Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Comparison of Methods for The Examination of Biofilms in Screed Insulation Layers Made of Polystyrene with Adhesive Tape Samples, CFU and Total Cell Count
*Corresponding author:Judith Meider, Broicher Str. 13, 51429 Bergisch Gladbach, Germany.
Received: March 23, 2024; Published: April 01, 2024
DOI: 10.34297/AJBSR.2024.21.002910
Abstract
In the case of water damage in floor structures, especially their insulation materials, the assessment of the biomass of the affected materials is based on microscopic analysis of adhesive tape samples. Current guidelines prescribe evidence of growth structures as the main criterion for assessment. This study was used to check whether the adhesive tape analysis method is the most suitable for determining the biomass and whether the growth structures are a good indicator. It was found that examination with adhesive tape samples alone would miss many instances of damage. Even when combined with examination for colony-forming units, only a small amount of damage could be detected. In comparison, the examination of the total cell count with the acridine orange direct count was able to show a significantly higher detection rate.
Synopsis: If inappropriate analytics are selected after water damage, microbial biofilm in screed insulation layers may be overlooked and human health may be compromised. This study compares existing test methods to investigate the biofilm in the screed.
Keywords: Spores, tape samples, total cell count (TCC), Acridine Orange Direct Count (AODC), water damages, polystyrene
Introduction
Water damage in indoor spaces is a widespread and complex problem that raises monetary and organisational questions on the one hand, while on the other hand the preventive health protection of the occupants must not be disregarded. In the case of water ingress into a floor construction, the question arises as to whether microbiological contamination in the insulation materials necessitates the removal of the screed. The Federal Environment Agency's mould guide (UBA, 2017) describes the situation as a decision with far-reaching consequences. On the one hand, the health of the room occupants should be protected; on the other hand, exaggerated assessments and unnecessary deconstruction should be avoided from an indoor hygiene perspective.
In most residential buildings, a floating screed is processed with the insulating material polystyrene. For the assessment of the polystyrene, the Federal Environment Agency (UBA, 2017) recommends a microscopic examination of the biomass of the material by means of an adhesive tape sample and, if necessary, an assessment of the colony-forming units (CFU). As the cultivation of CFU takes time, this procedure is often dispensed with, and an assessment of the damage is carried out based on the results of the microscopy. The main criterion for the assessment is the detection of growth structures, e.g., hyphae. From a microbiological point of view, the question is how long these structures can be detected on a nutrient-poor material. Hyphae and mycelia decompose when no more nutrients are available to serve as nutrients for the microflora itself. This raises the question of whether this method is suitable for assessing mould damage on polystyrene.
The aim of this work is to investigate whether adhesive tape analysis is a suitable method for detecting the biomass and the microbiological growth on the insulation material polystyrene or whether other methods are better suited for this purpose.
Material and Methods
For this study, polystyrene samples from floor assemblies were randomly selected from a routine laboratory.
In the first step, a sample of adhesive film was taken from the surface of the material by pressing an approximately 6 cm long adhesive strip onto the material and pulling it off. This adhesive tape was transferred to a microscope slide, numbered, and stained with Cotton blue. All samples were analysed with a transmitted light microscope with a magnification of 1000. To obtain comparable results, 900 fields of view were counted for each sample using the 3-line method (Meider/Messal 2021) [7]. The result was extrapolated to cm2 for comparability.
In the next step, a sample was taken from the material, weighed and, according to ISO DIN 1600-17 and 16000-21, a suspension was prepared that was needed for the next analytical steps.
In order to be able to carry out a further evaluation of the material, a serial dilution was prepared for each sample in accordance with DIN/ISO EN 16000-17 [4] and applied to the nutrient media DG 18 and malt. The dilution series was evaluated in accordance with DIN/ISO EN 16000-17 [5] after 7 days and calculated to the reference quantity CFU/gram.
The same suspension was used to determine the concentration of total cells counts (TCC) per gram by Acridine Orange Direct Count (AODC). 100μl of the sample was stained with Acridine Orange and the total cell count was evaluated by fluorescence microscopy (Meider, 2019) [8].
Subsequently, all analysis data obtained per sample were transferred to a table and evaluated. In the evaluation, separate evaluation criteria were applied for each type of analysis.
For the adhesive film sample and the CFU, the data in Table 6.2 and 6.3 of the Federal Environment Agency's mould guides were applied. German Federal Environment Agency (UBA, 2017) [10].
The samples were sorted into different categories.
The basis for the classification of the adhesive film and CFU was according to (Tables 1,2).
Subsequently, the results of all three analysis steps were compared with each other and it was investigated which analysis method best evaluates the sample and how.
Theory
Correctly identifying and assessing biomass and hence microbiological damage is a complex task that can have far-reaching consequences. Deconstruction of contaminated materials is carried out under protective measures to protect workers and residents alike. On the one hand, there is a high financial factor involved, so it should only be used when necessary. On the other hand, there is a duty of care to protect the health of the residents. Studies have shown that even dried-up damage is a health hazard [3,6] and that deconstruction for preventive health protection is an appropriate measure. Another study has shown that the load on the screed insulation layer has a relevant influence on indoor air [1,2]. Further research in this area is necessary.
If it is assumed that time and drought cause the growth structures on nutrient-poor materials to disintegrate and become difficult to detect microscopically, and that the number of CFU is also greatly reduced by these factors, the difficulty of the assessment becomes clearer.
The UBA evaluation criteria state that pure microscopy of the material using adhesive film analysis is sufficient to evaluate a material. An analysis of the CFU is not necessary. It is important to note here that a sample is only evaluated as an infestation if hyphae or spore carriers are detected.
In contrast to microscopy with adhesive film analysis, which only examines the surface of the material, the analysis of the total cell counts and CFU is an examination of the volume of the material. This means that the microorganisms in the depth of the material are also considered. For this reason, it must be noted that the results of the surface cannot be correlated with a volume examination, as was already shown in the publication by Meider / Trautmann 2018 [9].
The total cell count is a microscopic method like the adhesive film analysis, but like the CFU analysis it is based on the volume and on the same suspension. These results can therefore be related to each other [8].
Results
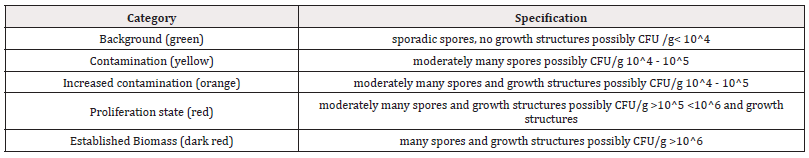
In the test set-up, 100 samples were analysed. For 9 samples, individual analyses could not be evaluated for various reasons. The basic population n is therefore 91 samples. 29% of the samples were in the range of the background values with all three types of analysis and are thus classified as inconspicuous. 27% of the samples showed abnormalities with all 3 methods of analysis and can thus be described as clearly conspicuous. This leaves 44% of the samples that are ambiguous, and the three methods produce different results (Figure 1).
Figure 1 shows the population of all samples colour-coded according to the corresponding classification. Some classifications were classified higher than the number would indicate. In these cases, additional growth structures of the moulds were detected.
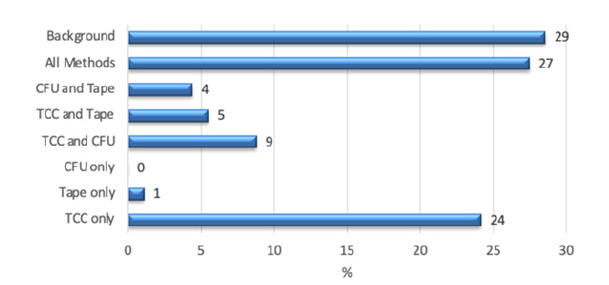
Figure 2 shows all samples. This graph already shows that only a very small area of the ambiguous samples was detected with adhesive film samples - only 1% with this method alone, and 10% if other methods were also conspicuous in parallel. The total cell count alone was able to reveal a conspicuous microbiological concentration of moulds in 24% of the samples. Detection with CFU alone did not. 13% were CFU conspicuous in combination with other methods, 4% CFU and tape samples, 9% CFU and TCC.
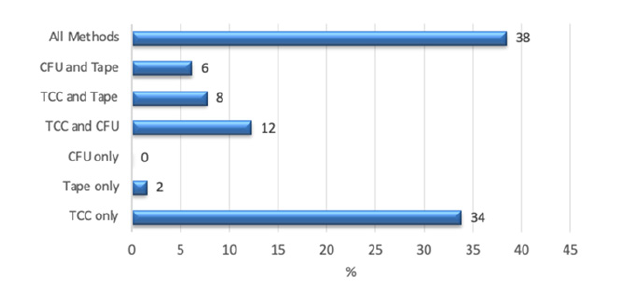
In the next figure, the inconspicuous samples have been omitted from the calculation. Here the figures become even clearer. 38% of the samples were clearly conspicuous with all three methods. In 34% of the samples, the load could only be detected with the total cell count, and in 46% the CFU was also conspicuous in addition to the TCC. Tape samples were only able to reveal a total of 16%, but only 2% on their own. CFU alone could not reveal any abnormalities, but in 13% the CFU was also conspicuous (Figure 3).
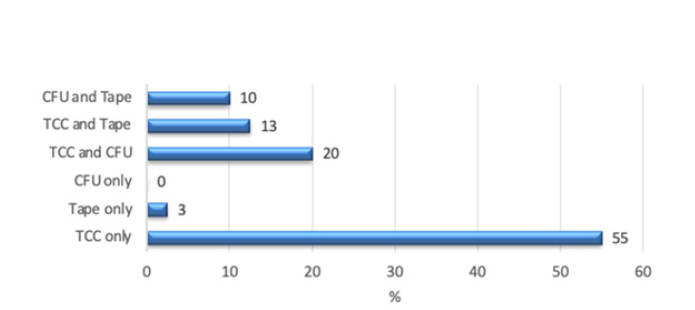
In Figure 4, only the ambiguous samples were compared. More than half of the samples could only be identified with TCC. 88% of the abnormal samples were indicated with TCC. In contrast, abnormalities were diagnosed with adhesive film alone in 3% of the tape samples, but with other methods in 26% of samples. CFU alone did not reveal any abnormalities; however, in 30% the CFU were also abnormal. Ambiguous samples examined with adhesive film and CFU were found to be abnormal in 13% with both methods and 16% overall (Figure 4).
All samples were analysed in triplicate. All samples were evaluated in triplicate and the mean value applied. The total cell count showed a standard deviation of 6-8 %. The CFU showed a standard deviation of 9-15 % and the tape samples of 10-13%.
Discussion
The analysis of insulation materials from floor constructions is a main criterion for deciding whether deconstruction is necessary or not. The previous practice of using adhesive film analysis as the sole basis for decision-making is not recommended according to these data. Even in the combination with adhesive film and CFU, damage is only found in 16% of ambiguous samples. From a microbiological point of view, this can be explained by the decay of the hyphae and the reduced cultivability due to disturbing factors such as drought. This is a clear advantage in the total cell count because this method is much less influenced by disturbing factors. This is especially because the vitality of the cells has no influence in this method of analysis. Dryness, time and also most biocides do not significantly reduce the total cell counts. Even in most samples that were only detected with TCC, hyphae could no longer be detected. However, since the spore concentrations in the material do not decrease, a high microbiological load could still be detected. This is particularly important if there is no active damage when the damage is detected, but an old infestation or after a biocide application. In addition, the agreed remediation goal should also be considered. In most cases, the removal of the biomass is agreed upon and it is then important to verify this goal with a method that works independently of disturbance factors.
Conclusions
The microbiological assessment of the biomass of screed insulation layers is often carried out with adhesive film samples. The detection of growth structures is a main criterion in the assessment. However, on nutrient-poor materials such as the insulation material polystyrene, the detection of these growth criteria is difficult with adhesive film samples. This is because these structures decay after absorbing the available nutrients and are difficult to detect microscopically. The investigation shows that many instances of damage in screed insulation layers are overlooked when they are examined exclusively with adhesive film samples. Even in combination with CFU, much damage is not detected. For a reliable damage assessment, the total cell count could detect microbiological loads more reliably, as this method is less influenced by interfering factors.
References
- Albrecht S (2012) Abschottung von Schimmelpilzbildung in der Estrich Dä Bachelor Thesis Akademie Bau - Bauen im Bestand Sommersemester.
- Albrecht S (2014) Hochsicherheitsestrich für Pilze und Sporen.; Abschottung von Schimmelpilzen in der Estrich-Dämmschicht. Bauen im Bestand. 67-69.
- Croston T, Green B, Lemons A, Barnes M, Goldsmith W, et al. (2018) Fungal fragmentation influences pulmonary immune responses following repeated exposure to Stachybotrys chartarum J. Allergy Clin. Immunol. 141(2): AB185.
- DIN ISO 16000-17: 2010, Indoor air - Part 17: detection and enumeration of moulds - culture-based method (ISO 16000-17:2008)
- DIN ISO 16000-17: 2010, Indoor air - Part 21: Detection and enumeration of moulds - Sampling from materials (ISO 16000-21:2013)
- Korkalainen M, Taubel M, Naarala J, Kirjavainen P, Koistinen A, et al. (2017) Synergistic proinflammatory interactions of microbial toxins and struc- tural components characteristic to moisture-damaged buildings. Indoor Air 27:13-23.
- Meider J, Messal C (2021) Faster Evaluation of Contaminated Surfaces for Mould Inspections by Tape Sampling. J Biomed Res Environ Sci 2(6): 516-522.
- Meider J (2019) Examination of total cell count in building materials by acridine orange direct count (AODC). Journal of Microbiological Methods 167: 105725.
- Trautmann C, Meider J (2018) Ableitung von Schimmelpilz- und Bakterienhintergrund-konzentrationen auf Baumaterialien aus Routineproben, Schimmelpilz-Handbuch. Hrsg. Irina Kraus-Johnsen, Bundesanzeiger Verlag, Kö
- Umwelbundesamt (UBA) (2017) Leitfaden zur Vorbeugung, Erfassung und Sanierung von Schimmelbefall in Gebäuden. Umweltbundesamt, Berlin.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.