Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
SIGLECs the Main Self-Pattern Recognition Receptors of the Immune System. A Promising Target for Inflammatory Modulation
*Corresponding author: Michael J Tolentino, Aviceda Therapeutics Cambridge Massachusetts, Department of Ophthalmology, Orlando College of Osteopathic Medicine, University of Central Florida School of Medicine, Orlando Florida.
Received: October 19, 2023; Published: October 27, 2023
DOI: 10.34297/AJBSR.2023.20.002714
Abstract
The existence of a Self- Pattern Recognition Receptor(sPRR) was postulated over 3 decades ago which would recognize and prevent immune response to self-cells covered with Self-Associated Molecular Patterns (SAMP). In the last few years, it has become evident that SAMPS are glycan patterns with end sialylation that activate complement factor H to deamplify the complement pathway and agonize immune resolving sialic acid binding Ig like lectins (Siglecs). The latter resolves inflammation via localized recruitment of a potent protein tyrosine phosphatase which dephosphorylates intracytoplasmic tyrosine kinase dependent activation pathways within immune cells. This mini review will recount the history behind this discovery and explain how SAMP mimetics can be used to therapeutically modulate the immune system.
Pattern Recognition Detection of Non-Self or Infectious Self
Charles Janeway’s and Ruslan Medzhitov’s compendium of research provides immunologist and scientist mechanistic schematic of how the immune system is regulated. Prior to 1989 the immune system function was considered exclusively modulated by T and B cells or what is termed, currently, the adaptive immune system. In a 1989 Cold Spring Harbor symposium, Janeway postulated that the immune system was more than just a defense system against foreign pathogens that discriminated “self vs non-self,” [1] but evolved to discriminate between “non-infectious self from infectious self” [2] He also implicated the innate immune system as the main effector of this additional discrimination [3].
This theory set the stage for 3 concepts to be demonstrated in the decades going forward. The first concept derived from the theory that Pathogen Associated Molecular Patterns (PAMP), non self, along with Damaged Associated Molecular Patterns (DAMPS), infectious or transformed self, are recognized by Pattern Recognition Receptors (PRR) found on innate immune cells that initiate and activate the immune response [4]. Secondly, this recognition by the innate immune cells would be transmitted as an activation signal for T and B lymphocyte which will develop an adaptive antibody response to these molecular patterns [5]. Thirdly he postulated the existence of a Self-Pattern Recognition Receptor(sPRR) that recognized Self-Associated Molecular Patterns (SAMP), self, that resolve inflammation and serve as a countervailing negative regulator to dampen or grade immune response and prevent autoimmunity [4].
Toll Like Receptors the First Identified PRR
Prior to the definitive identification of these microbial pattern recognition receptors, these receptors were characterized as transmembrane proteins that activated the NF-kB signaling pathway [6]. Medzhitov and Janeway, discovered that this yet to be identified candidate receptor contained Leucine-Rich Repeat (LLR) domain. Doing a proteomic search for LRR domain they discovered a Drosophila trans membrane receptor called Toll which was a protein identified to be involved in dorsal-ventral patterning in developing flies [7]. During the same time period, a loss of function mutation in this Toll receptor in drosophila was discovered to make flies susceptible to a deadly fungal infection implicating Toll receptors as a regulator of immune response [8]. This led to the discovery of human Toll Like receptors which were able to bind microbial lipopolysaccharide (LPS) to activate immune cells [9]. This seminal paper published in Nature in 1997 identified the first PRR postulated a decade earlier by Janeway [10] Toll like Receptor 4 was demonstrated to be the LPS sensing activator for immune cells by the Beutler [11]and colleagues who would go on to win the Nobel Prize with Hoffman in 2011 [12].
PRRs Discriminate Non-Self and Altered Self
In 2002 Medzhitov and Janeway also categorized functions of the immune system as recognition of “microbial non-self, missing self, and induced or altered self” [4]. The recognition of missing self relies on markers of normal self which activate inhibitory pathways that block initiation of immune response against self or autoimmunity. The recognition of altered or induced self in contrast to missing self, is based on detection of markers of abnormal self which mark the cells for immune elimination.
Janeway’s and Medzhitov’s collective research has elucidated how the immune system is activated and deactivated. Immune system activation is mediated by pattern recognition receptors that recognize both microbial moieties called PAMPs and infected, transformed, senescent cell markers of abnormal self-termed damage associated molecular patterns (DAMP). Conversely the immune system prevents autoimmunity by recognizing SAMP with sPRR. With these countervailing receptors, immune response can be tightly regulated.
PRRs Modulate Para Inflammation
In 2008 Medzhitov further postulated that these pattern recognition receptors in combination with the complement system not only regulated overt immune response but also the graded para inflammatory response. This response is initiated by tissue malfunction and is meant to restore tissue homeostasis. Parainflammation is an adaptive response that corresponds to the degree of tissue malfunction. In basal state the tissue resident macrophages response is muted and takes on a maintenance role [6]. Under noxious and stressful tissue environments the response borders on overt inflammation. While there are myriads of molecular patterns that bind and activate specialized pattern recognition receptors modulating para inflammatory response, the same SAMPs binding the same sPRRs, that modulate overt inflammation, modulate para inflammation as well.
Terminal Sialic Acid Determinant of Immune Self
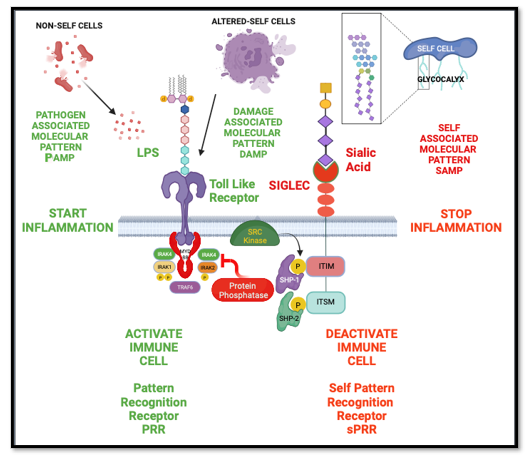
A prominent marker of normal self that was described by Medzhitov and Janeway was terminal sialic acid found on the glycocalyx of all normal heathy cells in vertebrate animals [4]. A family of sialic acid binding proteins called Sialic Acid Igg Like Lectin (Siglecs) at that time had been recently discovered. These Siglecs receptors were transmembrane proteins, that contain a cytoplasmic Inhibitory Tyrosine-Based Inhibitory Motif (ITIM). Siglecs recognize sialic acid markers of self which when agonized with a correct sialic acid pattern, would negatively modulate leukocyte function [13]. These Siglecs which are expressed on innate immune cells such as macrophages, dendritic cells, and neutrophils, would serve to prevent auto immune attack and be permissive to phagocytosis of microbial non-self-cells that do not possess the sialic acid biosynthetic machinery, as well as abnormal self-cells that have lost terminal end-glycan due to infection, injury, malignant transformation, or senescence [14] (Figure 1).
Pattern recognition receptors like Toll Like receptors recognize non-self-cells (Pathogen associated molecular patterns (PAMP)) or altered-self cells (Damage associated molecular patterns (DAMP) to activate an immune cell and initiate inflammation. Self-pattern recognition receptor (sPRR) called Siglecs Recognize Self-Cells (Self Associated Molecular Patterns (SAMP)) which are complex end termina sialic acid glycan patterns found on the glycocalyx of healthy host cells to resolve inflammation and prevent immune activation.
CFH and SIGLECs Represent the Main sPRRs
The identification of sialic acid as the critical component of Self-Associated Molecular Patterns (SAMPS) was first put forth by Varki in 2011 [15]. At this time he argued that there were 2 Self -Pattern Recognition Receptors (sPRRs) that detected Sialic acid SAMPs. The first described sPRR was factor H, a protein that required binding to sialic acid to restrict the alternate complement pathway when encountering a healthy host cell. The second class of SPPRs were the Siglecs receptors that Varki had discovered [16]. What made Siglecs likely to be the major sPRR is the number of Siglec Receptors that contained the immune receptor Tyrosine–Based Inhibitory Motif (ITIM) and immune receptor Tyrosine–Based Switch Motif (ITSM) domain. Furthermore, the differential display and the specificity of these Siglecs to a particular Sialic Acid presentation provides the immune system the ability to discriminate between normal self, missing self, and abnormal/transformed self. This complexity provides the immune system with a non-binary bar code that the differential display of SIGLEC receptors can interpret to modulate the immune system.
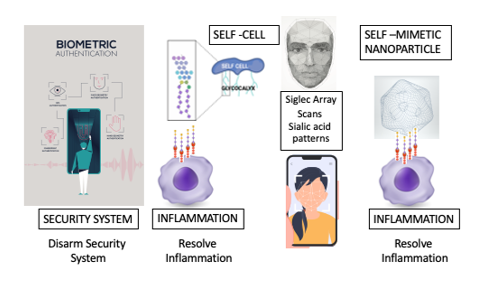
Consider this synonymous with a security system (immune system) with a facial recognition biometric authentication sensor (Siglec). Presenting the correct sialic acid molecular pattern to the Siglec terminal, is synonymous to recognizing the correct vector points on the facial scan (complexity and density of end terminal sialic acid glycan being scanned by the array of Siglec receptors) to disarm the security system. (Figure 2) The correctly presented SAMP, that binds and agonizes a particular Siglec will disarm a particular immune cell type. For example, to resolve microglial polarization, you need to present a SAMP (end sialic acid glycan pattern or glyco-code) that binds and agonizes Siglec-7,9,11 expressed by activated CNS resident macrophages called microglia. The sialic acid glycan pattern (SAMP) that activated microglial cells express to modulate the level of parainflammatory response is Polysialic Acid (PSA) presented on Neuropilin-2 and E-selectin ligand-1 [17]. Microglia secrete these PSA presenting proteins to provide negative feedback modulation immediately after activation in order to provide a graded para inflammatory response to activatory stimuli (Figure 2).
An array of Siglec receptors must recognize a three-dimensional pattern of end terminal sialic acid glycans to deactivate the immune cell and immune response. This is synonymous with facial recognition biometric scans. Creating glycomimetics to deactivate the immune system must also be presented in a multidimensional fashion to effectively resolve inflammation by agonizing these sPRRs.
Siglecs Powerful Immune Cell Deactivators
When SIGLECs were first postulated by Janeway as a potential discriminator of self vs non self or abnormal self, there were only a few Siglecs identified that possessed the ITIM/ITSM domain and served as negative regulators of immune response. Currently there are nine of 16 Siglec family members that constitute the largest family of immunosuppressive ITIM/ITSM domain containing receptors. These Siglecs that contain ITIM/ITSM are Siglec 2,3,5,6,7,8,9,10, and 11 [18]. The binding of an appropriately presented sialic acid pattern that binds a particular Siglec receptor activates ITIM/ITSM to recruit Src homology 1/2 domain–containing phosphatase (SHP1/2), encoded by the gene ptpn11 a powerful protein phosphatase [19]. This localized recruitment of this potent protein phosphatase mechanistically allows for dephosphorylation of all the activatory tyrosine phosphorylation.
Phosphorylation and Dephosphorylation of tyrosine residues are major regulators of protein activity especially as it relates to signal transduction, cytoskeletal remodeling, cell survival and cell proliferation [20]. Protein tyrosine kinases add a phosphate group to the aromatic rings of tyrosine residues while protein tyrosine phosphatases (PTP) remove these phosphate groups from the aromatic rings. In essence these PTPs are pan specific tyrosine kinase inhibitors and deactivators. Unlike therapeutic tyrosine kinase inhibitors which are known for their off-target toxicities, the localized recruitment of SHP2 to the agonized ITIM/ITSM domain provides precise specificity to only the Cell where the SPPR is located. Because tyrosine phosphorylation is critical to so many intracellular processes, non-specific inhibition or dephosphorylation would prove detrimental to many cellular processes and likely toxic. Evolution has created an emergency shut off switch to immune cell activation. This switch can also be used to modulate a graded response such as what is seen in para inflammation.
The ITIM/ITSM intracytoplasmic domain found in Siglecs, represent the most potent pathway for suppressing immune activation and inflammation and how the healthy host immune system prevents autoimmunity and resolves inflammation once pathogens or abnormal self-cells have been cleared. The ITIM/ITSM motif was first discovered in 1995 in a family of low-affinity immunoglobulin G (IgG)receptors named FcgRIIB [21]. These IgG receptors when co aggregated with BCR by IgG immune complexes had previously been shown to inhibit mouse B-cell activation [22]. Since that discovery, multiple ITIM/ITSM containing receptors have been discovered. These receptors, except for a few exceptions, are cell surface receptors belonging to the Ig superfamily or are C-type lectins [19].
Siglecs are Checkpoint Receptors but much More
While Siglecs constitute the largest family of receptors that possess this immune resolving motif, other ITIM/ITSM containing receptors, that have been nominated to be considered sPRRs bind specific proteins rather than binding molecular patterns. Signal Regulatory Protein (SIRP) and its well described ligand CD47 had been postulated to be a SAMP/sPRR. CD47 is considered a marker of self on erythrocytes and prevents premature clearance from circulation [23]. While SIRP was initially considered a possible sPRR, the predominant binding of proteins not patterns disqualified it from being the putative sPRR. Of all the ITIM/ITSM containing receptor ligand pairs only Siglec receptors binding specific glycan patterns with end terminal sialic acids remain the only ITIM/ITSM domain containing checkpoint receptor that meets the criteria for the putative sPRR.
The most well characterized ITIM/ITSM domain containing receptor ligand system is the programmed cell death protein 1 (PD-1) / programmed cell death protein ligand 1/2. PD-1 is an immunoglobulin (Ig) superfamily member that is the target for inhibition which has revolutionized the field of immunotherapy in cancer [24]. While PD-1 is most associated with checkpoint inhibition it was first thought to be involved in cell death hence its name. The PD-1 gene was found initially upregulated in a T-cell hybridoma line undergoing cell death [25]. It was later discovered to possess an ITIM domain which recruited SHP-2 to inhibit B cell receptor signaling [26]. It has 2 known ligands PD-L1 and PD-L2. PD-L1 is a 290 amino acid type 1 transmembrane protein expressed on hematopoietic cells mainly on T and B lymphocytes, and natural killer cells and non-hematopoietic cells such as vascular endothelium, multiple tumor cells and tumor infiltrating cells. epithelial, astrocytes, neurons and in cells of immune privileged organs. PD-L2 is a 270 amino acid transmembrane protein which has a threefold higher affinity for PD-1 than PD-L1. PD-L2 is predominantly expressed on macrophages, and dendritic cells [27]. Both PD-L1 and 2 are found on multiple tumors [28].
While PD-1 has been clinically validated as a checkpoint inhibitor involved in cancers, PD-L1 and PD-L2 do not meet the definition of self-associated molecular patterns. A SAMP is a molecular pattern not an individual protein, SAMPs need to have diversity, SAMPS need to be found on all healthy cells not just in select cells [15]. While PD-1 with its ITIM/ITSM domain meets the criteria for a powerful checkpoint inhibitor, its ligands do not support its role as a critical self-pattern resolution receptor that discriminates self from non-self, abnormal self, or missing self.
The nine ITIM/ITSM immune resolving Siglecs combined with sialic acid binding complement pathway deactivating factor H constitute the major self-pattern recognition receptors of the immune system making end terminal sialic acid glycan patterns the putative self-associated molecular pattern.
SAMP Mimetics Promising Immune Modulating Therapeutics
A promising strategy for modulating inflammation and parainflammation would be to mimic these sialic acid self-associated molecular patterns. An early indication that this strategy would prove effective is the use of polysialic acid to coat short peptides which would increase the circulatory half-life of the peptide directly correlated with the length of the PSA chain [29]. Polysialylation was also shown to reduce immunogenicity and antigenicity of protein enzymes like asparaginase [30]. PSA mimetics have been demonstrated in experimental models to promote therapeutic effect in spinal cord injury, [31,32] PSA also shows benefit in animal models of macular degeneration and Alzheimer’s [33,34].
To mimic a self-associated molecular pattern requires appropriate presentation. A nanoparticle decorated with diasialic acid was able to abrogate sepsis in a mouse model [35]. This nanoparticle sialic acid presentation was the designer SAMP mimic. Based on this technology, a nanoparticle decorated with polysialic acid has entered the clinic for the treatment of geographic atrophy second to age related macular degeneration [36].
This nanoparticle decorated with sialic acid represents a new therapeutic drug class that may finally allow immune modulation utilizing the body’s own holistic mechanism. Unlike cytokine depletion strategies which will produce immunosuppressive side effects, this strategy in contrast is a normalization strategy which will not only down modulate pathologic inflammation but will convert immune cells into resolution healing state. The ability to effectively agonize sPRRs will revolutionize how we will treat diseases of immune dysregulation and immune evasion.
Conclusion
The understanding of how the immune system discriminates between and responds to self, non-self, altered self and stress self has led to the identification of Sialic acid patterns (SAMP)and Siglecs (sPRRs) as the main negative regulator of immune response. This understanding coupled with innovations in automated glycan synthesis, high throughput glycan-lectin binding screens, biorthogonal conjugation, and nanoparticle technology glycomimetic therapeutics can be designed to selectively or pan-selectively agonize or antagonize Siglecs for the purpose of finely regulating immune response therapeutically. Thanks to pioneering work by visionaries like Drs. Janeway, Medzhitov and Varki who elucidated this understanding, we may soon have effective and safe therapies for diseases of para inflammatory overactivation such age-related macular degeneration, diabetic complications, and Alzheimer’s, as well as therapies for overt inflammatory diseases like arthritis, colitis, lupus, fibrosis, and allergies.
Acknowledgement
Figures were Created with BioRender.com
Conflict of Interest
AJT,MJT,AK and MAG are full or part time employees of Aviceda Therapeutics.
MJT,AK and MAG are inventors of technology mentioned in this article.
References
- Janeway CA (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54(1): 1-13.
- Janeway CA (1992) The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13(1): 11-16.
- Medzhitov R, Janeway CA (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91(3): 295-298.
- Medzhitov R, Janeway CA (2002) Decoding the patterns of self and nonself by the innate immune system. Science 296(5566): 298-300.
- Medzhitov, R, Janeway CA Jr (1999) Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol 64: 429-435.
- Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454(7203): 428-435.
- Miyake K, Yamashita Y, Ogata, M, Sudo, T, Kimoto M (1995) RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol 154(7): 3333-3340.
- Lemaitre, B, Nicolas, E, Michaut, L, Reichhart, JM, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86(6): 973-983.
- Janeway CA Jr, Medzhitov R (1999) Lipoproteins take their toll on the host. Curr Biol 9(23): R879-882.
- Medzhitov, R, Preston Hurlburt, P Janeway CA (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388(6640): 394-397.
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085-2088.
- Weiss HJ, O Neill L AJ (2022) Of Flies and Men-The Discovery of TLRs. Cells 11(19): 3127.
- Crocker PR, Varki A (2001) Siglecs sialic acids and innate immunity. Trends Immunol 22(6): 337-342.
- Crocker PR, Varki A (2001) Siglecs in the immune system. Immunology 103: 137-145.
- Varki A (2011) Since there are PAMPs and DAMPs, there must be SAMPs? Glycan "self-associated molecular patterns" dampen innate immunity, but pathogens can mimic them. Glycobiology 21(9): 1121-1124.
- Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, et al. (1998) Siglecs: a family of sialic-acid binding lectins. Glycobiology 8(2): v.
- Thiesler H, Beimdiek J, Hildebrandt H (2021) Polysialic acid and Siglec-E orchestrate negative feedback regulation of microglia activation. Cell Mol Life Sci 78: 1637-1653,
- Angata T, Varki A (2023) Discovery, classification, evolution and diversity of Siglecs. Mol Aspects Med 90: 101117.
- Daeron M, Jaeger S, Du Pasquier L, Vivier E (2008) Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev 224: 11-43,
- Marasco M, Berteotti A, Weyershaeuser, J, Thorausch N, SikorskaJ, et al. (2020) Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv 6(5): eaay4458.
- Daeron M, Latour S, Malbec O, Espinosa E, Pina P, et al (1995) The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 3(5): 635-646,
- Sinclair NR, Chan PL (1971) Relationship between antibody-mediated immunosuppression and tolerance induction. Nature 234: 104-105.
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, et al. (2000) Role of CD47 as a marker of self on red blood cells. Science 288(5473): 2051-2054.
- Ai L, Xu, A, Xu J (2020) Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv Exp Med Biol 1248: 33-59.
- Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11): 3887-3895,
- Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T (2001) PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A 98(24): 13866-13871.
- Ahmad SM, Martinenaite E, Holmstrom M, Jorgensen MA, Met O.; Nastasi, C. et al. (2018) The inhibitory checkpoint, PD-L2, is a target for effector T cells: Novel possibilities for immune therapy. Oncoimmunology 7(2): e1390641.
- Chen RY, Zhu Y, Shen Y-Y, Xu Q-Y, Tang H-Y, et al. (2023) The role of PD-1 signaling in health and immune-related diseases. Front Immunol 14: 1163633.
- Gregoriadis G, McCormack B, Wang Z, Lifely R (1993) Polysialic acids: potential in drug delivery. FEBS Lett 315(3): 271-276.
- Fernandes AI, Gregoriadis G (2001) The effect of polysialylation on the immunogenicity and antigenicity of asparaginase: implication in its pharmacokinetics. Int J Pharm 217(1-2): 215-224.
- Marino P, Norreel J-C, Schachner M, Rougon G, Amoureux M-C A (2009) Polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Exp Neurol 219(1): 163-174.
- Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, et al. (2010) Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther 18(1): 34-43.
- Varbanov H, Jia S, Kochlamazashvili G, Bhattacharya S, Buabeid MA, et al. (2023) Rescue of synaptic and cognitive functions in polysialic acid-deficient mice and dementia models by short polysialic acid fragments. Neurobiol Dis 180: 106079.
- Karlstetter M, Kopatz J, Aslanidis A, Shahraz A, Caramoy A, et al. (2017) Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol Med 9(2): 154-166.
- Spence S, Greene MK, Fay F, Hams E, Saunders SP, et al. (2015) Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med 7(303): 303ra140.
- Aviceda Therapeutics Inc. (2023) A Multiple Dose Study of AVD-104 for Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration (AMD) (SIGLEC).



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.