Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Transcatheter Aortic Valve Implantation in Pure Aortic Insufficiency, An Option for High-Risk Patients
*Corresponding author: José Bohórquez Rivero, Doctor. GIBACUS Research Group, University of Sinú, Cartagena, Colombia.
Received: November 23, 2023; Published: December 06, 2023
DOI: 10.34297/AJBSR.2023.20.002755
Abstract
Transcatheter Aortic Valve Implantation (TAVI) is a technique of widely known cardiac structural interventionism, which has been become one of the mainstays of treatment for patients with stenosis aortic, with evidence supporting its use regardless of risk surgical. For patients with severe symptomatic aortic regurgitation, establishes surgical intervention as the first option; however, the current management guidelines do not provide management recommendations percutaneous. Advances in valve technology and experience accumulated have encouraged the use of TAVI in other settings outside its indication by technical sheet, when the patient is not eligible for surgery due to discharge comorbidity, and one of these off-label indications is aortic insufficiency. We report the first case of transcatheter aortic valve implantation in patient diagnosed with pure aortic regurgitation on the caribbean coast Colombian.
Keywords: Aortic valve regurgitation, Aortic regurgitation, Transcatheter aortic valve replacement (DeCS).
Introduction
Transcatheter Aortic Valve Implantation (TAVI; Transcater Aortic Valve Implantation) has become the technique of more widespread cardiac structural interventionism [1]. In particular, the advancement of the technique has made it possible to perform a considerable number of procedures with good clinical and hemodynamic outcomes, comparable to the procedure surgery in selected patients. In the last 15 years, there have been approximately more than 350,000 valves in more than 70 countries and the number continues to increase [2]. The first implant was performed by Dr. Alan Criber in France, in 2002, initially, the procedure was performed only in patients with severe symptomatic aortic stenosis, with very high surgical risk; that is, with prohibitive risk for surgical aortic valve replacement (inoperable); however, currently the candidate patients have grown progressively with clinical studies that include subjects with high risk, intermediate and low [1,2]. In addition, there are some controversial indications that mark the frontier in the evidence for the use of TAVI, including patients at risk lower-intermediate surgical and bicuspid aortic valve, valve procedures in valve, cases of pure aortic regurgitation (AI) and patients with sequelae. Severe valvular lesions after “cured” infective endocarditis [1]. AI is one of the valve diseases with the highest prevalence and highest mortality. Currently, the established management is surgical with aortic valve replacement [3]. For patients with said valve disease, current management guidelines do not provide specific recommendations for percutaneous management [3,4]. The first case of successful TAVI in a patient with Pure AI diagnosis on the Colombian Caribbean coast.
Case Description
Male, 87 years old, who consulted for clinical symptoms of approximately 2 years of evolution, consisting of progressive deterioration of functional class, moderate functional class, dyspnea on moderate exertion that worsens with physical activity and improves with physical activity and improves with rest, and lower limb edema that leaves fovea. As the only pathologic history, she reported arterial hypertension, diagnosed 35 years ago, managed with losartan 50mg tablets orally every 12 hours. At physical examination the only positive findings were blood pressure of 154/72mmhg and auscultation of a diastolic murmur in aortic focus grade IV, decrescendo, radiating to the apical region. Due to this finding, a transthoracic echocardiogram transthoracic echocardiogram was performed, which revealed severe aortic insufficiency with a contractile vein width of 0.8cm, hemi-pressure time 247ms, EROA (Effective Regurgitant Orifice Area) and Effective Regurgitant Orifice Area (EROA) 0.36cm 2. Cardiac magnetic resonance imaging revealed end-diastolic diameter of 57mm, end-systolic diameter 40mm, septum thickness 16mm, Left Ventricular Ejection Fraction (LVEF) of 51%, trivalve aortic valve with trivalve aortic valve trivalve aortic valve with valve area 3.5cm 2 , severe aortic valve insufficiency with central regurgitant jet of central regurgitation, mild mitral insufficiency, aortic valve plane 26mm, ascending aorta 33mm, sino tubular junction 28mm, valsalva sinuses 36mm. Coronary arteriography was performed and showed no angiographically significant lesions. The case was discussed in the cardiology medical board and the age, the surgical risk, and the absence of findings suggesting the need for aortic root the need to intervene the aortic root, percutaneous management was chosen. percutaneous management was chosen; therefore, under general anesthesia via the right femoral artery, a guidewire is guidewire was advanced until the SAPIEN 3 valve was positioned in the aortic valve (Figure 1), the correct position of the valve was checked, and it was released. control aortogram and echocardiogram were performed, which showed a mean gradient of 6 and peak gradient of 10mmHg, the valve implantation was corroborated and there were no images of paraesophageal leakage. without images of paraprosthesis.
Discussion
The main recommendation for surgical intervention of the aortic valve is given for patients with severe symptomatic aortic regurgitation and those with left ventricular dysfunction (with LVEF≤50%) or end-diastolic diameter of 50mm [3,4]. In the present case, a patient with insufficiency is described. Severe symptomatic aortic disease, with advanced age and high surgical risk; therefore, he attended with an indication to operate on the aortic valve. There is currently no strong recommendation for the percutaneous management of AI. With the use of TAVI in this scenario, the rates of moderate and severe AR have been significantly reduced, although the results have not been equivalenting to those obtained in aortic stenosis [5,6]. In particular, pure AI pathophysiological differs from aortic stenosis due to dilation of the aortic annulus, associated aortopathy, and relative lack of calcium in the leaflets and annulus, conditions that increase the difficulty. When delimiting the annular plane leading to a greater exposure to contrast that could trigger nephropathy due to contrast medium. All the changes typical of pure AI make it difficult to adequately anchor and stabilize the device during deployment, which increases the risk of embolization or misuse. Position of the prosthesis with subsequent moderate to severe post-procedure failure; therefore, the transesophageal echocardiogram can be used to aid proper positioning of the valve, but more importantly, to assess post-procedure aortic regurgitation [5,7]. Given the pathophysiological changes produced by volume overload and dilation of the structures, when choosing the valve, the selection of the size of the device must be taken into account, requiring an index of oversizing of 15-20% to avoid residual aortic regurgitation and para leaks [8].
In addition, it is worth noting that the use of TAVI for the treatment of pure native AI with first-generation devices was associated with a higher procedural complication rate and that the development and use of newer or newer generation devices improved procedural outcomes, with lower rates of need for a second valve implantation or significant postoperative AI (≥grade 2) [1]. De Backer, et al., [9], in a study that included 254 patients with pure native AI, from 46 centers, demonstrated greater safety and efficacy with the new generation devices. The overall success of the device according to the Valve Academic Research Consortium (VARC-2) criteria was 67%, being higher with new generation devices 82% vs 47% compared to previous generation valves [9]. New generation devices such as Core Valve Evolut R, ACURATE neo, LOTUS VALVE and SAPIEN 3 have features that set them apart from their predecessors such as retrievability, repositionability, fewer mishap valve position and less post-procedure aortic regurgitation [5,10]. In the present, a self-expanding SAPIEN 3 valve was used allowing a safer procedure. In contrast to what was previously mentioned, published studies have recently shown that a significant reduction in the degree of AI is not enough, since postoperative AI≥2 is associated with higher rates of rehospitalization and all-cause mortality [1]. This points out that a strong level of evidence is currently lacking for TAVI in the treatment of patients with pure AI. In this sense, the use of TAVI using the devices currently commercially available in the United States for AI is considered an off-label indication [5]. The genesis of new devices on the pathophysiology of AI is required but, otherwise, at this time, it should be reserved only for selected cases of non-calcified AI and clinical and imaging evaluation [1] to define the type and size of the valve and its proper placement, thus avoiding prosthetic dysfunction and leaks. We do not ignore the fact that there has been an increase in off-label indications for this technology and that the accumulated international experience with the use of TAVI it has also grown significantly, which has caused clinical practice guidelines to gradually include more recommendations; therefore, we provide a case to strengthen the medical literature.
Conclusion
There is currently no established recommendation to support the indication for TAVI for patients with pure AI. TAVI for patients with pure AI. The pathophysiological substrate of this is different from that of aortic stenosis, which increases the difficulty of using this type of device in this setting. device in this scenario. It is important to perform an adequate prior assessment which should include the realization of cardiac imaging to select the patient adequately the patient, bearing in mind that there is an important risk of complications such as valve migration complications such as valve migration, embolization, or valve malposition. Therefore, in this scenario, TAVI is still indicated as an off-label recommendation for high-risk patients. off-label recommendation for high-risk patients (Figure 1).
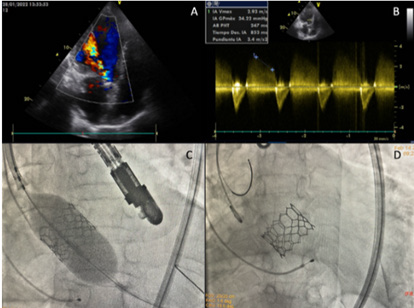
Figure 1:
A. Apical 5-chamber transthoracic echocardiogram view, severe eccentric aortic regurgitation jet.
B. Pulsed Doppler with THP aortic regurgitation jet. Pulsed Doppler with THP (Time of Hemi Pressure) 247ms.
C. Transcatheter aortic valve implantation.
D. Transcatheter aortic valve implantation.
Acknowledgements
None.
Conflict of Interest
None.
References
- Amat Santos IJ, Santos Martínez S (2020) TAVI en indicaciones especiales. REC Interv Cardiol 2(3): 206-214.
- Lièvano J, Villegas J, Acosta G, Sánchez J, Avila Y, et al. (2018) Transcatheter aortic valve implantation in severe aortic stenosis. REPERT WITH CIR 27(2): 109-113.
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, et al. (2021) ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143(5): e35-e71.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. (2022) ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 43(7): 561-632.
- Arias EA, Bhan A, Lim ZY, Mullen M (2019) TAVI for Pure Native Aortic Regurgitation: Are We There Yet? Interv Cardiol 14(1): 26-30.
- Pesarini G, Lunardi M, Piccoli A, Gottin L, Prati D, Ferrero V, et al. (2018) Effectiveness and Safety of Transcatheter Aortic Valve Implantation in Patients with Pure Aortic Regurgitation and Advanced Heart Failure. Am J Cardiol 121(5): 642-648.
- Arora S, Lahewala S, Zuzek Z, Thakkar S, Jani C, et al. (2021) Transcatheter aortic valve replacement in aortic regurgitation: The U.S. experience. Catheter and Cardiovasc Interv 98(1): E153-E162.
- Dvir D, Webb JG, Piazza N, Blanke P, Barbanti M, et al. Multicenter evaluation of transcatheter aortic valve replacement using either SAPIEN XT or Core Valve: Degree of device oversizing by computed-omography and clinical outcomes. Catheter Cardiovasc Interv 86(3): 508-515.
- De Backer O, Pilgrim T, Simonato M, Mackensen GB, Fiorina C, et al. (2018) Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am J Cardiol 122(6): 1028-1035.
- Bruschi G, de Marco F, Martinelli L, Klugmann S (2013) CoreValve® transcatheter self-expandable aortic bioprosthesis. Expert Rev Med Devices 10(1): 15-26.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.