- Joanna Kowal, Mercedes Tkach, Clotilde Théry (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29: 116-125.
- Xiao Ming Wu, Yan Bin Gao, Fang Qiang Cui, Na Zhang (2016) Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open 5(4): 484-491.
- Xiaoyun He, Gaoyan Kuang, Yongrong Wu, Chunlin Ou (2021) Emerging roles of exosomal miRNAs in diabetes mellitus. Clin transl med 11(6): e468.
- Cristina Solé, Josefina Cortés Hernández, Maria L Felip, Marta Vidal, Josep Ordi Ros (2015) miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol dial transplant 30(9): 1488-1496.
- Lin Li Lv, Yu Han Cao, Hai Feng Ni, Min Xu, Dan Liu, et al. (2013) MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305(8): F1220-1227.
- Lin Li Lv, Yu Han Cao, Ming Ming Pan, Hong Liu, Ri Ning Tang, et al. (2014) CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin chim acta 428: 26-31.
- Bo Wang, Kevin Yao, Brooke M Huuskes, Hsin Hui Shen, Junli Zhuang, et al. (2016) Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther24(7): 1290-1301.
- Haidong Wang, Bin Wang, Aiqing Zhang, Faten Hassounah, Yiqi Seow, et al. (2019) Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Molecular Ther 27(3): 571-583.
- Cheng Ji, Jiahui Zhang, Yuan Zhu, Hui Shi, Siqi Yin, et al. (2020) Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis 11(5): 327.
- Yan Zhang (2021) Research progress on the mechanism of action of exosome-derived miRNAs in fibrotic diseases and their related signaling pathways Shandong Medicine 61(17): 92-94.
- Sun Yang (2015) Pathogenesis of renal fibrosis and progress in treatment. Journal of Practical Clinical Medicine 19(09): 173-176.
- Yuhan Cao, Yuanhui Shi, Yanlang Yang, Zhangli Wu, Nana Peng, et al. (2023) Urine exosome-derived circular RNA as a biomarker of renal fibrosis. Ann Med 54(1) :1966-1976.
- Lanlan Song (2022) The role of fibrogenic gene expression in urinary exosomes in the diagnosis of early diabetic nephropathy. New World of Diabetes 25(18): 32-35.
- Yu Jing (2022) Umbilical cord mesenchymal stem cell-derived exosomes repair renal interstitial fibrosis in rats with unilateral ureteral obstruction by inhibiting the TGF-β1/Smad 3 pathway Journal of Modern Urology 27(07): 593-599.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.