Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Rapid Response Innovation of Healthcare Delivery During the Covid 19 Pandemic
*Corresponding author: Bernhard Fassl MD, Director - Center for Global Health Innovation, College of Graduate Studies Professor of Pediatrics, College of Medicine, USA
Received: September 06, 2023; Published: September 12, 2023
DOI: 10.34297/AJBSR.2023.20.002675
Abstract
Introduction: In 2020, the coronavirus pandemic brought new challenges to healthcare systems as rising demands led to resource inequalities around the globe. The inability to safeguard healthcare workers (HCW) led to increased infection rates and deaths worldwide. The purpose of this report is to showcase the response of an in-house innovation committee to meet the unforeseen needs faced by healthcare systems.
Methods: Housed directly within a major health system, The Centre for Medical Innovation (CMI) teamed up with administration, BME, and occupational health to create an innovative think-tank to tackle the challenges brought in by the coronavirus pandemic. While prioritizing acuity, frequency, and urgency, CMI used human-cantered design to engineer solutions from commercially available materials and test reimagined products against known gold-standards.
Results: The close-working relationship between CMI and the health system allowed for the rapid innovation and engineering of products during the months following the COVID pandemic. These included aerosol containment tents, powered air-purifying respirators, and self-testing stations. In anticipation of future needs, CMI engineered 20 additional rapid-response products, such as a bubble CPAP, containment boxes, and re-usable PPE. From these, several open-source manuals were shared with global partners to address equipment inequities.
Discussion: The rapid development of low-cost solutions for acute clinical needs is improved via partnership between health systems and medical innovation center. Future expansions of rapid innovation may include anticipatory identification of products facing similar shortages, including sharing open-source materials with LMIC entities to improve rapid-response innovation locally and abroad.
Summary Box:
i. During the onset of covid-19, supply shortages led to a glaring need for personal protective equipment due to rising infection rates among healthcare workers.
ii. By bringing together major stakeholders, one healthcare system was able to quickly define problems and ideate solutions, including timely prototyping, testing to industry standards, and manufacturing.
iii. Rapid response innovation leads to new solutions and protocols in acute settings, which can be shared with global partners to address the differences and disparities experienced in diverse healthcare systems
iv. The rapid development of low-cost solutions for acute clinical needs is improved via partnership between health systems and medical innovation centre.
Keywords: Healthcare innovation, Biomedical engineering, Healthcare economics, Public health, Occupational health
Introduction
The coronavirus pandemic brought new challenges to healthcare systems as the rising demand for Personal Protective Equipment (PPE) and respiratory machinery led to unprecedented product shortages [1]. Additionally, rising infection rates among frontline providers led to increased risks by Healthcare Workers (HCW), provoking a growing inability to safeguard the front lines [2]. As the supply chain deficiencies fluxed in constant chaos, the entire healthcare system went into a state of crisis, along with the ability to anticipate future needs for individual patients appropriately. Furthermore, the unequal distribution of healthcare resources, exacerbated by this acute demand, increased healthcare inequity among low- to middle income countries (LMICs) around the globe. These inequities resulted in significant disruptions of health services available to meet the needs of HCW infected by Covid-19 [3].
Frontline HCW’s, such as those within emergency departments or inpatient settings, were reported to be 6% of total covid infections and more than seven times as likely to have a severe illness [4,5]. Additionally, some areas showed 19% of documented COVID infections within HCW, including a severely high death rate in this group of 5.7% [6]. This increased infection rate and mortality within HCW exacerbated workforce shortages and the already limited healthcare resources caused by the increased strain on healthcare systems across the US. Furthermore, HCW in low resource environments were disproportionately affected in the US and globally [7,8].
In this report, we detail the steps taken by an academic centre within a major health system to leverage institutional capacity to respond to the covid-19 pandemic via the design of a Rapid Response Innovation (RRI) system. We will also describe how, through the creation of this system, our facility responded effectively and timely to the clinical needs of health workers and their ever-changing, emergent healthcare needs, both for the institution and throughout the state. Additionally, we outline the dissemination of these innovations to other community facilities, especially in low resource, rural regions and neighbouring states. By sharing this information, we hope to highlight how the design and development of RRI systems are replicable in other clinical settings, which can lead to a better and more effective response to future pandemics while also improving disaster response preparedness.
Methods
Building Administrative Capacity and Response Planning to Covid-19
In March of 2020, as news from Europe slowly spread about the Covid-19 pandemic, the University of Utah (UU) determined to establish an in-house rapid response innovation committee to meet any future, unforeseen needs faced within its healthcare system (UU-Health). With the goal to (1) quickly respond to the needs of frontline clinical care and (2) disseminate knowledge, tools, and materials to local and global partners, the UU Rapid Response Innovation committee (UU-RRI) was formed. The core members that made up UU-RRI consisted of the Center for Medical Innovation (CMI), Rocky Mountain Center for Occupational and Environmental Health (RMCOEH), and the UU Covid Task Force (CTF) (Table 1).
By bringing together a diverse, multidisciplinary group of academic minds, UU-RRI worked on projects within UU-Health to define their problems and ideate feasible solutions, including processes such as concept generation, prototyping, testing/validation, mass production, clinical integration, and business development.
Intake Process
The process of innovation identification started primarily with the physicians working directly with those infected by the coronavirus. Once identified, clinical needs from these providers, and often from non-clinical departments such as environmental services, were shared with administration through daily briefings scheduled with each department within UU-Health. Although these needs could be presented to any of the three core stakeholders within UU-RRI, they were primarily funnelled through the CTF.
After identification, evolving clinical needs were catalogued and stratified by the Pandemic Innovation Team (PIT)-housed within CMI-based upon acuity, frequency, and urgency. At the same time, UU-RRI also discussed speed, possible solutions, and next steps on an ad hoc basis. Lastly, the committee would determine problem feasibility based on resources (e.g., materials and workforce) and financial needs, including any engineering or prototyping necessary to carry out the determined innovation.
Ideation of Feasible Solutions, Concept Generation, Prototyping
Due to the nature of supply shortages, UU-RRI focused on mobilizing existing resources at UU to respond to a specific need. The process included meeting with departments to understand clinical needs, identifying standard industry practices, engineering comparable solutions through easily obtainable materials, customizing the product usage and experience to the requesting party, and, ultimately, validating innovations through direct comparison to industry gold standards (Figure 1).
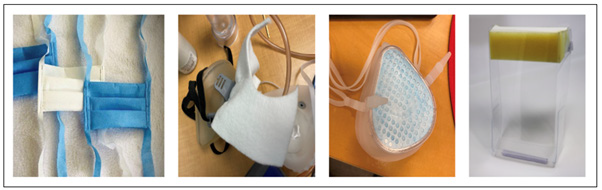
i. Example 1: During the initial stages of the pandemic, face shields were in short supply. In response, PIT developed a variety of prototypes options, which were evaluated by clinicians within 24 hours. After receiving feedback on the various prototypes, a design with the most positive feedback was then marked for mass production. By utilizing its existing design team and prototyping facility, PIT developed a variety of models that were then evaluated by the clinical work force and UU-RRI for user friendliness and clinical applicability, which ultimately led to the selection, production, and usage of a new face shield in practice.
This example helped define the ultimate process through which PIT progressed through subsequent needs-based analysis, explicitly focusing on iterative, human-cantered design to meet clinical demands while planning for estimated equipment shortages. Additionally, PIT would rapidly integrate end-user design feedback in subsequent prototype versions until consensus among clinical users and administrators was achieved. Depending on the project’s complexity, this process would take anywhere between forty-eight hours to two weeks (Figure 2).
ii. Example 2: Aerosol containment tents used during intubation underwent a series of significant design changes based on end user feedback and direct observation of design team members in clinical settings until a final design blueprint was completed.
Of note, an essential aspect of this process includes the research of available supplies to produce prototypes in large numbers. The ever-increasing shortages in raw material supply (i.e., plexiglass for face shields) played a pivotal role in the progressive supply chain deficiencies faced during the onset of the pandemic. These are specific and unique problems associated with the pandemic, which led to developing an individual and novel production plan to solve known and joint issues.
Testing/Validation
Using existing resources and expertise at the RMCOEH, PIT performed safety testing following standard NIOSH/OSHA procedures under the supervision of RMCOEH experts. Testing was carried out in the presence of the multidisciplinary team members, and design changes to mitigate testing failures were applied commonly within the same day. Once testing and safety certification was completed, the prototype was advanced to production.
Production Capacity Building
CMI operates a prototyping facility that was used for design and testing but its capacity for mass production is limited. For devices required in large numbers UU-RRI engaged with regional industry leaders until its own production capacity was able to meet the clinical demand. After a certain design was validated and approved by the committee, PIT connected with other entities with UU and local industry to fulfill mass production needs. For example, 3D printing for frames/shields and tubing connectors. Using this strategy over 1000 face shields were available to the workforce in clinical settings within 4 weeks.
Transitioning Ideas to Business Development
The UU-RRI engaged with the UU commercialization and business creation entity, the PIVOT Centre (Partners for Innovation, Venture, Outreach, Technology) on an ad hoc basis to review innovations and identify opportunities for IP, patentability, and business creation.
Clinical Integration and Dissemination of Innovations
For dissemination within the UU-Health network, UU-RRI engaged with clinicians to plan and assist with integration into clinical care and provided end-user support (i.e., education around use and cleaning), troubleshooting and maintenance support, and regular safety re-testing of PPE devices. For external dissemination, UU-RRI engaged with health facilities/systems located in the region, especially in rural locations, as well as global health partners in Asia and Africa and provided support in two ways: (1) direct supply of RRI materials (regional) and (2) provision of design and manufacturing blueprints to international partners to use in prototype, test, and implement design concepts within their environment.
Results
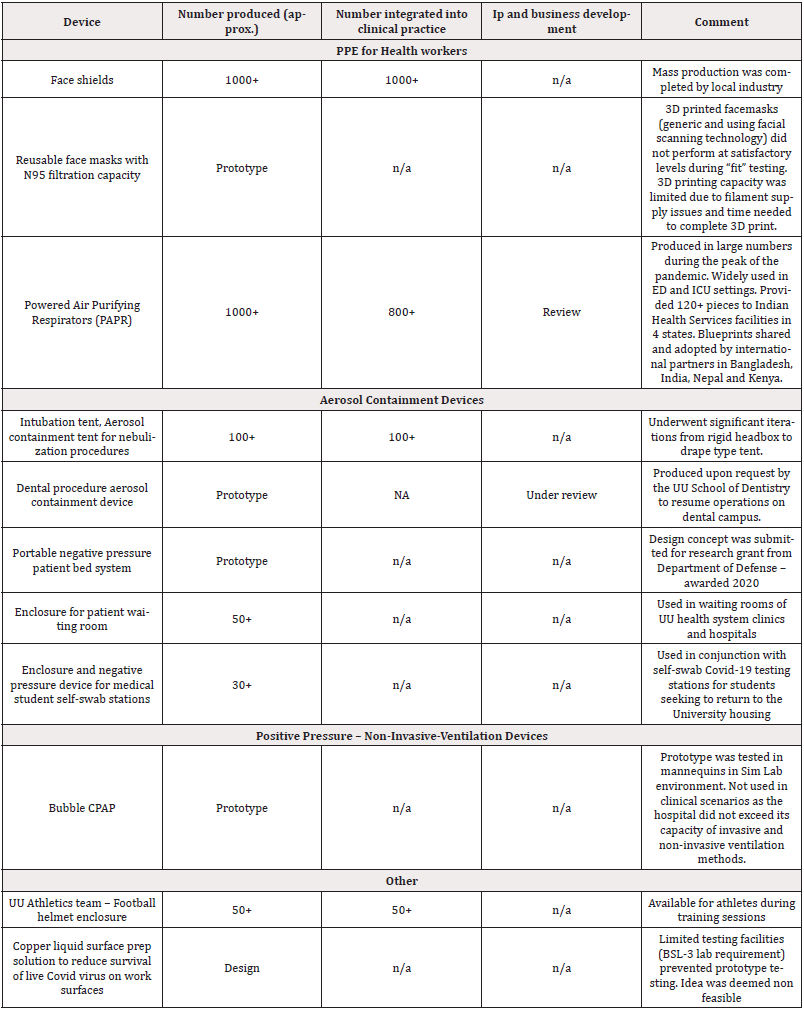
As a result of the clinical needs identified by HCW, four categories were recognized as highest need during the initial months of the pandemic. These include: (1) PPE for health workers; (2) Aerosol containment; (3) positive pressure – non-invasive-ventilation devices; (4) Other miscellaneous needs (Table 2).
Real-World Clinical Use
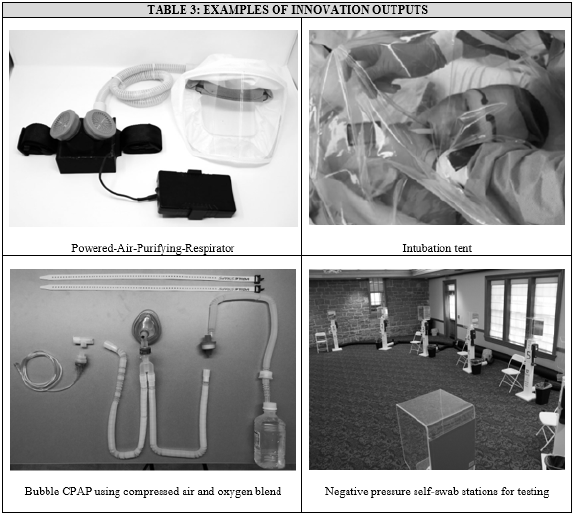
As the ideas that ultimately led to innovative devices were clinical driven, they generally experienced high rates of adoption. This was further assisted by the process of PIT, who engineered solutions from commercially available materials that were highly comparable to standard products. This efficacy was further validated by testing reimagined products against known gold-standards and its generalizability improved via the creation of open-source assembly guides that allowed others facing similar shortages to do the same.
Beyond their use locally, the engineering process was meticulously documented and tailored to be easily reproducible and, as a result, multiple, “Improvised Personal Protective Equipment’’ manuals were created (Table 3). The end goal of maintaining these manuals for COVID needs includes sharing their contents with both local and global partners to address the growing need of equitable healthcare access during this pandemic.
Safety
Products were developed and tested according to OSHA standards under supervision of the RMCOEH. A safety event reporting system was instituted to proactively identify and track safety events. In one case, a faulty lithium-ion battery, used as part of the PAPR device, led to a house fire in a UU clinic while charging multiple stacked up batteries on a single charging station. The innovation team responded by designing dedicated battery charging stations and separating batteries to avoid overheating.
Discussion
As the coronavirus changed health system approaches to healthcare delivery and supply chain management, the collaboration between hospital administrators within the UU-Health and PIT at CMI allowed for the development of easily producible, low-cost solutions to acute clinical needs. This relationship allows for the rapid development of new solutions and protocols in acute settings, which can be shared with global partners to address the differences and disparities experienced in diverse healthcare systems. This includes the mentorship and collaboration of best practices with collegiate, non-academic, or low-income systems who lack the resources or structure found within a major healthcare system, including the approach to identifying clinical problems, expand upon interdisciplinary innovation, and produce equipment that meets the unforeseen, critical needs of an ongoing equipment shortage or pandemic.
Future expansions of CMI will include the establishment of teams focused on anticipatory equipment shortages for the likes of future pandemics or disaster crisis. Additionally, these teams will create any additional manuals outlining the step-by-step protocols for new, improvised solutions developed by CMI, including specifications detailing their construction and assembly. Ultimately, these will be packaged and shared with UU-Health partners, both among low-income communities locally and LMIC entities across the globe. In this way, the innovations developed from this relationship will allow for improvement of the rapid-response capability of hospital systems at a time of acute need both in local communities and abroad.
Conclusion
The rapid development of low-cost solutions for acute clinical needs-especially those faced by the equipment shortages during a pandemic-is improved via partnership between health systems and medical innovation centre. Future expansions of rapid innovation may include anticipatory identification of products that may face similar shortages, including sharing open-source materials with LMIC entities to improve rapid-response innovation inequities locally and abroad.
References
- Ranney M L, Griffeth V, Jha A K (2020) Critical Supply Shortages-The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. New Engl J Med 382(18): e41.
- Nguyen L H, Drew D A, Graham M S, Amit D Joshi, Chuan Guo Guo, et al. (2020) Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 5(9): e475-e483.
- Chemali S, Mari Sáez A, El Bcheraoui C, Heide Weishaar (2022) Health care workers’ experiences during the COVID-19 pandemic: a scoping review. Hum Resour Health 20(1): 27.
- Kambhampati A K, O’Halloran A C, Whitaker M, Michael Whitaker, Shelley S Magill, et al. (2020) COVID-19-Associated Hospitalizations Among Health Care Personnel-COVID-NET, 13 States, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep 69(43): 1576-1583.
- Mutambudzi M, Niedzwiedz C, Macdonald E B, Alastair Leyland, Frances Mair, et al. (2021) Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med 78(5): 307-314.
- Bandyopadhyay S, Baticulon R E, Kadhum M, Muath Alser, Daniel K Ojuka, et al. (2020) Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health 5(12): e003097.
- Mehta S, Machado F, Kwizera A, Laurent Papazian, Marc Moss, et al. (2021) COVID-19: a heavy toll on health-care workers. Lancet Respir Med 9(3): 226-228.
- Tan Torres Edejer T, Hanssen O, Mirelman A, Paul Verboom, Glenn Lolong, et al. (2020) Projected health-care resource needs for an effective response to COVID-19 in 73 low-income and middle-income countries: a modelling study. Lancet Glob Health 8(11): e1372-e1379.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.