Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Development and Validation of a Generic IP-LC-MS/ MS Based Quantification Method for Lecanamab - A Therapeutic Antibody for Treatment of Alzheimer’s Disease in Human Serum and CSF
*Corresponding author:Zhongping John Lin, PhD, Frontage Laboratories, Inc. 700 Pennsylvania Drive, Exton, PA 19341.
Received: February 01, 2024; Published: February 27, 2024
DOI: 10.34297/AJBSR.2024.21.002869
Abstract
Although immunoassay is still the standard methodology for antibody quantitation due to its advantages of high-throughput and sensitivity, liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) has increasingly become an alternative to immunoassay for absolute quantitation of antibody drugs in biological matrices. Here we present a generic LC-MS/MS method for quantitation of antibodies in human serum and CSF. Anti-IgG1 antibody is immobilized to magnetic beads and services as a capture reagent for therapeutic antibodies in human serum and CSF. After digestion, a specific tryptic peptide for a target antibody is selected to serve as a surrogate peptide in multiple reaction monitoring (MRM) for the absolute quantitation of the antibody. A second antibody is used as an internal standard to monitor the whole sample process from capture through digestion to analysis (through its unique tryptic peptide). As a proof of concept, BAN2401 (Lecanamab), a therapeutic antibody is quantitated in human serum using this approach. A second antibody, MORAB-022, is used as an internal standard. The assay is fully validated and cross validated using blinded quality control and incurred study samples with an ELISA method. The variation of the assay is generally less than 12.9%. This hybrid LC-MS/MS assay is 12 times more sensitive than the original ELISA method that employed an anti- BAN2401 polyclonal antibody. This hybrid assay has been used to quantitate BAN2401 in human serum and human CSF to support global clinical trials including US and China. This approach of hybrid LC-MS/MS can be applied to any antibody quantitation in biological matrices as long as there is a unique surrogate peptide from the antibody.
Keywords: Lecanamab, Alzheimer’s disease, IP-LC-MS, Absolute quantitation, Immunocapture, Anti-IgG1 antibody, Cross validation of LC-MS vs. LBA
Abbreviations: BAN2401: Lecanamab; CSF: Cerebrospinal Fluid; ELISA: Enzyme-Linked Immunoassay.
Introduction
Therapeutic monoclonal antibodies (mAbs) are the largest class of biological therapies under development and the highest earning category of all biological drugs in biotechnology industry [1-2]. There are more than 130 therapeutic antibodies approved by FDA and EMA by 2023 and currently there are more than 300 mAbs in various stages of trials worldwide [3]. Immunoassay is currently the standard method for antibody quantitation in biological matrices because of its advantage of high sensitivity and high throughput. However, due to dependence on specific antibody reagents, this approach requires lengthy methods development time. In addition, nonspecific binding issues can often impact assay lower limit of quantitation. This has prompted enormous interest in liquid chromatography coupled tandem mass spectrometry (LC-MS/MS) based methods [4-19]. These drawbacks are especially evident when critical antibody reagents are not available in the early stage of drug development or, when critical antibody reagent is not good due to its non-specific binding to structurally similar epitopes. In contrast, LC-MS/MS based approach is usually faster in method development, and it does not depend on a specific reagent. Moreover, it also provides a way to differentiate even close epitopes by monitoring both parent and daughter ion of a molecule. The lack of selectivity in immunoassay sometimes results in high background noise, which can greatly reduce its sensitivity. LC-MS/MS based approaches have been reported for antibody quantitation in biological matrices [19-22]. These approaches include albumin removal with detection at mg/mL level [20], direct digestion with no pre-digestion separation [4, 21] differential dimethyl labeling during derivatization [22] and protein A enrichment plus subsequent SDS-PAGE separation [23]. Although quantitation of intact protein through high resolution mass spectrometry has been also reported [24], this paper will focus on the protein quantitation through surrogate peptides by triple quandrupole mass spectrometry. Generally, sample cleanup procedure for LC-MS/MS based approach usually involves three possible steps: separation of protein of interest from matrix proteins (pre-digestion separation), breaking down of protein into peptides (digestion) and separation of surrogate peptides from other peptides (post-digestion separation). Because of the sheer number of endogenous proteins in biological matrices, the effectiveness of pre-digestion separation will have a vital impact on the method performance, which includes method selectivity and sensitivity. No pre-digestion separation step will limit the sample volume that can be processed due to large number of endogenous proteins [4].
As a result, samples are diluted, and which results in lower sensitivity. Moderate pre-digestion separation by taking advantage of difference in the unique physical properties of the analyte protein and the endogenous protein increases the sample volume and thus increase method sensitivity [4-8]. Highly specific pre-digestion separation achieves much cleaner samples, which reduces the burden of downstream sample process. It usually has a higher sensitivity in part through concentrating samples and reducing the interfering proteins during sample processing. But it requires the availability of tight interaction reagents such as antibody-antigen interaction [5,8,12,17], which may not be available. In this paper, we propose a generic and practical approach for absolute quantitation of antibody drugs by using a commercially available monoclonal anti-IgG1 antibody as a capture reagent as procedures shown in Figure 1.
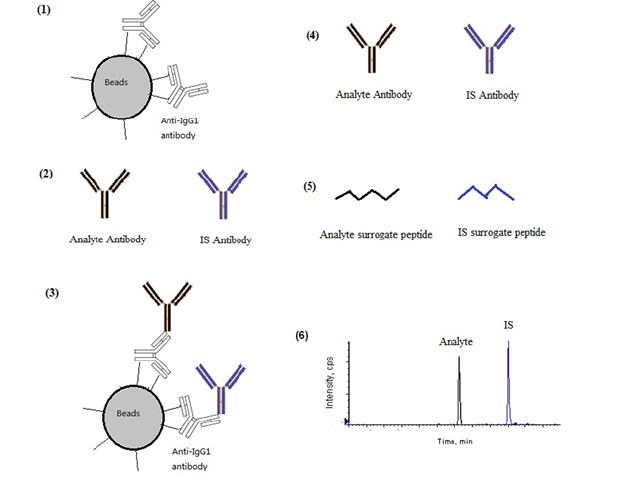
Figure 1: A scheme for the current generic approach of absolute quantitation of antibodies: (1) Immobilized Anti-IgG1 monoclonal antibody magnetic beads are added to (2) analyte and IS antibodies spiked biometrics (3) Analyte and IS antibodies (plus other endogenous IgG1 antibodies) are captured by immobilized beads (4) analyte and IS antibody (plus other endogenous IgG1 antibodies) are eluted out (5) surrogate peptides for analyte and IS generated after antibody reduction and digestion (6) MRM chromatograms generated from the surrogate peptides in the processed samples.
This approach provides a generic and moderate pre-digestion separation for any antibody drug in biomatrices. As a proof of concept, this approach was applied to BAN2401 (Lecanemab), a therapeutic monoclonal antibody that has been recently approved as a treatment for early-stage of Alzheimer’s disease. A second IgG1 monoclonal antibody, MORAB-022 is selected as the internal standard to monitor the entire sample process including immunocapture, trypsin digestion and mass spectrometric detection.
Materials and Methods
Materials
BAN2401 and MORAB-022 stock solutions were obtained from Eisai Inc. (Nutley, NJ). Anti-IgG1 monoclonal antibody was purchased from Mabtech (Nacka Strand Sweden). Sequencing Grade Trypsin was purchased from Life sciences (Wisconsin, WI). Blank human serum and CSF from both healthy volunteers and Alzheimer disease patients were purchased from BIOIVT Inc. (Hicksville, NY). Calcium Chloride, Ammonium Sulfate, Sodium Hydroxide and dithiothreitol (DTT) were purchased from (Sigma-aldrich, St Louis, MO).
Sample Preparation
BAN2401 stock solution with a concentration of 10 mg/mL was serially diluted in pooled human serum to give 0.5, 1, 3, 6, 20, 40, 120 and 150 µg/mL. Quality control (QC) samples were prepared in the same way with final concentration of 1.5, 12, 112.5 and 450µg/mL for low, medium, high and dilution QCs. Internals standard working solution was prepared by diluting MORAB-022 stock solution in PBS buffer to 50 µg/mL.
BAN2401 stock solution with a concentration of 10 mg/mL was serially diluted in pooled human CSF to give 5, 10, 20, 50, 100, 200, 400 and 500 ng/mL. Quality control (QC) samples were prepared in the same way with final concentration of 15, 75, 150, 375 and 3750 ng/mL for low, medium 1, medium 2, high and dilution QCs. Internal standard working solution was prepared by diluting MORAB-022 stock solution in PBS buffer to 20 µg/mL.
Identification of Tryptic Peptides and Selection of a Surrogate Peptide for BAN2401 and MORAB-022
In-silico identification of tryptic peptides selective for BAN2401 and MORAB-022:
Antibody sequences were interrogated applying the following rules for surrogate peptide selection: (i) cleavage must be fully tryptic and no missed cleavages are allowed; (ii) peptides must not contain methionines and cysteines and their mass should be between 800-3500 Da. Peptides that fulfilled all criteria were analyzed against the NCBI nr database using the blast algorithm and only peptides selective for the antibody in question were followed up.
Peptide characterization with neat antibodies:
Digestion and solid phase extraction
Antibodies were precipitated using CHCl3/MeOH (25) and protein pellets resuspended in 8M urea in 25 mM Tris/HCl, pH 8.8. Proteins were reduced (DTT), alkylated (iodoacetamide) and then digested with Lys-C (protein: enzyme ratio = 50:1) for 4 hours at 25°C. Samples were diluted 1:4 using 100 mM (NH4)2CO3, supplemented with trypsin (protein: enzyme ratio = 50:1) and digestion continued over night at 25°C. Protein digests were then purified on C18 Stage tips as described [26], the eluates were dried down completely and stored at -80°C until analysis.
Nanoflow-liquid chromatography tandem mass spectrometry (LC-MS/MS)
LC-MS/MS experiments were performed on a LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Hemel Hempstead, UK) connected to an Ultimate 3000 system (Dionex UK Ltd, Camberley, UK). The LTQ-Orbitrap was equipped with an in-house made nanospray source. Peptide separation was achieved using a 15 cm long 100 mm inner diameter “stone-arch” capillary needle packed with reverse-phase ReproSil-Pur C18-AQ 3 mm resin [27] (Nikkyo Technos Co. Ltd, Tokyo, Japan). Obtained protein digests were loaded using a trap column (loading was achieved in 0.06% TFA in 2% ACN at a flow rate of 25 l/min) and eluted with a gradient of 5% B (0-5 mins), 5-30% B (5-35 min), 30-100% B (35-40 min), 100% B (40-50 min) at a constant flow of 300 nl/min. Solvent A was 0.5% acetic acid and solvent B 0.5% acetic acid in 80% ACN. The spray voltage was set to 2 kV and the temperature of the heated capillary to 1500C. The mass spectrometer was operated in data-dependent mode to automatically acquire MS and MS/MS spectra. Full scan survey spectra (m/z 400-1600) were acquired in the Orbitrap with a resolution of 30000 at m/z 400 after accumulation of 500000 charges. The five most intense ions were sequentially isolated and fragmented in the linear ion trap by collision induced dissociation after accumulation of 5000 ions (normalized collision energy 35%). Maximum inject times were 700 ms for full scans and 100 ms for MS/MS scans. Dynamic exclusion was enabled with the exclusion list restricted to 500 entries, an exclusion duration of 40 seconds and a mass window of 10 ppm. Data were acquired using the Xcalibur software (version 2.0.5).
Bead immobilization with Anti-IgG1 Antibody and Sample Process
The manufacture suggested procedure was followed to immobilize antibodies to M-280 tosylactivated magnetic beads (Dynabeads®) from Invitrogen (Carlsbad, Ca). Briefly, the magnetic beads (50 mg equivalent to 1675 µL suspension) were washed with 1 mL of borate buffer (0.1 M, pH 9.5) using a magnetic separator. The anti-IgG1 monoclonal antibody (0.5 mg in 750 µL) in borate buffer and 500 µL 3M ammonium sulfate in borate buffer were added to the beads. The mixture was then incubated overnight (14-18 hours) at 37°C oven with rotation. The reaction supernatant was removed using a magnetic separator. The beads were washed twice with 1ml wash buffer (50 mM Tris buffer pH8.5 with 100 µg/mL BSA) and then incubated at 37°C for 1 hour on a roller in 1 mL of 50 mM Tris buffer pH 8.5 with 5 mg/mL BSA. After washed twice and re-suspended in 1.25 mL wash buffer, 40 mg/mL beads with immobilized antibody are achieved.
The Anti-IgG1 antibody immobilized beads (4.5 mg (110 µL)) were added to each serum sample (10 µL) in a 1.5 mL Eppendorf tube. The samples were then incubated at room temperature for 1.5 hours with gentle rotation. The samples were washed twice with 1 mL wash buffer and eluted with 70 µL Elution buffer (50 mM Tris pH 2.0 with 100 µg/mL BSA). The eluates were transferred to a new set of tubes and then reduced by adding 5 mM DTT in 0.5 M NaOH and incubating at 80°C for 30 min. 20 µL (0.1µg/mL) trypsin and 6 µL of 10 mM CaCl2 was added to each sample and digest overnight at 37°C in a water bath. 10 µL of 2% formic acid in water was added to each sample and samples were then injected to LC-MS/MS system.
Quantitation of Surrogate Peptide by Liquid Chromatography Coupled with Tandem Mass Spectrometry
The processed samples were analyzed on an LC-MS/MS system comprised of a Shimadzu LC-20AD system (Shimadzu Corporation, Columbia, MD) coupled to an AB Sciex API 5500 mass spectrometer (Foster City, CA). A 25 µL sample was injected onto a phenomenex (Torrance, CA) Gemini C6-phenyl column (3µm, 50 mm x 3.0 mm) for separation with a flow rate of 700 µL/min without split. The mobile phase A and B were 1% formic acid in water and 1% formic acid acetonitrile respectively. The mobile phase gradient is as follows (%B/min), 0%/0, 20%/5, 100%/5.1, 100%/6, 0%/6.1, and 0%/7.5. The resolved peptides were analyzed on the API 5500 mass spectrometer with a TurboSpray. The vaporization temperature of the TurboSpray ionization source was set at 500 °C, and the spray voltage was set at 5.0 kV. The samples were analyzed using the multiple reaction monitoring (MRM) scan with collision energy set at 27 V and declustering potential set at 60 V. The MS/MS transition for the HT9 peptide of BAN2401 was 482.8 m/z (triply charged) à 558.2 m/z (singly charged, y7 ion), with 440.3 m/z (doubly charged) ---->508.4 m/z (singly charged, y7 ion) monitored for tryptic peptide ANSVWFR of MORAB-022. The ratio of the peak area of HT9 peptide to the peak area of ANSVWFR peptide was used for quantification of BAN2401 in human serum.
Full validation of the LC-MS/MS method
The method has been fully validated according to US FDA Guidance for Industry: Bioanalytical Method Validation [28] with extended acceptance criteria (20% deviation is allowed for regular standards and QCs, and 25% for LLOQ). In addition to the usual experiments, the LC-MS/MS assay was cross validated with the original ELISA assay (based on an anti-BAN2401 polyclonal antibody) using blinded quality control samples and also incurred clinical study samples. The cross validation of the established LC-MS/MS assay in human serum between different labs was also successfully conducted using blinded quality control samples to support the BAN2401 phase 3 global clinical trial [29].
Results and Discussion
Selection of Surrogate Peptides for BAN2401 and MORAB-022
In order to identify suitable tryptic peptides for monitoring MRM assays, BAN2401 and MORAB-022 sequences were investigated in-silico for sequences that were selective for the respective antibody and complied with a set of rules (fully tryptic and no missed cleavages, sequences must not contain methionines and cysteines and peptide mass should be between 800-3500 Da). For BAN2401, two tryptic peptides and for protein IS MORAB-022, four tryptic peptides were identified that met all criteria. Tryptic digests of both antibodies were analysed by LC-MS/MS on a LTQ-Orbitrap XL instrument in order to check for liberation of peptides of interest and to obtain relevant fragmentation data. This fragmentation data was then used to construct MRM assays for the respective peptides on an API 5500 instrument. For each antibody one surrogate peptide (EGGYYYGR (HT9) for BAN2401 and ANSVWFR for MORAB-022) was chosen on the basis of superior LLOQ levels and chromatographic behaviour.
Comparison of Peptide and Protein Internal Standard
Theoretically, any human antibody with a selective tryptic peptide can serve as an internal standard for this generic assay. Nonetheless, some practical aspects have to be taken into account. First of all, it has to be stable at various digestion and reconstitution conditions. Secondly, the similarity to the surrogate peptide of analyte will help reduce the matrix effect and variation in MS detection. The deuterated (EGGYYY-d7GR) form of surrogate peptide was also synthesized to assess its performance as an internal standard. The antibody IS has performed better than the deuterated surrogate peptide IS (data not shown). This is likely in part due to the antibody IS compensating for the variation in capture step and digestion step as well as LC and MS detection steps. This becomes obvious in cases when the digestion step is not optimized. In that case, bigger variation can be seen in the samples with deuterated peptide as IS, while the variation is generally smaller and within acceptance criteria when a protein IS is used. Based on these results, a second antibody is used as an internal standard for this generic approach due to its readily availability. A deuterated protein IS will be ideal for its ability to monitor both sample process procedures and LC-MS/MS detection, but it is not a requirement.
The performance of LC-MS/MS Serum Assay for BAN2401
The overall performance of the assay is summarized in Table 1. The Intra-Run and Inter-Run QC in-precision is generally less than 9.2%, except 18.8% at LLOQ level. The overall recovery of analyte and IS is 25.9% and 12.3% (n=3, CV=6.4%) respectively. They are quite low according to the criteria of similar methods for small molecule drugs, but the precision of the recovery proves to be more important. At three QC levels, the BAN2401 recoveries are 25.5% (n=6, CV=4.4%), 26.1% (n=6, CV=6.7%) and 26.2% (n=6, CV=1.7%) for low, mid and high QC respectively. The high precision of interaction between anti-IgG1 antibody and BAN2401/MORAB-022 is fundamental to the performance of this assay. This is the reason why anti-IgG1 antibody was chosen over protein A or protein G as the capture reagent, besides it is slightly more selective than the other two capture reagents (data not shown). Anti-BAN2401 polyclonal antibody was also explored as a capture reagent in the early method development. It not only has the tight precision of recovery, but also greatly improves the sensitivity through its concentration effect. For this specific capture reagent using anti-BAN2401 polyclonal antibody, 50 times more sample volume (500 uL versus 10 uL used in anti-IgG1 mAb as the capture reagent) can be used, thus yielding roughly 50 times more sensitive assay (Data not shown here). With 500 uL sample volume, the LC-MS/MS based assay using the anti-BAN2401 polyclonal antibody can achieve more than 200 folds of the sensitivity (data not shown here) that the ELISA assay has using the same antibody (the primary antibody for ELISA assay). However, considering the heterogenicity of polyclonal antibody and the expected lot-to-lot variations in a long period of time, the assay performance including assay sensitivity and assay specificity may be impacted by the availability of the good quality of this critical reagent in a lengthy clinical development period if polyclonal antibody was used, which most LBA assay suffers. For this reason, the generic anti-IgG1 mAb was selected instead in this assay in order to maintain the LC-MS/MS method performance in a long period of time due to the consistent performance and the reliable availability of monoclonal antibody.
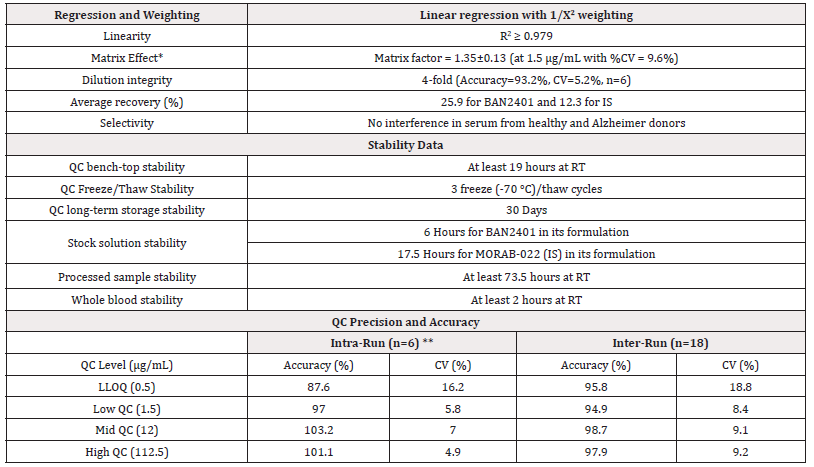
Table 1: Summary of the performance for BAN2401 LC-MS/MS serum assay.
Note*: *Matrix effect was determined by peak area ratio of post extraction spiked samples/neat solution. **Data from Intra-Run Day 1.
The MRM chromatograms of a typical double blank sample and a LLOQ (500 ng/mL) sample are shown in Figure 2. At LLOQ, the MRM transition for surrogate peptide EGGYYYGR of BAN2401 has a decent signal. Although this is a generic approach for any antibody, the assay sensitivity may vary quite a lot pending on the physical property such as ionization efficiency and fragment pattern of each individual surrogate peptide for a specific antibody. The observed matrix effect at QC-Low level (Table 1 & Table 4) is not because the matrix enhancing effect. Rather, it was because the matrix has a bigger suppression effect on the surrogate peptide of IS. If an isotope labelled antibody is available as an internal standard, it will improve the assay performance, at least on the parts of chromatographic separation and mass spectrometric detection. Optimization of antibody reduction (in 5 mM DTT with heating at 80°C for 1 hour) and digestion (for 4-16 hours incubation at 37°C with 2 μg trypsin) was based on a similar assay developed previously (Chen Z. manuscript not submitted yet, data not shown).
Performance of LC-MS/MS Assay vs the ELISA Assay
Both blinded quality control samples and incurred clinical study samples were used as cross validation samples to assess the performance of the LC-MS/MS serum assay and its consistency with the ELISA assay from another lab. After un-blinding, the results of 12 cross validation QC samples tested were within 11.4% of nominal values, which indicates the good performance of this assay. The cross validation of incurred clinical study samples as shown in Table 2 has clearly shown the consistency of this assay with the ELISA assay for those samples with drug concentration high enough to be detected by ELISA assay. The LC-MS/MS assay, however, can extend the measurement of additional samples that were below the ELISA LLOQ (6 ug/mL), but above the LLOQ of current assay (0.5 ug/mL). Compared to the existing ELISA assay for this analyte using generic anti-IgG1 mAb in this LC-MS/MS assay, the LC-MS/MS assay is 12-fold more sensitive.

Figure 2: Chromatograms of a representative double blank (A) and LLOQ, 500ng/mL (B). MRM Chromatograms on the left are BAN2401 surrogate peptide and MRM Chromatograms on the right are IS (MORAB-022) surrogate peptide.
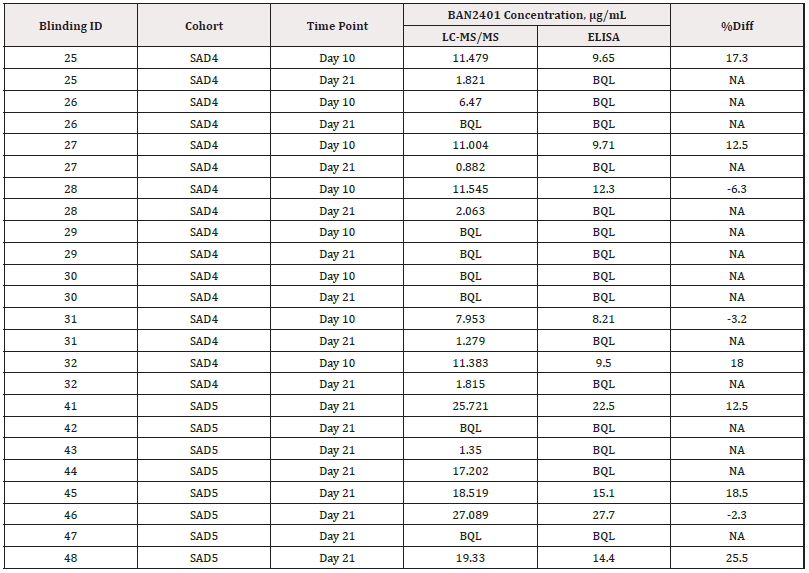
Table 2: The results of clinical samples cross validation between the current LC-MS/MS method and the ELISA method from Lab C.
Note*: The calculation for %difference used was:

Performance of LC-MS/MS CSF Assay for BAN2401
A similar approach was used to quantitate BAN2401 in human CSF. Since there is no IgG in the human CSF matrix, the more convenient protein G beads were used for the CSF assay with high sensitivity and accuracy. The assay was fully validated. The overall performance of the assay is s summarized in Table 3. The Intra-Run and Inter-Run QC in-precision is generally less than 9.8%, except 16.0% at LLOQ level. The overall recovery of analyte and IS is 34.8% (n=4, CV=6.3%) and 12.3% respectively. The MRM chromatograms of a typical double blank sample and a LLOQ (5.0 ng/mL) sample are shown in Figure 3.
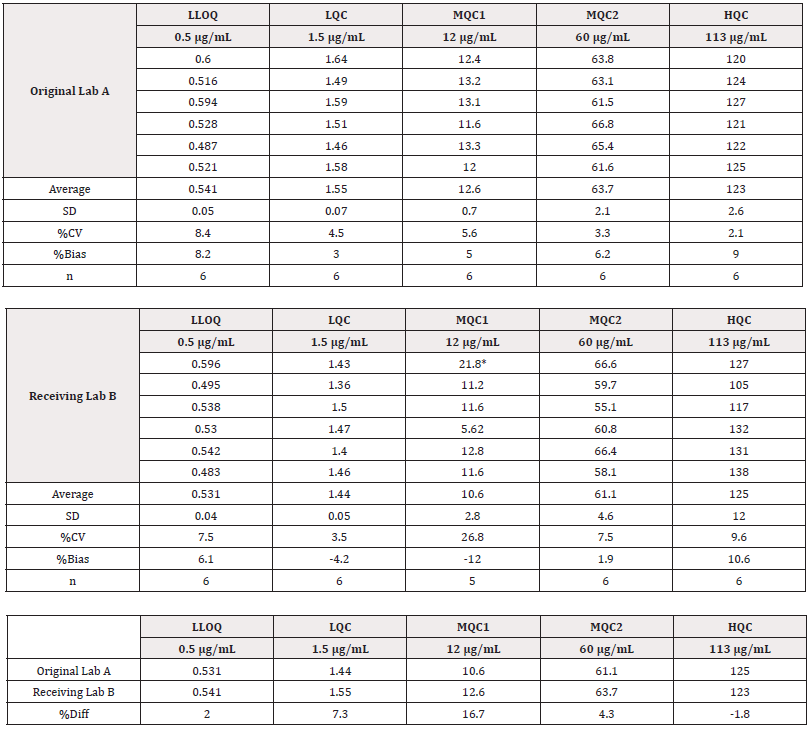
Table 3: Summary of the performance for BAN2401 LC-MS/MS serum assay.
Note*: *Matrix effect was determined by peak area ratio of post extraction spiked samples/neat solution. **Data from Intra-Run Day 1 ***n=17 for LLOQ (one LLOQ was excluded from summary statistics because the value is a statistical outlier).
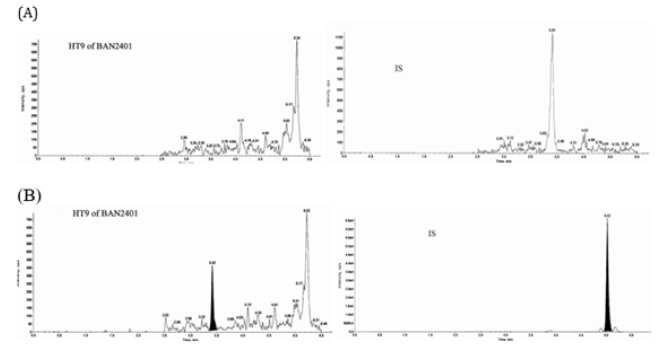
Figure 3: Chromatograms of a representative double blank (A) and LLOQ, 5.00ng/mL (B). MRM Chromatograms on the left are BAN2401 surrogate peptide and MRM Chromatograms on the right are IS (MORAB-022) surrogate peptide.
Cross validation of LC-MS/MS Serum Assay in Different Laboratories
Following the successful method validation of the established generic LC-MS/MS serum assay, the method was transferred to another laboratory to support the global phase 3 clinical trial of BAN2401. Per M10 requirements, cross validation was then conducted to evaluate the consistency of the method performance in different laboratories. Blinded quality control samples were used in this cross validation. After un-blinding, the cross-validation results were shown in Table 4. The %Diff between Lab A and Lab B are within ±20% of theoretical concentration of each QC level for 30 cross validation samples. Based on the data, this generic LC-MS/MS method is considered robust to maintain consistent method performance when transferred to different labs. The use of generic reagents in the assay and the intrinsic selective property of LC-MS/MS platform renders the assay consistent performance in over 10 years with different laboratories.
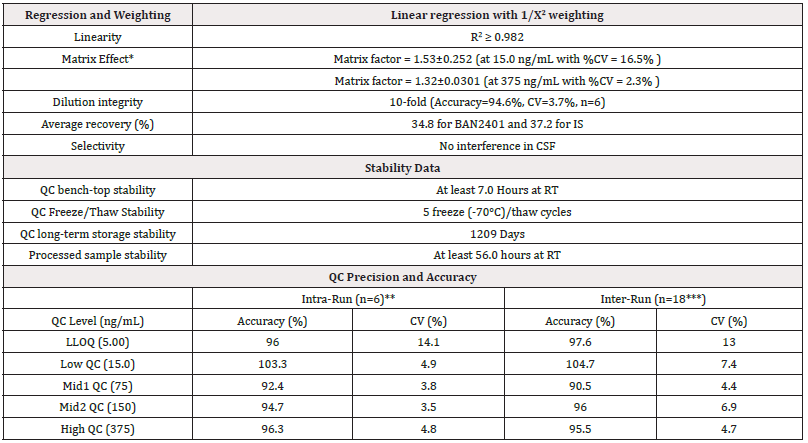
Table 4: Cross validation of LC-MS/MS Serum Assay between two laboratories.
Note*:
Note*: *Note*: %Bias = 100*(Mean – Theoretical Conc)/Theoretical Conc
Note*: Note*: *Outlier, which was out of mean ±3SD, excluded in the statistical calculation.
Note*: Note*: *%Diff is calculated with formular: 100*(Original lab A Mean-Receiving Lab B Mean)/Theoretical Conc. QC samples were prepared and quantitated with lab A. These QC samples were blinded and shipped to lab B for cross validation. %Bias = 100% x (Average -theoretical concentration)/Theoretical Concentration.
Conclusions
A generic LC-MS/MS approach for antibody quantitation is presented through immunocapture with Anti-IgG1 antibody (serum assay), protein G (CSF assay) and surrogate peptide quantitation. The three steps of this generic approach include: 1) Identify a unique tryptic peptide within the analyte antibody and an internal standard antibody, 2) capture both analyte and internal standard antibody using anti-IgG1 antibody immobilized magnetic beads (serum assay) or protein G (CSF assay), 3) quantitate analyte antibody through its surrogate peptide by LC-MS/MS. As a proof of concept, a therapeutic antibody, BAN2401 is quantitated by this approach in human serum and human CSF. The serum assay is 12 times more sensitive than the ELISA assay developed on an anti-BAN2401 antibody. The cross validation shows that serum LC-MS/MS assay agrees well with the ELISA assay and therefore has replaced the ELISA assay. The use of generic reagents in the assay and the intrinsic selective property of LC-MS/MS platform render the assay consistent performance in over 10 years with different laboratories for the support of BAN2401 global clinical trials. The presented generic approach here can be applied to any antibody that has a unique surrogate peptide that can be used as a surrogate peptide for quantitation.
Acknowledgments
None.
Conflict of Interest
None.
References
- Scolnik PA (2009) Mabs: a business perspective. MAbs 1(2): 179-184.
- Leavy O (2010) Therapeutic antibodies: past, present and future. Nat Rev Immunol 10(5): 297.
- Lyu XC, Zhao QC, Hui JL, Wang T, Lin M, et al. (2022) The global landscape of approved antibody therapies. Antib Ther 5(4): 233-257.
- Yang Z, Hayes M, Fang X, Daley MP, Ettenberg S, et al. (2007) LC-MS/MS Approach for quantification of therapeutic proteins in plasma using a protein internal standard and 2D-solid-phase extraction cleanup. Anal Chem 79 (24): 9294-9301.
- Dubois M, Fenaille F, Clement G, Lechmann M, Tabet JC, et al. (2008) Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal Chem 80(5): 1737-1745.
- Wu ST, Ouyang Z, Olah TV, Jemal M (2011) A strategy for liquid chromatography/tandem mass spectrometry based quantitation of pegylated protein drugs in plasma using plasma protein precipitation with water-miscible organic solvents and subsequent trypsin digestion to generate surrogate peptides for detection. Rapid Commun Mass Spectrom 25(2): 281-290.
- Yang Z, Ke J, Hayes M, Bryant M, Tse FL (2009) A sensitive and high-throughput LC-MS/MS method for the quantification of pegylated-interferon-α2a in human serum using monolithic C18 solid phase extraction for enrichment. J Chromatogr B Analyt Technol Biomed Life Sci 877 (18-19): 1737-1742.
- Winther B, Nordlund M, Paus E, Reubsaet L, Halvorsen TG (2009) Immuno-capture as ultimate sample cleanup in LC-MS/MS determination of the early stage biomarker ProGRP. J Sep Sci 32(17): 2937-2943.
- Barnidge DR, Goodmanson MK, Klee GG, Muddiman DC (2004) Absolute quantification of the model biomarker prostate-specific antigen in serum by LC-Ms/MS using protein cleavage and isotope dilution mass spectrometry. J Proteome Res 3(3): 644-652.
- Barnidge DR, Dratz EA, Martin T, Bonilla LE, Moran LB (2003) Absolute Quantification of the G Protein-Coupled Receptor Rhodopsin by LC/MS/MS Using Proteolysis Product Peptides and Synthetic Peptide Standards. Anal Chem 75(3): 445-451.
- Seegmiller JC, Barnidge DR, Burns BE, Larson TS, Lieske JC (2009) Quantification of Urinary Albumin by Using Protein Cleavage and LC-MS/MS. Clin Chem 55(6): 1100-1107.
- Berna MJ, Zhen Y, Watson DE, Hale JE, Ackermann BL (2007) Strategic use of immunoprecipitation and LC/MS/MS for trace-level protein quantification: myosin light chain 1, a biomarker of cardiac necrosis. Anal Chem 79(11): 4199-4205.
- Barr JR, Maggio VL, Patterson DG Jr, Cooper GR, Henderson LO (1996) Isotope dilution--mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem 42(10): 1676-1682.
- Kuhn E, Wu J, Karl J, Liao H, Zolg W (2004) Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics 4(4): 1175-1186.
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP (2003) Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci USA 100(12): 6940-6945.
- Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW (2004) Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA). J Proteome Res 3(2): 235-244.
- Berna M, Schmalz C, Duffin K, Mitchell P, Chambers M (2006) Online immunoaffinity liquid chromatography/tandem mass spectrometry determination of a type II collagen peptide biomarker in rat urine: Investigation of the impact of collision-induced dissociation fluctuation on peptide quantitation. Anal Biochem 356(2): 235-243.
- Barnidge DR, Hall GD, Stocker JL, Muddiman DC (2004) Evaluation of a cleavable stable isotope labeled synthetic peptide for absolute protein quantification using LC-MS/MS. J Proteome Res 3(3): 658-661.
- Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K (2008) Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem 80(11): 4200-4207.
- Hagman C, Ricke D, Ewert S, Bek S, Falchetto R (2008) Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography-tandem mass spectrometry. Anal Chem 80(4): 1290-1296.
- Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K (2008) Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem 80(11): 4200-4207.
- Ji C, Sadagopan N, Zhang Y, Lepsy C (2009) A universal strategy for development of a method for absolute quantification of therapeutic monoclonal antibodies in biological matrices using differential dimethyl labeling coupled with ultra performance liquid chromatography-tandem mass spectrometry. Anal Chem 81(22): 9321-9328.
- Lu Q, Zheng X, McIntosh T, Davis H, Nemeth JF (2009) Development of different analysis platforms with LC-MS for pharmacokinetic studies of protein drugs. Anal Chem 81(21): 8715-8723.
- Ruan Q, Ji QC, Arnold ME, Humphreys WG, Zhu M (2011) Strategy and its implications of protein bioanalysis utilizing high-resolution mass spectrometric detection of intact protein. Anal Chem 83(23): 8937-8944.
- Wessel D, Flügge, UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138(1): 141-143.
- Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2(8): 1896-1906.
- Ishihama Y, Rappsilber J, Andersen JS, Mann M (2002) Microcolumns with self-assembled particle frits for proteomics. J Chromatogr A 979(1-2): 233-239.
- (2001) Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, US FDA, Centre for Drug Evaluation and Research, Rockville, MD, USA.
- Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, et al. (2023) Lecanemab in early Alzheimer’s Disease. N Engl J Med 388(1): 9-21.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.