Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Sum - Up of Covid-19 Successful Vaccination Implementation in Nigeria: An Atomistic Overview of Events
*Corresponding author: Elemuwa Chris O, National Primary Healthcare Development Agency, 2, Uke Street, off Ahmadu Bello Way, Area 11, Garki, Abuja, Nigeria, Tel: + (234) 8033164487.
Received: September 28, 2023; Published: October 16, 2023
DOI: 10.34297/AJBSR.2023.20.002702
Abstract
Background: COVID-19 is a respiratory disease caused by a new strain of coronavirus. It spreads through airborne droplets and contact with contaminated surfaces. The pandemic has overwhelmed healthcare systems worldwide. Nigeria experienced a growth in cases after relaxing lockdown measures. The UK was the first Western country to approve the Pfizer-BioNtech vaccine. Nigeria implemented public health measures and trained health workers to control the spread of the virus. Vaccination started in March 2021, with Johnson and Johnson being the available vaccine. As of June 2023, 65.8% of the eligible population in Nigeria have been fully vaccinated. Since the outbreak of COVID-19 in Nigeria, the country has made significant efforts to implement successful vaccination programs to curb the spread of the virus.
Objective: This paper aims to provide an atomistic overview of the successful vaccination implementation of COVID-19 in Nigeria, highlighting the key events and strategies used.
Methods: The data for this study was gathered from various sources, including government reports, academic papers, and news articles. The information was analyzed to identify the key events and strategies employed during the vaccination implementation.
Results: The successful vaccination implementation in Nigeria was a result of several key events and strategies. Firstly, the government launched an extensive vaccination campaign that targeted different population groups, prioritizing frontline healthcare workers, the elderly, and individuals with underlying health conditions. This approach ensured that the most vulnerable populations were protected. Additionally, the government established vaccination centers across the country, making the vaccine easily accessible to the public. To encourage vaccine uptake, the government launched an extensive public awareness campaign, using various channels such as television, radio, and social media. This helped dispel vaccine hesitancy and misinformation, resulting in increased vaccine acceptance. Moreover, a robust monitoring and evaluation system to track vaccine distribution, adverse events, and vaccine effectiveness were implemented. Thees allowed for timely adjustments and improvements in the vaccination program.
Conclusion: The successful vaccination implementation in Nigeria can be attributed to a combination of targeted prioritization, accessibility, public awareness campaigns, and a robust monitoring and evaluation system. These strategies can serve as important lessons for other countries facing similar challenges in vaccine implementation. However, ongoing efforts are needed to ensure equitable distribution and address vaccine hesitancy to achieve widespread protection against COVID-19.
Abbreviations: ACSM: Advocacy Communication and Social Mobilisation; AFENET: African Field Epidemiology Network; CDC: Centre for Disease Control; COVID-19: Corona Virus 2019; CorPREP; Covid-19 Preparedness and Response Project; CHIPs: Community Health Influencers and Promoters Service; EMID: Electronic Management of Immunization Data; FP: Focal Person; HFs: Health Facilities; HWCs: Health Care Workers; HIV: Human Immunodeficiency Virus; JTF: Joint Task Force; LGA: Local Government Area; M&E: Monitoring and Evaluation; NPHCDA: National Primary Health Care Development Agency; PSA; Public Service Announcement; PHC: Primary Health Care; PHEIC: Public Health Emergency of International Concern; PSI: PHC Services Integrated with Covid-19 Vaccination; RI: Routine Immunization; RRT: Rapid Response Team; SCALES: Service Delivery Communication Accountability Logistics Emid Supervisory; SIAs: Supplemental Immunization Services; T: Traditional Microplanning and Vaccination Campaign Approach; E: Electronic Self- Registration; A: Assisted Electronic Registration of Eligible Nigerians; C: Concomitant Vaccination alongside Electronic Registration; H: House-to-House Electronic Registration; TOR: Terms of Reference; TB: Tuberculosis; UNICEF: United Nations Children’s Emergency Fund; USAID: United State Agency for International Development; USG PEPFAR: United States Government President’s Emergency Plan for Aids Relief; WHO: World Health Organisation; WB: World Bank
Background
The Corona Virus disease 2019 (COVID-19) is a communicable respiratory disease caused by a new strain of corona virus that causes illness in humans also known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2). The disease spreads from person to person through infected air - droplets that are projected during sneezing or coughing. It can also be transmitted when humans have contact with hands or surfaces that contains the virus and touch their eyes, nose or mouth with the contaminated hands. The pandemic shocked the world, overwhelming the health systems of even high-income countries [1]. The World Health Organization (WHO) declared the novel human coronavirus disease outbreak which began in Wuhan, China on December 8, 2019, a public Health Emergency of International concern (PHEIC) 0n January 30, 2020. With the first case of COVID-19 was reported in Nigeria on 27th February, 2020. The incidence grew steadily in Nigeria, moving from an imported case and elitist pattern to community transmission. The country recorded an upsurge (52%) of total cases) in the transmission during the short period the lockdown was relaxed [2].
On December 2nd 2020, the United Kingdom Medicines and Health Care Product Regulatory Agency (MHRA) gave temporary regulatory approval for the use of Pfizer-BioNtech vaccine. Becoming the first country in the western world to approve the vaccine and first country in the western world to approve the use of Covid-19 vaccine [3].
Nigeria instituted many public health measures including social distancing, lockdown, intensive testing etc in effort to control the spread of the disease. Nigeria through the National Primary Healthcare Development Agency (NPHCDA) speedily trained over 260,000 health workers across the national and subnational levels on how to handle the pandemic and ensure provision of essential health services at the PHCs [4]. The National Primary Healthcare Development Agency (NPHCDA) is a Semi -autonomous parastatal of the Federal Ministry of Health (FMoH) with the mandate to provide technical and programmatic support to states and LGAs on the development and implementation of Primary Healthcare (PHC) in Nigeria. The core mandates are: - Control Preventable Diseases, improve access to basic Health Services, Improve quality of care, Strengthen the institutions, Develop high performing Health work force, Strengthen Partnerships, Strengthen Community structures and engagements [4].
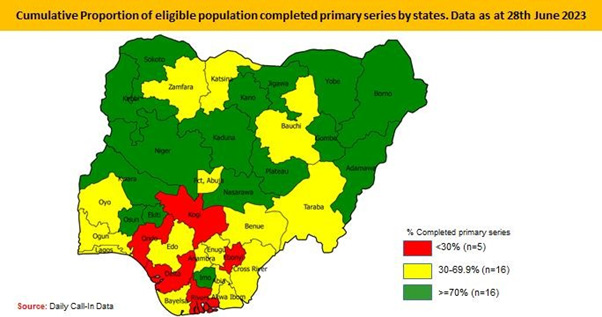
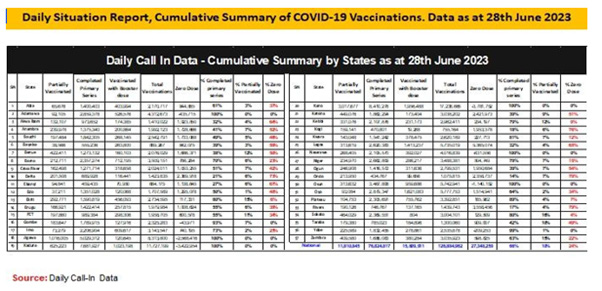
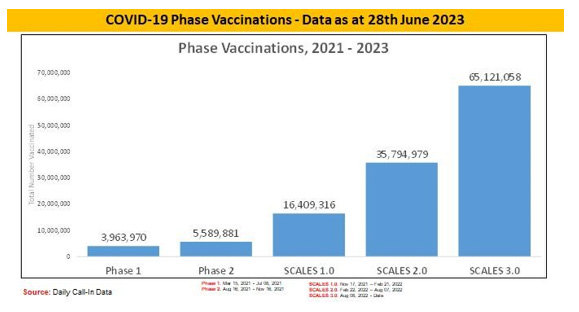
Nigeria Commenced COVID-19 vaccination implementation on 5th March 2021. Target population is 18 years and above. The Federal Government of Nigeria through the NPHCDA has deployed several strategies including to ramp-up integrated COVID-19 vaccination and PHC services across the country5. Nigeria approved AstraZeneca, Pfizer-Bio-N-Tech, Moderna and Johnson and Johnson COVID-19 vaccines to vaccinate her population. However, only Johnson and Johnson COVID-19 vaccine is currently available in-country. As of 14th June 2023, 65.8 percent of the total eligible population have been fully vaccinated translating to 76,315,303 persons; 74.9 percent of the total eligible population (86,922,395) have had at least one dose of the vaccine, 15 states (Nasarawa, Jigawa, Osun, Kaduna, Kano, Adamawa, Gombe. Yobe, Pateau, Kwara, Ekiti, Sokoto, Kebbi, Niger and Imo) have vaccination coverage of over 70%; 8 states (Zamfara, Borno, Oyo, Enugu, FCT, Rivers, Cross River and Edo) have fully vaccinated >50>70%; 9 states (Bauchi, Anambra, Taraba, Katsina, Ogun, Bayelsa, Benue, Akwa-Ibom and Lagos) have fully vaccinated 30%; and the remaining 5 states (Ebonyi, Delta, Kogi, Rivers and Ondo) have less than 30 percent coverage. Nigeria achieved 2-3m vaccinations/month in PSI COVID, 5-6m/month in SCALES, ~7m/month in SCALES 2.0 and with SCALE 3.0 strategy launched in August 2022, Nigeria achieved a 500,000/day vaccination rate at the peak of implementation [5] (Figures 1-3).
On the 4th May, 2023, the WHO DG declared that the COVID-19 is now an established and ongoing health issue which no longer constitutes a public health emergency of international concern (PHEIC). This document summaries what the journey has been as we continue to strive for the country to attain the herd immunity.
Strategies of COVID-19 Vaccination Phases and Rollout to Target populations
Nigeria has continued to adopt innovative strategies in line with the prevailing situation to ramp-up coverage in the states across workers, front line workers and ports of entry (air, land, and sea). The vaccine roll out were carried out in 4 phases:
I. PHASE 1: Healthcare ports), Military, COVID 19 rapid response team (RRT), laboratory network, policemen, petrol station workers. These groups were considered to be the high risk individuals based on their exposure and susceptibility to the virus. For instance, Health workers treat patients with Covid-19, therefore immunizing health providers first is the best way to curb the spread of the virus [6].
II. PHASE 2: Older adults aged 50 years and above and those with co-morbidities aged 18 - 49 years of age. These groups are more likely to get very sick or die from Covid-19, the more health conditions older adults have, the higher the risk of becoming sick if infected with Covid-19.
III. PHASE 3: Those in states/LGAs with high disease burden and who missed phases 1 and 2.
IV. PHASE 4: Other eligible population (Figure 4).
Strategies
T.E.A.C.H Strategy: An Indigenous Approach to COVID-19 Vaccination in Nigeria. The rollout of the COVID vaccine employed the TEACH strategy. The TEACH strategy is underpinned by the benefits of traditional micro planning methods and the advantages of the use of appropriate technology to deploy COVID vaccines across the country. The TEACH strategy is characterized by:
T: Traditional Micro planning and Vaccination campaign approach involving the conduct of a comprehensive desk review of micro-plan to identify the resources to vaccinate the eligible population. The use of Electronic self -registration, Assisted registration, Concomitant Community and House to House vaccination.
E: Electronic Self- Registration by Eligible Nigerians; healthcare workers using the URL shared through appropriate platforms.
A: Assisted Electronic Registration of Eligible Nigerians; Healthcare workers and eligible people who are unable to complete self-registration due to lack of android device, network, or other related issues.
C: Concomitant Vaccination alongside Electronic Registration: e-registration and vaccination of the eligible population, especially when the HCW self-registration has stopped to capture missed beneficiaries.
H: House-to-House Electronic Registration: teams explicitly deployed to areas where there is inadequate enrollment or registration of eligible population with low coverage as an added push to register beneficiaries [7].
PSI Covid Strategy: PHC Services Integration with COVID-19 Vaccination: This is a family centered integrated PHC approach; it was launched in November 2021, to improve access to basic health services, integrating the COVID-19 vaccination program with the provision of PHC services including routine immunization. This largely did not achieve the desired outcomes.
Optimized Scales Strategy: Is a systematic integration of covi-19 vaccination with other health services to rapidly accelerate Covid-19 vaccine coverage. Scales strategy provides an opportunity to leverage on the covid-19 vaccination structures and resources to improve routine immunization coverage with improved efficiency and reduced duplication of efforts by health care workers. SCALES is an acronym that stands for Service Delivery, Communication, Accountability, Logistics, Electronic Management of Immunization data and supportive supervision.
S: Service delivery system that delivers vaccines to all eligible persons without compromising efficiency.
C: Communication strategy involving targeted advocacy, intensive media engagement, community engagement and participation.
A: Accountability system to track vaccination activities, detect and promptly address inappropriate and fraudulent activities.
L: Effective Logistics system to adequately forecast, efficiently distribute and track vaccines and ancillary supplies.
E: EMID (Electronic Management of Immunization data) a platform that provides a harmonised data system for reporting programmatic and logistic data to inform decision making.
S: Supervisory system that is robust, leveraging multi-agency collaboration at all levels.
In implementing the Scales strategy, five service delivery approaches were adopted to ramp up COVID- 19 vaccines uptake in Nigeria.
a. Expansion to all public health facilities (primary, secondary and tertiary facilities:
b. Establishment of Mass vaccination sites.
c. Temporary posts - drive through, community pop-ups, outreach RI sessions.
d. Expansion to corporate institutions (public and private, including selected pharmacy stores).
e. Expansion to private health facilities.
Scales 2.0: This strategy was launched in February 2022. It is an Integrated COVID 19 vaccination approach for PHC strengthening. This was intended to ramp up covid-19 vaccination, with the use of Johnson and Johnson singles dose vaccine and the introduction of the vaccination site finder. With this strategy, all childhood vaccination including administration of vitamin A, was done alongside covid-19 vaccination sites, where adult receive covid-19 vaccines [9].
Scales 3.0: This strategy was launched in August 2022. It was designed, using mobile special teams to take vaccines to the door steps were people live and to work places. It is an evidenced based update that fixes the bugs in Scales 2.0 and uses human centered demand generation design to address low Covid-19 risk perception in the country. It was a Refocused 3- month Campaign mode, Performance based incentives, data use for action and decentralized demand generation [9].
The SCALES 3.0 strategy was anchored on six guiding principles:
1) Intensive campaign
2) Performance-based incentives
3) Optimized integrated package of services
4) Decentralized & incentivized demand generation strategies
5) Partners coordination and accountability
6) State specific context and strategies and 3 enabling factors;
a) Prompt remunerations of vaccination teams and program managers
b) Use of EMID for action and team performance
c) Decentralized demand generation
SCALES 3.0 focused on an intensive campaign across all states and FCT leveraging fixed and mobile sites concurrently Intensive campaign. The Mobile teams deployed during the intensive campaign conducted vaccination of eligible persons not reached by HFs. The supervision of the vaccination teams strengthened the campaign to ensure that teams deployed worked to achieve the set targets [10];
I. Integrated PHC services with COVID-19 vaccinations are conducted in all public health facilities (and some private health facilities (HFs) [11].
II. Leveraging the fixed RI sessions and outreach sessions at high-traffic facilities, caregivers will be targeted for COVID-19 vaccination thus optimizing any R.I visits to health facilities [12].
III. Mobile teams were deployed to places with a high population with a potentially good flow of clients and
IV. Rural and hard-to-reach areas who are generally cut-off from the provision of COVID-19 vaccinations and other immunization services.
SCALES 3.0 addressed the identified implementation gaps in SCALES 2.0 and promptly promotes accountability for performance. S C A L E S Service delivery focused more on routinization of COVID 19 vaccination using more fixed posts, teams Communication, targeted advocacy, intensive media engagement, community engagement/ participation, Accountability system to track vaccination activities, detect and promptly address inappropriate and fraudulent activities using JTF Logistics system to adequately forecast, efficiently distribute and track vaccines and ancillary supplies. The main enablers for SCALES 3.0 were comprehensive resource and partner mapping, targeted and robust demand generation, strengthened electronic data reporting, continuous performance management plan for efficiency, and states/context specific approaches.
Optimization of vaccination strategy;
a. Establishment of mass vaccination sites
b. Involvement of the private sector; and
c. Expansion of vaccination sites to all public health facilities (primary, secondary, tertiary).
Optimization policy shift: Roll-out of vaccine mandate, Announcement of vaccine mandate in some states (Ondo, Edo, Kaduna) resulted to a surge in vaccine uptake, Implementation of policy shift and/or a strategy optimization to ramp up uptake of the vaccines.
EMID platform that provides a harmonised data system for reporting programmatic and logistic data to inform decision making Supervisory system that is robust, leveraging multiagency collaboration at all levels Focusing more on intensification and integration using campaign mode and more mobile teams Incentivized performance and optimized integration Decentralized demand generation targeting health workers, gatekeepers, grassroots demand generation & community referrals, Mutual accountability (timely logistics and team payment - Only teams who have achieved thresholds and targets are paid Performance based payments based on EMID reporting Optimized Integrated Vaccine Supply Chain.
Last Mile Stock Visibility to avoid stock out or close vial expiry
a. Team specific visibility on EMID
b. EMID visibility and use for action at subnational levels
c. Data access and use for action at subnational levels Results Based payment for supervisors and monitors Revampe supportive supervision and performance management (Figures 5 & 6).
The campaign utilized the full complements of teams in the service delivery points Intensive campaign;
A. 2 vaccinators, 2 recorders 1 validator 1 mobilizer
B. Preparation and administration of vaccines
C. Adherence to infection control practices between individuals
D. Educate vaccinee on possible side effects of the vaccines and possible treatment
E. Disposal of clinical waste and adherence to infection control practices
F. Monitor vaccinees after vaccine administration
G. Ensure availability of data tools - recording sheets, tablet/ phones, vaccination cards etc.
H. Take proper record of vaccinees exercise (on both paper and electronic tools)
I. Fill and give vaccination cards to vaccines and
J. Educate client on other services available and/or return period for subsequent doses (if applicable)
K. Validates vaccination on EMID platform
L. Ensures uniformity between existing data tools; - Paper tools (vaccine register, tally sheet) - EMID Platform - Vaccine utilization register
M. Create awareness of integrated COVID-19 vaccination around the vaccination sites ▪ Disseminate proper information about COVID19 vaccination roll out process
N. Build wider community partnership
O. Help community understand the process and respond to their questions to create a positive environment for the vaccination process. The vaccinators are expected to be competent with the delivery of RI vaccines and use of RI tools to ensure integrated service delivery at the vaccination sites.
Integration approach by thematic areas: The government and partners, recognized that it no longer make sense for Covid-19 vaccination services, including other immunization services to exist in isolation. Hence, the potential integration with other services as the way forward for covid-19 vaccination [13].
Service delivery integration occurs where managerial or operational changes to health systems bring together inputs, delivery, management and organization of the particular service functions in ways that are contextually appropriate and person-centered with the aim of improving coverage and address physical access barriers, inconvenience, quality, acceptability, effectiveness and cost effectiveness” [14].
Integration approach was anchored on the following thematic areas [14];
Leadership and coordination: Harmonize coordination structures for SIAs, VAS, RI & COVID-19 Task Force in all states, adopting One country, one team, one plan, and one budget approach. Data Management M&E: Joint data reporting and feedback to all levels. To provide a harmonised system for data reporting. Programmatic and logistic data for decision making at all levels. Supportive Supervision: Review the TOR of supervisors for the integrated campaign.
Service Delivery: Integration of SIA, COVID - 19, RI, VAS in all fixed posts and temporary fixed post and integrate RI antigens in all temporary, fixed post across the Zero-dose LGAs in states identified. Advocacy Communication and Social Mobilisation Activities: Integrate all ACSM activities at national and state levels. Mobilization by CHIPS & other community structures for all interventions.
Logistics and supply: Harmonize logistics movement of SIA, RI, Covid -19 vaccines & VAS to the last mil
Training: Integrated Implementation training at all levels.
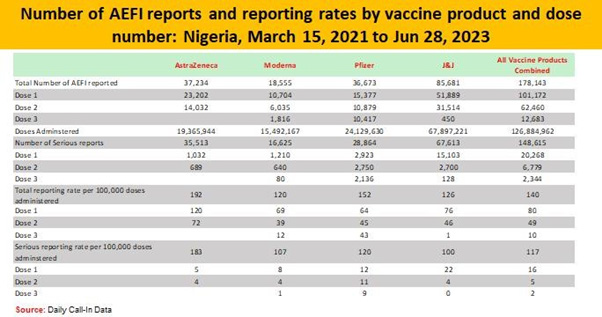
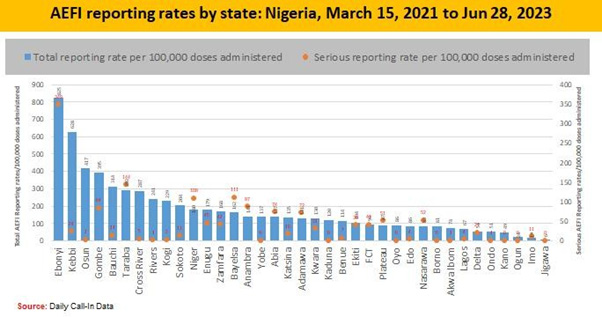
Adverse Events Following Immunization (AEFIs)
This can be defined as any untoward medical occurrence following immunization which does not necessarily have a causal relationship to the vaccine [15]. The adverse may be due to any unfavourable or unintended sign, abnormal laboratory finding, symptoms or disease [16].
Classification of AEFIS
There are two broad classification of AEFI:
The regulatory classification (Non-serious and serious) and
The cause specific classification includes five categories
i. Vaccine product related reaction
ii. Vaccine quality defect related reaction
iii. Immunization error related
iv. Anxiety related reaction and
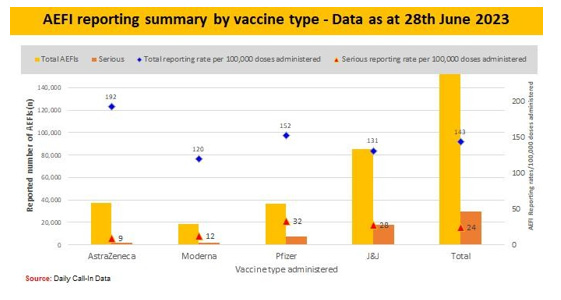
v. Coincidental [26] (Figures 7-9)
Throughout the process of Covid-19 vaccination, emphasis were made on the vaccination teams to consistently report, monitor and manage AEFIs. Some common AEFIs were; Pain, Erythema, Swelling at the site of injection, low grade fever, fatigue, chills, muscle pains and headache that occurs within 3 days of injection and it resolves within 3 days of appearance of symptoms.
Reporting and Monitoring of AEFI was important in other to track the quality of service delivery during the immunization sessions to detect, investigate and treat all AEFI cases and prevent loss of life. Reporting is done using the appropriate tools for the purpose of investigation and line listing. When AEFI is detected, management is essential to maintain the credibility of the immunization services. During the Covid-19 vaccination period all vaccination posts were equipped with AEFI kits and health workers can recognize, manage and refer AEFI cases to the designated health facility/ laboratory appropriately.
Mapping of Partners Support across the 36 states and FCT
Partners support were in two forms; Complete state-wide support to Mobile & fixed post teams, data bundle, supervision, trainings, demand generation and supply chain. Partial support; to Mobile teams, data bundle, supportive supervision, trainings, and demand generation.
i. UNICEF, WHO and Africa CDC support will cover the 36 states for SCALES3.0 surge and complement in USG states
ii. US CDC support will cover mobile teams (service delivery), data bundle,
iii. USAID will support vaccine supply chain to LGAs
a. USAID will also provide partial support (demand generation, service delivery and partial support in states
b. USG PEPFAR will provide indirect additional support for sub populations (TB/HIV, Malaria, FP etc)
c. WB Coprep funds will cover funding gaps across all 36 states and FCT in implementing the integrated work plans.
iv. Challenges
The COVID-19 vaccination program is the most extensive vaccination program held in Nigeria to date. As of June 28, 2023, more than 31 million people have been fully vaccinated which made up 15% of the entire population. Following the global COVID-19 vaccination goal, Nigeria was expected to vaccinate 40% of its population in 2021 and reach the 70% vaccination threshold before the end of 2022. Currently, Nigeria is nowhere near the global vaccination goal due to various challenges encountered by the program. Challenges such as distrust in government, poor cold-chain management, and poor communication during the onset of the program all contributed to the inability to attain the set goal. It is important that Nigeria, in preparation for upcoming vaccination programs, learn from some of the challenges that the COVID-19 vaccination program encountered and take actions to ensure greater success in future vaccination programs in the country [17]. This paper aims to give an overview of the COVID-19 vaccination program in Nigeria, highlight the challenges encountered, and provide recommendations for better future vaccination initiatives in the country [17].
Funding
Delayed payment of teams due to delay in the release of funds by donors.
Delay in the payment of the vaccination team mobilisers.
Vaccine hesitancy with associated slow uptake of covid-19 vaccine (as noted during the just concluded integrated RI intensification) [18-22].
Data Management
i. Initial challenges with EMID
ii. Inadequate capacity of the team (EMID) recorders at early stages of implementations
iii. Supportive supervision at subnational levels is yet to be optimal
iv. Inadequate capacity of ACSM personnel in developing a robust integrated ACSM work plan.
v. Poor ownership/ political- will and accountability at State and LGA levels30.
Recommendations
a. Robust and functional integrated platform for visualizing real time COVID-19 and R.I.
b. Early release of funds from donors before program implementation
c. Conduct of regular meetings engagements amongst relevant stakeholders for immediate course correction.
d. State specific integrated ACSM work plan to be adhered to for a tailored demand generation.
e. Continuous engagement of the state leadership to ensure commitment and ownership
f. Identification of the low performing states for targeted supports including supportive supervision
g. Continuous capacity building for the EMID recorders [23-34]
Lessons Learned
a. Lessons Learned Regular review meetings across various levels encouraged and/fostered ownership of integrated vaccinations. It enhanced coverage of basic PHC services especially immunizations Over ambitious integration of different antigens and programs of different age groups.
b. The integrated approach led to greater efficiency; leading to optimized use of available resources. Supportive supervision at LGA level is yet to be optimal.
c. Delay in the release of operational funds from donors resulted in sub-optimal performance in affected states
d. Inadequate capacity of Advocacy,Communication,Social Mobilization (ACSM) personnel in developing a robust integrated ACSM work plan.
e. Improved monitoring and management of vaccines
f. Ancillary supplies and waste management processes.
g. Strong leadership and coordination are very key.
However, there will be need to review and airing of Public Service Announcements (PSAs) and jingles on COVID-19 as well as reviewing the COVID-19 vaccination guidelines to posit appropriate preparedness plans and strategies.
Ramping - up campaigns in selected states is highly recommended.
References
- (2020) Novel Corona Virus Global Epidemic. Africa CDC.
- (2020) Cross River State Leverages Polio Campaign Structure to integrate Covid-19 Vaccination. Global Polio Eradication initiative.
- (2023) An update of COVID-19 outbreak in Nigeria. NCDC.
- National Library of Medicine (2023) Journal of Family Medicine and Primary Care.
- (2022) Considerations for integrating covid-19 Vaccinations in Nigeria.
- (2022) Covid-19: Nigeria launches new vaccination strategies-voice.
- (2020) Novel Coronavirus (2019-nCoV) Global Epidemic.
- (2020) Corona Outbreak in Nigeria, Burden and Socio-economic Medical Response during the first 100 Days. National Institute of Health.
- (2021) Nigeria Launches Strategy for Effective Covid-19 Vaccination. Premium Times.
- (2022) A rapid review of vaccine acceptance rate and the associated factors. Plos One.
- (2020) COVID- 19 Outbreak in Nigeria: Situation Reports. NCDC.
- Oyadiran (2021) Journal of Global Health Reports: Towards effective and Efficient Covid-19 vaccination in Nigeria.
- (2022) Nigeria Government to integrate covid-19 vaccination, childhood immunization campaigns. Premium Times.
- (2022) Nigeria Government to integrate covid-19 vaccination, childhood immunization campaigns. Premium Times.
- (2022) Nigeria Government to integrate covid-19 vaccination, childhood immunization Premium Times.
- (2023) Regional Office for Africa. World Health Organization, Geneva, Switzerland. WHO.
- (2022) FG unveils Scales 0 Strategy for COVID-19 vaccination. Nigeria. Worldstagenews
- (2021) Training Manual For Covid-19 Vaccine Introduction, Nigeria. National Primary Health Care Development Agency. NPHCDA.
- (2020) Corona Virus Update. World Health Organization, Geneva, Switzerland. WHO.
- (2020) Corona Virus Disease (COVID-19). Africa CDC.
- Afenet Nigeria (2022) Launch of Scales 3.0 Strategy: Acceleraton Covid-19 Vaccination and PHC Services in Nigeria.
- GAVI The Vaccine Alliance (2021) Combining Covid-19 Vaccination: Nigeria Implements’ a whole family approaches’.
- (2023) Medical world Nigeria.
- Butler N (2022) Social Science in Humanitarian Action: Vaccination services: Insights from Iraq and Syria for the MENA Region.
- (2020) Nigeria’s Public Health response to the COVID-19 pandemic. National Centre for Biotechnology Information, Nigeria. NCBI.
- (2022) Qualitative analyses of the CPVID-19 vaccination roll out in Lagos, Nigeria: Client and Provider Perspectives on the plan, the process and the progress. National Institute of Health.
- (2020) First COVID-19 Vaccine Doses to go Health Workers. NPR.
- (2022) FG Launches Scales 0 Strategy, Single dose J&J Vaccination Site Finder. Science Nigeria.
- (2023) Considerations for integrating COVID-19 vaccinations into immunization TechNet-21.
- (2021) Nigeria Scales up its Covid-19. The World bank.
- (2020) Covid -19 Pandemic in Nigeria.
- (2023) COVID-19 vaccination in Nigeria Challenges and recommendations for future vaccination initiatives.
- (2021) Regional Office Africa Nigeria’s Health workers take the Country’s first jabs of Covid-19 World Health Organization, Geneva, Switzerland. WHO.
- (2018) Weekly epidemiological Record. World Health Organization, Geneva, Switzerland. WHO.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.