Short Communication 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Gene of Resveratrol Synthase transformation to Cordyceps militaris
*Corresponding author: DU Juan, Zhuhai College of Science and Technology, Zhuhai, China.
Received: October 28, 2023; Published: November 13, 2023
DOI: 10.34297/AJBSR.2023.20.002727
Abstract
Resveratrol synthase (RS) is a key enzyme in Resveratrol (Res) synthesis pathway. RS genes have been transformed and expressed in many plants and microorganisms and play biological roles in plant metabolism and regulation. In this study, the full-length sequence of resveratrol synthase RS gene was transformed into Cordyceps militaris by Agrobacterium tumefaciens infestation with 1179bp. The RS gene was integrated into Cordyceps militaris genome by PCR, Southern blot hybridization, Northern hybridization and other molecular biological tests. Objective To improve the content of RS gene in into Cordyceps militaris by transgenic technology, improve the resistance of Cordyceps militaris and promote the content of resveratrol downstream products, provide a theoretical basis for studying the biological function and regulation mechanism of RS gene metabolites, and provide an effective way to improve species, resistance and increase the yield of metabolites.
Keywords: Resveratrol synthase gene, Agrobacterium tumefaciens, Transgenic, Cordyceps militaris
Introduction
Resveratrol (Res) is a natural polyphenol plant antitoxin with significant anti-cancer activity, mainly found in Polygonum cuspidatum, grape and Cordyceps militaris. Resveratrol has antioxidant, anti-proliferation, apoptosis promoting, anti-aging and immune regulatory effects, and is widely used in the treatment of cancer and immune system diseases [1-6]. Resveratrol was first obtained from the root extract of Veratrum lancifolium and has been obtained from more than 70 plants. The results showed that the contents of Polygonum cuspidatum, grape was higher. Resveratrol synthase (RS) is a key enzyme that catalyzes the reaction of malonyl coenzyme A and coumarinyl coenzyme A, and the final product of the catalysis is resveratrol [7]. The catalytic reaction of RS enzyme is a series of condensation reactions, and also an important regulatory enzyme affecting resveratrol biosynthesis [8,9]. RS gene is transformed into corresponding plants or microorganisms through gene transformation technology, which has improved the stress resistance of plants or microorganisms and the yield of resveratrol [10]. In 1990, Hain, et al., first transformed resveratrol synthase RS gene of peanut into tobacco and detected the expression of resveratrol in transgenic tobacco suspension cells [11]. Zheng Shigang and others reported that the disease resistance and yield of RS transgenic crops were relatively improved [12]. Lo, et al., measured the contents of three Res related metabolites in the crude extract of Arabidopsis thaliana with RS gene of sorghum, namely Res trans hexyl glucoside, diglucoside and cis hexyl glucoside, by liquid chromatography tandem mass spectrometry (LC/MS/MS). The results showed that the accumulation of cis hexyl glucoside and cis hexyl glucoside in Res was large, and trans hexyl glucoside could not be detected [13]. RS transgenic fruit and vegetable crops, such as apple (Pyrus malus L.) and strawberry (Fragaria vesca L.), have significantly improved their stress resistance, such as disease resistance and insect resistance. Some researchers have transferred RS gene into Chinese herbal medicine, such as Salvia miltiorrhiza. The RS gene transformed Salvia miltiorrhiza not only has increased stress resistance, but also has enhanced pharmacological activity. It can be seen that RS gene has a good application prospect in the transformation of plant and microbial functions.
Experimental Materials and Methods
Experimental Materials:
1) Medium for Cordyceps militaris Culture and RS Gene Transformation
CYM medium: glucose 20g/L, tryptone2g/L, yeast extract 2g/L, potassium dihydrogen phosphate 0.46g/L, magnesium sulfate heptahydrate 0.5g/L, potassium hydrogen sulfate1.0g/L. Screening medium: CYM+0.1 mg/L IBA+2 mg/L PPT+350 mg/L Cb; Fruit body culture medium: 20g rice, 15g sorghum rice, 30mL sterile water.
2) Strain and Expression Vector:
Agrobacterium tumefaciens, LBA4404(Rif R, SmR), expression vector pCAMBIA3300, RS gene recombinant vector pCAMBIA3300- RJ39-RS-Tnos were constructed and preserved by the State Key Laboratory of Plant Genetic Engineering and Protein Engineering, Academy of Life Sciences, Peking University.
3) Primers, Enzymes and Reagents:
Primers for RS gene amplification:RSF: 5 ‘-CGGGATCCGCCATGGCTTCAGTTGAGAAATTTAG- 3’: RSR: 5’- GTGAGCTCGAAGGGTAAACCATTCTCTTTTAT- 3’, synthesized by Shanghai Sangong Biotechnology Co., Ltd; T4 DNA ligase, Taq DNA polymerase, DNA restriction endonuclease, pMD18T carrier, dNTPs, gel recovery kit, etc. were purchased from Takara Company (Japan); Kanamycin, streptomycin and rifampicin were equally purchased from Beijing Dingguo Biotechnology Co., Ltd; Sequencing was completed by Shanghai Bioengineering Co., Ltd.
4) Cordyceps militaris:
Isolation and identification of Cordyceps militaris species from Changbai Mountain wild fungi.
Test method
1) Isolation and Identification of Cordyceps militaris Fungi:
a) Separation and purification of Cordyceps militaris
Wipe and disinfect the wild cordyceps militaris collected from Changbai Mountain with 75% alcohol, pick up the spores on the fruiting bodies, inoculate them on the CYM medium, pick out the robust mycelia, inoculate them on the CYM solid medium, and conduct subculture for many times until the purified mycelia of cordyceps fungi are obtained.
b) Genomic DNA extraction of Cordyceps militaris fungi
Take Cordyceps mycelium, inoculate it on CYM liquid medium, place it in a constant temperature shaker at 28℃, shake it at 150r/ min for5 to7 days, and obtain a single mycelium of Cordyceps fungi. The fungal genomic DNA extraction kit was used to extract the DNA of Cordyceps mycelia.
c) Molecular biological identification of mycelium DNA
PCR reaction system (50μL) : Mycelial DNA 2μL;ITS 3′Primer 2μL;ITS 5′ primer 2μL;TagE 25μL;ddH2O19μL. PCR reaction conditions: 94℃ pre denaturation for 4 min; Denaturation at 94℃ for 30s, annealing at 52℃ for 30 s, extension at 72℃ for 1.5min, 30 cycles; Extend at 72℃ for 10min and keep at 4℃ for 1 min. PCR product electrophoresis, gel recovery of electrophoresis products,- connection of gel recovery products, transformation of Escherichia coli, and PCR detection of bacterial fluid; Send the PCR amplified positive clone of the bacterial solution to the Sequencing Department of Bioengineering (Beijing) Co., Ltd. for sequencing.
2) Agrobacterium Mediated Transformation:
The plasmid pCAMBIA3300-RJ39-RS-Tnos was transformed into the competent state of Agrobacterium tumefaciens EHA104 by liquid nitrogen freeze-thaw method (liquid nitrogen freeze-thaw for 3min and maintained at 37℃ for 5min). The genetic transformation of Cordyceps militaris was carried out by agrobacterium co culture method. The positive single colony was selected and inoculated in CYM liquid medium, and the culture was oscillatory at 28℃ for 3 days to the logarithmic growth period.
3) Identification and Detection of RS Transgenic CORDYCEPS Sinensis:
a) PCR detection of RS transgenic Cordyceps militaris
The mycelia of RS transgenic Cordyceps militaris and non RS transgenic Cordyceps militaris were selected, and the genomic DNA of transformants and non transformants was extracted by CTAB method. The PCR reaction system (20μL) Is: template DNA1μL;Primer RSF1μL;Primer RSR1μL;ten×buffer 2μL; dNTPs (2.5 mmol/L) 2μL;Taq DNA polymerase (10000 U/mL) 0.2μL.The reaction conditions were: pre denaturation at 94℃for 4 min; 94℃for 30 s, 50℃ for 1 min for 30 s, 72℃ for 1 min for 30 s, 30 cycles; Then 72℃ for 10min. PCR products were electrophoretic on 0.7% agarose gel, and the size of the target gene was observed by gel imager.
b) Detection of RS transgenic Cordyceps militaris by Southern blot hybridization
CTAB (cetyltrimethylammonium bromide) method was used to extract the total DNA of Cordyceps militaris, PCR, membrane transfer, preparation of probes, hybridization and detection [14].
c) Northern hybridization test of RS transgenic Cordyceps militaris
Trizol method was used to extract total RNA from young leaves of transgenic and non transgenic Cordyceps militaris, electrophoresis, membrane transfer, probe preparation, hybridization and detection [14].
Results & Discussion
PCR Identification of Transgenic Cordyceps militaris
Resveratrol synthetase RS gene was cloned from Parthenocissus tricuspidata from the State Key Laboratory of Plant Genetic Engineering and Protein Engineering, Academy of Life Sciences, Peking University. The gene sequence is1179bp long and encodes 392amino acids. The plant expression vector pCAMBIA3300-RJ39- Tnos was constructed with strawberry fruit specific promoter RJ39 (Figure 1).
The plant expression vector pCAMBIA3300-RJ39-RS-Tnos was transformed into Agrobacterium tumefaciens LBA4404. The marker gene was selected as herbicide resistance gene (bar). Through Agrobacterium tumefaciens mediated Cordyceps militaris, 50 resistant strains were obtained. Genomic DNA was extracted, and PCR amplification was performed with primers RSFP and RSRP. Electrophoresis results showed that 15 strains were positive, with a conversion rate of 30%. The PCR amplification band size of RS gene positive Cordyceps militaris was about 1.1kb, while the negative control did not. The PCR results of some RS transgenic Cordyceps militaris plants are shown in Figure 2.

Figure 2: PCR identification of transgenic pCAMbiA3300-RJ39-RS-TNOS Cordyceps militaris. 1-8: Positive Result of PCR identification of transgenic Cordyceps militaris.—: DNA of wild-type Cordyceps militaris was the negative control template.+: Plasmid pCAMBIA3300-RJ39-RS-Tnos was the positive control of the template. M: DL2000marker.
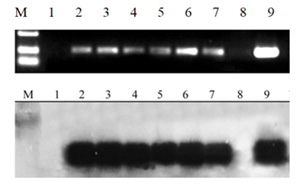
Figure 3: PCR amplification and PCR-Southern hybridization Results of RS transgenic Cordyceps militaris. 1: RS-negative; 2-7: RS positive; 8: negative control (non-gm materials); 9: positive control; M: DL2000marker.
Southern Blot Hybridization Detection of Transgenic Cordyceps militaris
Seven of the 15 RS positive strains were randomly selected for Southern blot hybridization. The total DNA of Cordyceps militaris was extracted by CTAB method, PCR, membrane transfer, probe preparation, hybridization and detection. Results Six strains were all transgenic Cordyceps militaris strains, and one was RS negative strain (Figure 3).
Northern Hybridization Detection of Transgenic Cordyceps militaris
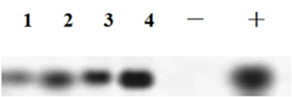
Figure 4: Northern Blotting analysis Results. 1-4: RS positive; -: negative control (non-gm material) ; +: positive control.
The total RNA of RS transgenic Cordyceps militaris with positive PCR identification was extracted and transferred to the Hybond N+membrane by formaldehyde denaturation electrophoresis. The RS gene was hybridized with digoxin labeled full-length RS gene fragment as probe, and the transcriptional level of RS gene in transgenic Cordyceps militaris was detected by Northern hybridization. Northern blotting analysis results showed that the exogenous RS gene could be normally transcribed in the transgenic Cordyceps militaris mycelia, while non transgenic materials did not have hybridization signals (Figure 4).
Conclusions
Resveratrol (Res for short), a secondary metabolite of plants, is a kind of plant protectant, which has a defensive effect on pathogenic bacteria. Studies have found that Res has anti-cancer, anti-inflammatory, cardiovascular disease prevention and other health functions, but the content of Res in plants is very low. The biosynthetic mechanism of Res has been clarified, so it is possible to use microorganisms to express Res heterologously. Res biosynthesis is catalyzed by phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumaric acid: coenzyme A ligase (4CL) and resveratrol synthase (RS). Resveratrol synthase is the most critical enzyme in Res biosynthesis, which is responsible for catalyzing the production of Res from 1 molecule coumarinyl coenzyme A and 3 molecules malonyl coenzyme A [15]. In this study, the nucleotide sequence of resveratrol synthase RS gene cloned from Parthenocissus tricuspidata is 1179 bp long and encodes 392 amino acids [16]. The homology between this gene and the resveratrol synthase gene of Cordyceps militaris FM955393 registered in GenBank is 84.41%. Since promoters play an important role in regulating gene expression and physiological and biochemical characteristics of receptor plants, this experiment used strawberry fruit specific promoter RJ39 to construct pCAMBIA3300-RJ39-RS-Tnos plant expression vector and transform Cordyceps militaris. After screening on selected culture medium, 18 transformants were verified and 6 positive transformants were obtained through PCR, Southern and Southern hybridization. The RS gene expression of Parthenocissus tricuspidata was obtained from the fruiting body of Cordyceps militaris, which improved the economic value of Cordyceps militaris. In recent years, great progress has been made in the research on the function, development and utilization of resveratrol. With the development of sustainable agriculture and the continuous demand of human for drugs and health products, it is urgent to use plant genetic engineering technology to increase the yield of resveratrol. With the continuous cloning and improvement of resveratrol synthase RS gene, and the inheritance improvement of crops, drugs and microorganisms and the cultivation of new varieties by using RS gene are also in order, but how RS gene participates in stress response and its mechanism of action need to be further studied. In a word, the demand of Res in food, health care, medicine and other industries can only be met by transferring resveratrol synthase RS gene to improve the nutritional added value of receptor plants and the production of Res in recombinant microorganisms.
Acknowledgments
Fund Project
Guangdong University Characteristic Innovation Program (Natural Science) Fund (2021KTSCX173).
References
- Brenjian S, Moini A, Yamini N, Ladan Kashani, Maryam Faridmojtahedi, et al. (2020) Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol 83(1): e13186.
- Khafaga A F noreldin A E, Taha A E (2019) The adaptogenic anti-ageing potential of Resveratrol against heat stress-mediated liver injury in aged rats: Role of HSP70 and NF-kB signaling. J Therm Biol 83: 8-21.
- Alrafas H R, Busbee P B, Nagarkatti M, Prakash S Nagarkatti (2020) Resveratrol downregulates miR-31 to promote T regulatory cells during prevention of TNBS-induced colitis. Mol Nutr Food Res 64(1): e1900633.
- Khusbu F Y, Zhou X, Roy M, Fang-Zhi Chen, Qian Cao, et al. (2020) Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int J Biochem Cell Biol 118: 105644.
- Asadpour S, Yeganeh H, Khademi F, Hossein Ghanbari, Jafar Ai (2020) Resveratrol-loaded polyurethane nanofibrous scaffold: viability of endothelial and smooth muscle cells. Biomed Mater 15(1): 015001.
- Abd Aziz N A W, Iezhitsa I, Agarwal R, Roqiah Fatmawati Abdul Kadir, Azian Abd Latiff, et al. (2020) Neuroprotection by trans-Resveratrol against collagenase-induced neurological and neurobehavioural deficits in rats involves adenosine A1 receptors. Neurol Res 42(3): 189-208.
- A Schwekendiek, G Pfeffer, H Kindl (1992) Pine Stilbene Synthase c DNA, a Tool for Probing. Environmental Stress. FEBS Letters 301(1): 41-44.
- J Fliegmann, G Schröder, S Schanz, L Britsch, J Schröder (1992) Molecular Analysis of Chalcone and Dihydropinosylvin Synthase from Scots Pine (Pinus sylvestris) and Differential Regulation of these and Related Enzyme Activities in Stressed Plants. Plant Mol Biol 18(3): 489-503.
- T Yamaguchi, F Kurosaki, DY Suh, U Sankawa, M Nishioka, et al. (1999) Cross-reaction of Chalcone Synthase and Stilbene Synthase Overexpressed in Escherichia coli. FEBS Lettrs 460(3): 457-461.
- Zhang Zhen, Li Sheng, Liu Ai, et al. (2008) Application of biotechnology in resveratrol research. Journal of Gansu Agricultural University 43 (1): 119-125
- Hain R, Bieseler B, Kindl H, et al. (1990) Expression of a stilbene synthase gene in Nicotiana tabacum Results in synthesis of the phytoalexin Resveratrol. Plant Mol Biol 15(2): 325-335.
- Zheng Shigang, Li Zhen, Zhao Shancang, et al. (2014) Research progress on application and function of resveratrol synthase gene in genetic engineering. Journal of Biological Engineering 30(3): 341-354
- Lo C, Le Blanc JC, Yu CK, K H Sze, Dominic C M Ng, et al. (2007) Detection, characterization, and quantification of resveratrol glycosides in transgenic Arabidopsis over-expressing a sorghum stilbene synthase gene by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21(24): 4101-4108.
- Wang Guanlin, Fang Hongyun (2016) Plant Genetic Engineering Second Edition. Science Press.
- Han Jingjing, Liu Wei, Bi Yuping (2010) Cloning and prokaryotic expression of resveratrol synthase gene PNRS1. Cordyceps militaris Journal of Crops 36 (2): 341-346.
- Wang Xiaoli (2007) Regulation of resveratrol biosynthesis in transgenic plants. Shihezi University.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.