Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Editorial: Functionality-Armed Catalytic Antibodies (Antibody-Proteases) As Trans-Lational Tools of The Next Step Generation to Monitor Progression of Multiple Sclerosis to Predict, To Prognosticate and To Prevent Demyelination
*Corresponding author:Sergey Suchkov, The Russian University of Medicine and Russian Academy of Sciences, Moscow, Russia.
Received: January 04, 2024; Published: January 24, 2024
DOI: 10.34297/AJBSR.2024.21.002827
Abstract
Biomarkers as a part of the ligand-receptor tandems have induced an impulse to prompt the devel open of an upgraded concept of the targeted therapy. It is health indicators that justifying the necessity to create targeted drug of the next step generation to be implemented at the clinical and subclinical key stages of the disease pathogenesis and to be involved into a multi stage process to get the shifts appeared modified. Catalytic antibodies (CatAbs) have emerged as powerful tools for the efficient and specific catalysis of a wide range of chemical transformations. Generating Ab-driven catalysts that achieve enzymatic efficiency remains a challenging task to be implemented in the designed translational applications.
We also comment on recent developments in the screening CatAbs-related process that allow for a more efficient identification of Ab-based catalysts to be used further as native ones or engineered and/or designed bioproducts in clinical practice. The generation of an edited Abs, enzyme or artificial ABZYME through transition state stabilization by Abs has been thus demonstrated. So, we may consider Ab-proteases as unique translational probes to diagnose, to monitor, to control and to treat and rehabilitate MS patients at clinical stages and to prevent the disorder at subclinical stages in persons at risks to secure the efficacy of regenerative manipulations, High impact of Ab-proteases can be used to monitor both clinical and subclinical courses of chronic auto immune inflammation (in MS, for instance) to predict stepwise transformations of the MS course and to prognosticate the clinical illness finally. In this sense, Ab-proteases can be programmed and reprogrammed to suit the requests and standards of regenerative medicine and remyelination, in particular. This information can allow to design the algorithms for combinatorial (preventive, prophylactic, therapeutic and rehabilitative) treatment, whilst developing unique tools for individually therapy for a number of diseases, such as a group of autoimmune diseases which holds a particular position.
Introduction
Today, the society objectively requires a new approach to health care, based on the prevention of diseases, but not on endless and expensive treatment of chronic conditions and cases. And those grandiose events that occur today in the world of medical science and clinical practice, once again pay attention to the reconsideration their views on problems related with human health. Thus, an absolutely new model of healthcare services, which integrates the philosophy and principles of Personalized and Precision Medicine (PPM) and aims at identifying the disease in the pre-early (subclinical) stage whilst predicting and prognosticating features of the progression, is being created [1]. In this context, new and upgraded targeted therapies for autoimmune and inflammatory diseases and conditions would require greater understanding of a patient or a person at risk to get the therapy personalized for either of those subsets. And although there is currently no definite cure for MS, new therapies have recently been developed based on a continuous search for new biomarkers, being able to differentiate the MS course between clinical and subclinical ones.
Meanwhile, to identify the focus of subclinical pathology, it is necessary to create a special system of subclinical criteria and respective predictive diagnostic tests. But the lack of reliable MS-specific biomarkers often resulted in a delay in MS diagnosis and treatment while clinicians waited for a relapse to occur or for results of sequential neuroimaging studies to confirm the presence of disseminated lesions in the CNS. And the availability of reliable biomarkers could radically alter the management of MS at critical phases of the disease spectrum, allowing for intervention strategies that may prevent evolution to long term neurological disability. This article provides an overview of this research field and focuses on recent advances in blood based biomarker research.
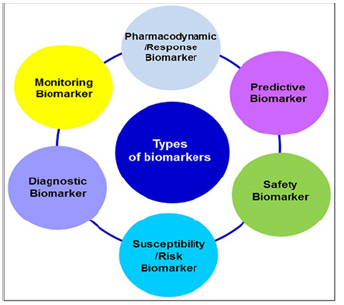
What tests will allow us to determine with high authenticity the genetic susceptibility to the occurrence of pathologies? First of all, it is genomic testing (or genomic profiling), which represents the complete picture of the genes and chromosomes of the particular clients (patients or persons at risk). This information can allow us to design the algorithms of targeted treatment, to create tools for individual tagging therapy for a number of diseases, such as the group of autoimmune (or inflammatory) diseases holds a unique position [1]. Accordingly, now it is time to identify biomarkers of newer generations and to create simultaneously a new strategy based upon subclinical recognition of those biomarkers long before the disease clinically manifests itself. And thus, the identification and implementation of panels of diagnostic, predictive and prognostic biomarkers become the Holy Grail of PPM and highly valuable for the worldwide markets (Figure 1). Since highly specific, informative and validated biomarkers enable evidence based pre-early (subclinical) diagnosis, guide targeted therapy and monitor the progression activity and therapeutic responses across the diseases.
Viewpoint
For instance, new biomarker based targeted therapies for autoimmune and inflammatory diseases would require greater understanding of a patient or a person at risk to get the therapy personalized for either of those subsets [2]. Biomarkers (Figure 2A&B) enable pre-early diagnosis, guide targeted therapy and monitor the activity and therapeutic responses across the broad scope of diseases [1,3]. Diagnostic biomarkers encompass a variety of biomarkers used to detect or confirm the presence of a disease or medical condition, and would contribute to improve PPM increasing the effectiveness of the therapeutic response. Monitoring biomarkers are analysed at different time points to monitor the status of a disease or medical condition, and as a marker of the response to an intervention, including exposure to a medical product or an environmental agent. Predictive biomarker is considered as a predictive biomarker when its presence or modification allows predicting which patient or group of patients are more likely to experience an effect as consequence of being exposed to a medical product or environmental agent. The use of predictive biomarkers facilitates the selection of specific patients more likely to respond or not to therapy.
Prognostic biomarkers are used to identify the probability of developing a clinical event in patients diagnosed with a disease or medical condition. Those biomarkers can provide information about treatment safety, guiding patient hospitalization or their entrance into intensive care units. Pharmacodynamic or response biomarker is modified in response to a medical condition or clinical intervention, including drug treatments. The main utility of this biomarker is to guide the clinical management, providing crucial information for deciding whether or not to continue the treatment.
Thus, those biomarkers determine the progression of the treatment. Safety biomarker can be assessed before and after the exposure to a medical intervention, or an environmental agent, allowing to identify the probability of developing signs of toxicity as an adverse event, to detect the presence of toxicity, and for monitoring its extension. Susceptibility or risk biomarker Is used as a risk measure to develop a disease or medical condition. Susceptibility/risk biomarkers are essential for the development of epidemiological studies aimed to evaluate the risk of developing a disease, contributing to establish preventive strategies in clinical practice. Biomarkers for cancer diagnosis, prognosis and prediction are important tools and an urgent need in PPM and PPM-related cancer management, including novel protein markers, antibodies and abzymes, cell free DNA, metabolome compounds, immune and stroma signatures and microbiome compositions as biomarkers for cancer related practice.
DNA
So, biomarker discovery would require a special multi step, highly complicated but promising strategy to identify, to characterize, to describe, to define the classification niche, to assess the scope of applications and to get them validated. Among the best validated predictive biomarkers are autoimmunity related ones to dominate (including anti bodies/Abs), and thus to predict and prognosticate risks of the chronification, complications and thus disabling of the autoimmune conditions. The latter is so valuable and important since chronic autoimmune inflammation courses are structured to consist from different stages including two key ones, i.e., subclinical and clinical ones (Figure 3) [1,4].
So, accurate prediction is crucially vital for prevention of auto aggression, and the targeted treatment being personalized could thus be given to those individuals who are most likely to develop the disease. Today, Abs are recognized to elicit an almost unlimited range of reactivity including responses to compounds only recently synthesized in the laboratory and not previously existing in nature. In addition, molecules differing in the smallest detail could be distinguished by their reactivity with different Abs. The highly evolved machinery of the immune system to produce structurally and functionally complex molecules like Abs offers tremendous opportunities for biologists, chemists, bio designers, bioengineers and practitioners. Whereas the latter provided the framework for understanding the molecular basis of biomolecular structure and function, the immune system provided a highly evolved synthetic and selective process of nature.
Multiple sclerosis (MS) is just one of the chronic (severe progressive) tissue specific autoimmune diseases with slight short-term relapses in its course. resulting in a destruction of myelin by different tools, including autoAbs [4]. Autoagression against vulnerable myelin associated Ags results in multiple lesions throughout the CNS. The crucial step in the pathogenesis of MS is primary myelin damage which is mediated by autoAbs to trigger a release of separate and pathogenically valuable myelin associated epitopes into the bloodstream. Those epitopes act as sensitizing factors to generate autoAbs (Figure 4), The MS clinical phenotypes, disease courses and responses to treatment that are associated with anti-myelin autoAbs are currently being defined.
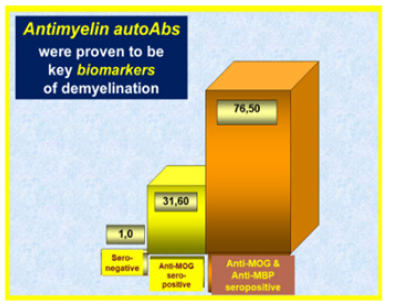
Figure 4: Key serum anti-myelin autoAbs in multiple sclerosis having a crucial role in the pathogenesis.
Anti-myelin basic protein (MBP) autoAbs
Anti-myelin basic protein (MBP) autoAbs have generally been considered to be absent from sera from healthy individuals, but to be detectable in sera from some patients with multiple sclerosis (MS). AutoAb biomarkers are useful in distinguishing subjects with the relapsing remitting form of MS from those with the secondary progressive subtype. And anti-MBP autoAbs are also a marker for MS-associated demyelination and appears to play a significant role in the etiology of MS.
Abs against myelin oligodendrocyte glycoprotein (MOG-Abs)
Abs against myelin oligodendrocyte glycoprotein (MOG-Abs) are associated with demyelinating syndromes of the CNS. Most patients with MOG-Ab-associated disorders have favourable outcomes, but a subset are left with permanent disability, usually as a result of the initial attack. Many MOG-Ab-positive MS patients develop relapsing disease; relapses usually involve optic neuritis and often occur during steroid weaning or soon after steroid cessation, suggesting that a longer initial treatment duration is required.
Analysis of serum autoAbs against MOG and MBP in MS patients with a clinically isolated syndrome (CIS) is a rapid, inexpensive, and precise method for the prediction of early conversion to clinically definite MS. This finding may be important for the counselling and care of patients with a first demyelinating event suggestive of MS. Although, in general, autoAbs against myelin are neither a specific nor a diagnostic feature of MS, it seems that specific demyelinating Abs are involved in the immunopathogenesis of the disorder in at least a subgroup of patients. And the analysis of those Abs can be used to estimate roughly the individual risk of an early first relapse and therefore of clinically definite MS. which, in turn, would drive the demyelination and thus the disease progression. Wide scale autoimmune attack in MS towards nervous tissues leads to a stepwise demyelination with concomitant release of myelin Ags (epitope spreading), formation of autoAbs and, consequently, systematization of proinflammatory responses. Monitoring of antimyelin autoAbs is just a brick for making the subclinical diagnosis of MS and timely implementation of predictive measures and preventive treatment.
The effort of establishing satisfactory biomarkers for MS has been proven to be very difficult, due to the clinical and pathophysiological complexities of the disease. Moreover, the precise pathogenesis and etiology of MS are still a mystery. This is mainly the reason why finding a biomarker with absolute surrogacy abilities remains elusive. And there is urgently need for candidate biomarkers, which could clarify pathology, monitor disease progression, response to treatment, and prognosis in MS. Different human compartments analysis using OMICS-portfolio and bioinformatics approaches has generated new information for further clarification of MS pathology, elucidating the mechanisms of the disease, finding new biomarkers and targets, and monitoring treatment response. Overall, OMICS approaches can develop different therapeutic and diagnostic aspects of complex disorders such as MS, from biomarker discovery to PPM [5].
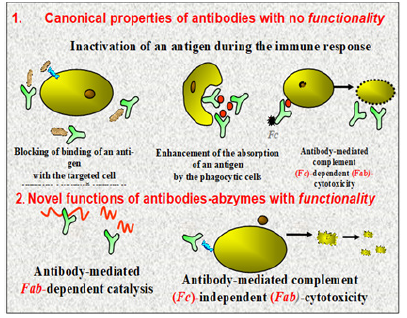
At present, a spectrum of myelin associated autoAbs occurring in MS patients and acting as biomarkers, has been confirmed to be very large. The versatility of Abs is demonstrated by the various functions that they mediate such as neutralization, agglutination, fixation with activation of complement and activation of effector cells. In addition to this plethora of functions, some Abs express enzymatic activity [5,6]. According to classical conception, Abs are specific proteins produced by the immune systems with exclusive function of Ag binding. But Abs against chemically stable analogues modelling the transition states of chemical reaction can catalyze many different reactions and were thus called catalytic Abs or abzymes (derived from Ab and enzymes), which thus to belong to Abs with a feature of functionality (Figure 5) [7]. Fab, is the region on an immunoglobulin molecule that binds to antigens Fc, is the region for binding cellular receptors, conferring its effector function.
Catalytic Antibodies (Catabs)
Catalytic Antibodies (Catabs) are capable of performing almost any type of reaction with high selectivity and stereo specificity and like enzymes process their substrates through a Michaelis complex in which the chemical transformation occurs, followed by product dissociation [8]. "Naturally occurring" CatAbs participate directly in the elimination of the biochemical wastes released by the metabolism of the organism and pointed towards an intrinsic protective role of Abs under physiological conditions. This role is independent of the capacity of Abs to neutralize circulating exogenous Ags, to facilitate their endocytosis by Ag presenting cells (APCs) and to participate in their elimination from the organism [9].
Abs endowed with enzymatic properties have been described in human autoimmune manifestations for more than a decade in a variety of disorders such as autoimmune thyroiditis, systemic erythematosus (SLE), scleroderma, rheumatoid arthritis (RA), and acquired hemophilia (AH). Abs isolated from these conditions were able to specifically hydrolyze thyroglobulin, DNA, RNA, and factor VIII (FVIII) or factor IX (FIX), respectively. Most of the data accumulated through studies on natural catalytic autoAbs indicate that production scales up markedly in pathological abnormalities. DNA- and RNA-hydrolyzing Abs (DNA and RNA-abzymes) have been isolated from the serum of patients with different systemic autoimmune diseases: systemic lupus erythematosus (SLE), scleroderma, rheumatoid arthritis (RA) [10,11]. The first example of an abzyme under pathological conditions in bronchial asthma patients was a case, in which the Abs were able to cleave the vasoactive intestinal peptide (VIP) [12] (Figure 6). The first example of Ab-proteases was an IgG found in bronchial asthma (BA) patients to hydrolyse VIP which played a major role in the respiratory disfunction VIP, vasointestinal peptide Proteolytic Abs specific for thyroglobulin (Tg) have been reported in patients with thyroiditis. Amyloid b peptide (Ab)-hydrolyzing IgM Abs were recently found in the sera of patients with Alzheimer’s disease (AD).

Figure 6: The anti-VIP catalytic antibody binds a seven-amino acid subsequence of VIP distant from the cutting site (shown as a gap).
The origin of disease associated CatAbs under pathological conditions is far from clear. Disease associated CatAbs may have been "induced" by the Ag implicated in the disease. Secondly, the increased occurrence of CatAbs in pathology may result from the loss of repressive control over CatAbs-producing clones generated spontaneously under physiological conditions. A third explanation for the origin of CatAbs in pathological conditions is based on idiotypic network and exacerbated self-recognition in autoimmune diseases. The CatAbs may complement the general alteration of the immune response. In this respect, the pathological immune response may be directed towards different Ags, some of which are directly relevant to the disease, some of which are not, or alternatively, may be directed against a single Ag that may not be related to the disease. In this sense, proteolytic Abs (Ab-proteases) as the second stage of the discoveries in the area mentioned, would represent Abs to provide proteolytic effects. It is known that proteases precisely control a wide variety of physiological processes and thus are important drug targets.
A protease is a canonical enzyme that performs proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in a polypeptide chain. Proteases have evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms [10,13]. So, critical for diverse biological processes, proteases represent one of the largest families of potential and promising and druggable targets. The precise pathogenesis of MS as a complex autoimmune disease is still a mystery. Despite many studies that have been aimed to identify biomarkers, no marker has yet been approved for MS. And as it is known, canonical autoAbs play neither predictive nor discriminative role to affect the pre-early and/or subclinical stage of MS. So, there is urgently needed for biomarkers, which could clarify pathology (including sub clinical one), monitor disease progression, response to treatment, and prognosis in MS.
The current findings in biomarker research for MS demonstrate exciting progress toward the identification and validation of clinically useful biomarkers for this complex disease. Of particular interest are the biomarkers that reflect the underlying neurodegeneration and intrathecal inflammation driving progressive disease. Those biomarkers are especially relevant as new therapies aimed at neuroprotection and neural repair are developed, including stem cell based regenerative therapies. As this new class of treatments enters clinical trials, biomarker discovery and analysis should be done in parallel. Furthermore, as new biomarkers are discovered and validated, more powerful tools for statistical analysis and pattern detection will be required to identify combinations of biomarkers that best reflect clinical status.
In this context, immunomic analysis is a set of powerful tools to identify putative and novel candidate biomarkers. Different human compartments analysis using proteomics, cytomics, and bioinformatics approaches has generated new information for further clarification of MS pathology, elucidating the mechanisms of the disease, finding principally new targets, and monitoring treatment response. Overall, omics approaches can develop different therapeutic and diagnostic aspects of MS, from biomarker discovery to PPM. Briefly! MS is one of the chronic autoimmune inflammatory conditions resulting in a destruction of myelin by different tools, including autoAbs. And a spectrum of myelin associated autoAbs occurring in MS patients has been confirmed to be very large.
The crucial step in the pathogenesis of MS is a primary myelin damage which is mediated by autoAbs to trigger a release of separate and pathogenically valuable myelin-associated epitopes into the bloodstream. AutoAb biomarkers are useful in distinguishing subjects with the relapsing remitting form of MS from those with the secondary progressive subtype [14]. Among canonical autoAbs, for instance, anti-ganglioside Abs are considered important biomarkers to differentiate MS from other diseases that exhibit similar symptoms and used as a new and effective platform to screen blood samples for potential diagnosis of MS by assessing ganglioside Ab interactions and quantifications [15]. Abs against myelin oligodendrocyte glycoprotein (MOG) antigens have been found in the immunoreactive brain lesions of MS patients and proven to be used as a prognostic marker in the course of disease. However, the serum levels of those autoAbs during different phases of MS activity or after an immunomodulatory therapy have been poorly investigated.
Myelin Basic Protein (MBP)
Myelin basic protein (MBP) is highly expressed on the surface of myelin and is involved in maintaining myelin structure. Increased MBP in the CSF and sera of MS patients was an early marker suggested in MS. However, with MRI monitoring of patients, there is no clinical utility in measuring MBP levels in the CSF. Meanwhile, the likelihood of autoAbs and biochemical evidence of MS has been proven to be proportional to the presence of antimyelin autoAbs, and anti-MBP Abs, in particular! So, determination of the level of antimyelin autoAbs in blood serum of patients with MS might serve as a biomarker of inflammatory and, probably, of the neurodegenerative processes of this disease and determine the dynamics of clinical course of the MS. Following the above mentioned, for instance, anti-MBP Abs play an important role in the pathogenesis of the MS and are an additional marker of the severity of the clinical course of neurological and some neuropsychological disorders.
Whereas the identification of an optimal MS biomarker will provide advantages in terms of choosing pre-early (subclinical) preventive treatment options for newly diagnosed patients, the design of personalized treatment for patients, and lower the cost of clinical trials substantially, the heterogeneity of disease in MS makes biomarker identification more challenging. And thus, for a complex and heterogeneous disease like MS, a single marker or a biomarker with its single function is not effective, and thus efforts should be made toward combinatorial analysis of biomarkers or multifunctional biomarkers to create signatures for MS disease diagnosis, prognosis, and treatment response [16].
In this context, along with canonical Abs, some of the families proven to occur are Abs possessing with catalytic activity (catAbs or abzymes) and thus to belong to Abs with a feature of functionality. As regard CatAbs, it is multivalent Igs, presumably, of IgG isotype, endowed with a capacity to hydrolyze the Ag substrate. The property mentioned is buried in the Fab fragment of the Ig molecule (Figure 7A&B), Whole IgG molecules are composed of two Ag-binding domains (Fab) and one effector domain (Fc). Cleavage with the appropriate enzymes can create either an FAb (left) or 2 Fabs joined by a disulfide bond (e.g., F(ab’)2, right). Fab’s and F(ab’)2s have one and two Ag binding domains, respectively. Neither has the Fc domain that mediates Ab effector function such as Fc receptor binding, complement mediated cytotoxicity or Ab-dependent cellular cytotoxicity.
Ig, immunoglobulin; Fab, is the region on an immuno globulin molecule that binds to antigens. A Fab fragment consists of four domains: VH, CH1, VL and CL1. The Fab fragment has 440-450 amino acids and its molecular weight is about 47-48 kDa. Monomeric immunoglobulins are cleaved by the enzyme papain into two Fab fragments and one Fc fragment. Each Fab fragment consists of two amino acid chains, a light chain and a heavy chain. They are each composed of a variable (V) and a constant (C) antibody domain. Fab fragments are used in medicine as diagnostics and therapeutics.
CatAbs (or abzymes) are multivalent Igs, presumably, of IgG isotype, endowed with a capacity to hydrolyze the Ag substrate. In this sense, proteolytic Abs (or Ab-proteases) as a significant portion of the big family of abzymes represent Abs endowed with a capacity to provide targeted proteolytic effect; Ig, immunoglobulin; Fab, is the region on an immunoglobulin molecule that binds to antigens. A Fab fragment consists of four domains: VH, CH1, VL and CL1. The Fab fragment has 440-450 amino acids and its molecular weight is about 47-48 kDa. Monomeric immunoglobulins are cleaved by the enzyme papain into two Fab fragments and one Fc fragment. Each Fab fragment consists of two amino acid chains, a light chain and a heavy chain. They are each composed of a variable (V) and a constant (C) antibody domain. Fab fragments are used in medicine as diagnostics and therapeutics.
And is appearing to sound as a functional property of the Ab molecule. In this sense, proteolytic Abs (or Ab-proteases) as a significant portion of the big family of abzymes represent Abs endowed with a capacity to provide targeted proteolytic effect and thus to belong to Abs with functionality (Figure 8)! The activity of Ab-proteases markedly differs between MS patients and healthy controls (Figure 9) The activity of Ab-proteases demonstrated significant correlation with scales of demyelination, neurological deficiency and thus with the disability of the patients (as ween from the EDSS scores) (Figure 10A &B).

Figure 8: Catalytic antibodies (abzymes) as bi-functional biomolecules integrating antibody specificity and catalytic power.
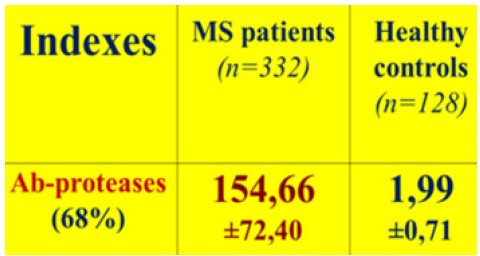
Figure 9: The activity of MBP-targeted Ab-proteases in MS patients.
Note*: MBP, myelin basic protein; Ab, antibody; MS, multiple sclerosis
Some of the MBP-targeted Ab-proteases were found to demonstrate their highest activity in MS patients with the most severe (progradient, progression phase and/or remittent course, exacerbation phase) clinical courses [17]. The other ones whilst being less immunogenic were shown to be attacked by Ab-proteases in MS patients with moderate (remission-type and/or stabilization) clinical courses (Figure 11).
Meanwhile, the greater value is a sequence specificity of Ab-proteases. Ab-mediated proteolysis of MBP was shown to be sequence-specific whilst demonstrating five sites of preferential proteolysis to be located within the immunodominant regions of MBP and to fall inside into 5 sequences fixed (Figure 12). Abs against MBP with proteolytic activity exhibiting sequence specific cleavage of MBP are of great value to monitor demyelination whilst in MS. As we have established, the most immunogeneic and encephalitoge-neic sites of immunodominant category responsible for generating aggressive bursts of demyelination are concentrated in three areas of MBP molecule (Figure 13A-C): Sequence-specific Ab-proteases have proved to be greatly informative and valuable as biomarkers to monitor MS at both subclinical and clinical stages! The activity of Ab-proteases was first registered at the subclini-cal stages 1-2 years prior to the clinical illness (Figure 14).
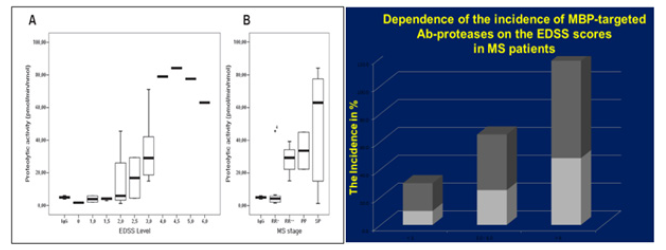
Figure 10: The correlation of BP-targeted Ab-associated proteolytic activity with scales of demyelination,neurological deficiency and thus with the disability of the patients.
Note*: And, finally, the activity demonstrated also significant correlation with scales of demyelination, neurological deficiency and thus with the disability of the patients (it is seen from the EDSS scores) EDSS, The Expanded Disability Status Scale (EDSS) is a method of quantifying disability in multiple sclerosis and monitoring changes in the level of disability over time. It is widely used in clinical trials and in the assessment of people with MS. The EDSS scale ranges from 0 to 10 in 0.5 unit increments that represent higher levels of disability.

Figure 11: Dependence of the incidence of MBP-targeted Ab-proteases on the EDSS score in MS patients.
Note*: MS, multiple sclerosis; Ab, antibody; MBP, myelin basic protein; EDSS, The Expanded Disability Status Scale (EDSS) is a method of quantifying disability in multiple sclerosis and monitoring changes in the level of disability over time. It is widely used in clinical trials and in the assessment of people with MS. The EDSS scale ranges from 0 to 10 in 0.5 unit increments that represent higher levels of disability. Scoring is based on an examination by a neurologist.
The activity of Ab-proteases is first registered at the pre-early (subclinical) stages of the disease, when Ab-proteases are low-active, the inflammation is minimized, and the manifestations are thus moderate. Apart from MS patients with the diagnosis confirmed, about a quarter of the direct MS-related relatives were initially seropositive for anti-MBP autoAbs having no proteolytic activity (“disarmed” Abs) and MBP-specific but low-active Ab-proteases, respectively. Neither of those seropositive relatives regardless to type of Abs demonstrated neither clinical manifestations nor instrumental or laboratory signs of MS [18]. At the initial steps of MS and in moderate forms, Ab-proteases would attack presumably low-immunogeneic epitopes. And thus low-active Ab-proteases are typical for either subclinical stages of MS (Figure 15) or for MS ost of the seropositive relatives established, were demonstrating a stable growth of the MBP-targeted Ab-associated proteolytic activity during the evolution of the disease (Figure 16A &B).
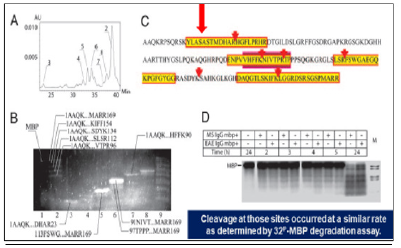
Figure 12: Sequence specificity of the MBP-targeted proteolysis by anti-MBP autoAbs harvested from MS patients.
Note*: Ab-mediated proteolysis of MBP results in generating a set of peptides with MW ranged in various but fixed boundaries to suit common principles of the molecular architectonics of MBP. The final statistical data revealed FIVE sites of preferential proteolysis MS, multiple sclerosis; MB, myelin basic protein; Ab, antibody.
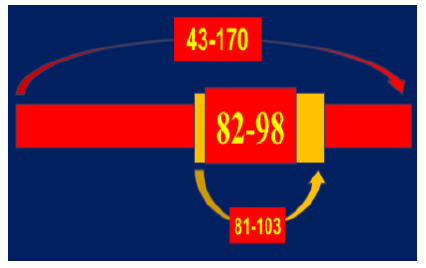
Figure 13: Sequence-specific architecture of MBP mole-cule for MBP-associated proteolytic attacking. (A) The strongest sites for MBP-targeted Ab-proteases-mediated attacking is against the smallest 82-98 subsequence in MBP molecule. (B) The weaker site for MBP-targeted Ab-proteases-mediated attacking is formed by a longer 81-103 sub-sequence in MBP molecule. (C) A site with the lowest immunogeneic and encepha-litogeneic properties for MBP-targeted Ab-proteases-mediated attacking is formed by a 143-170 sequence defined
Note*: MBP, myelin basic protein; Ab, antibody

Figure 14: The activity of MBP-targeted Ab-proteases during the evolution and progression of MS: at the pre-early (sub-clinical) and clinical stages.
Note*: MS, multiple sclerosis; Ab, antibody; MBP, myelin basic protein.y
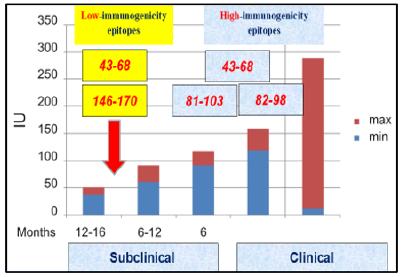
Figure 15: The distribution of the activity of MBP-targeted Ab-proteases in different courses of MS.
Note*: MS, multiple sclerosis; Ab, antibody; MBP, myelin basic protein.

Figure 16A: Stable growth of the MBP-targeted Ab-associated proteolytic activity during 2-3 years under the study.
Note*: TWO THIRD of the MS relatives being seropositive for Ab-proteases monitored for 2-3 years have been demonstrating stable growth of the MBP-targeted Ab-associated proteolytic activity during the time span being under the study IU, international units; Ab, antibody; MBP, myelin basic protein.

Figure 16B: Evolution of MBP-targeted Ab-associated proteo-lytic activity at the subclinical and clinical stages of MS progression.
Note*: IU, international units Clinical, the stage of a disease that is rich of clinical manifestations and based on laboratory, clinical and instrumental tests, including physical exams, imaging tests, laboratory tests and biopsies;
At the progression of MS and/or in the aggressive forms, Ab-proteases would attack presumably high-immunogeneic epitopes. And thus, highly active Ab-proteases are typical for the stages with full-scale clinical manifestations or/and for MS aggressive types. Moreover, in exacerbations of the remittent course (ERP) or in secondary-progredient course progression phase (SPPP), the highest activity of Ab-proteases to attack the highly immunogenic epitopes occur!!! And when bursts of the proteolytic activity were evident, the pre-early stages of the exacerbation could be predicted, even at no seeing any clinical manifestations (Figure 17).
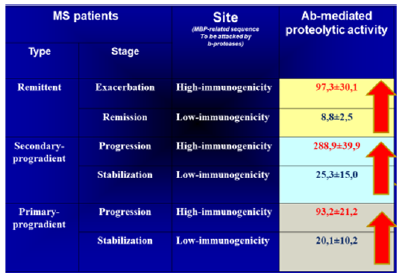
Figure 17: Distribution of MBP-targeted Ab-associated proteolytic activity in different courses of MS.
Note*: MS, multiple sclerosis; MBP, myelin basic protein. The red arrow indicates a tendency of MBP-targeted Ab-associated proteolytic activity to grow in a direction of transforming of moderate (remission or stabilization courses with dominating low-active Ab proteases) into more aggressive (exacerbation and progression with dominating highly active Ab proteases) ones.
Registration in the evolution of highly immunogenic Ab-proteases would illustrate either risks of transformation of subclinical stages into clinical ones, or risks of exacerbations to develop. And along with the evolutionary changes of the sequence specificity, when we saw an extensive growth of the activity, we could predict transformations into the clinical course, i.e., changing of a remitting/stabilization course (moderate one) into the exacerbation/progression course (aggressive one) prior to changing of the visible clinical manifestations (Figure 18)! That “immune escalation” illustrating reorienting of the sequence specificity to accent the more important targeted sites for proteolysis might be an early prognostic and predictive sign of progressing demyelination and thus the clinical illness to come. Therefore, the proposed predictive value of MBP-targeted Ab-proteases for the development of MS is being challenged! So, the activity of Ab-proteases and its dynamics tested would confirm a high subclinical and predictive value of the tools as applicable for personalized monitoring protocols [1,20].

Figure 18: MBP-targeted Ab proteases as biomarkers to predict and to prognosticate the evolution and progression of MS following changes of subclinical and clinical stages on one hand, and stages of immune-related conditions on the other one.
Note*: MS, multiple sclerosis; MBP, myelin basic protein; Ab, antibody.
Discussion
The primary translational potential of this knowledge is in the rational design of new diagnostic tools and therapeutics to exploit the role of the key pathways in influencing disease. In this sense, catAbs (abzyme) is a type of Abs with catalytic activity being found not only in healthy humans and but also in patients with autoimmune diseases. Studying abzymes and Ab proteaes, in particular, can provide important insights into bifunctional reaction mechanisms and the immune system itself. Naturally, it is the emergence of nanotechnology, specifically, the design of new diagnostic tools and new targeted therapeutics based on principles of natural and artificial bifunctional biocatalysts and drug design [21].
The traditional goal of Ab engineering is to combine various Ab domains to generate customized Abs that show specialized binding properties, optimal half-lives and desirable effector functions. Abs can be engineered to make proteins of higher affinity or smaller molecular variants that retain or change the functional properties of the original Ab. So, Ab-proteases as biomarkers and potential targets on one hand, and integration of diagnostics with therapeutics on the other one, are becoming important for the selection and monitoring of individualized (targeted) treatments [22,23]. And thus MBP-targeted Ab-mediated proteolysis could be applied to isolate from Ig molecules catalytic domains directed against encephalitogenic autoepitopes or domains containing segments to exert proteolytic activity.
Of tremendous value are Ab-proteases directly affecting remodeling of tissues with multilevel architectonics (for instance, myelin). By changing targeted sequence specificity, one may reach reduction of a density of the negative proteolytic effects within the fixed sites of myelin sheath and thus minimizing scales of demyelination. So, further studies on Ab-mediated MBP degradation and other targeted Ab-mediated proteolysis may provide biomarkers of new generations and thus a supplementary tool for assessing the disease progression and predicting disability of the patients and persons at risks. Abs can be engineered to make proteins of higher affinity or smaller molecular variants that retain or change the functional properties of the original Ab. For instance, prodrug activation by CatAbs conjugated with targeted Abs, called Ab-directed abzyme prodrug therapy (ADAPT), might be proposed as a strategy for site specific drug delivery systems for myelin degradation preventing drugs. To achieve ADAPT, we should focus on specific requirements for prodrugs and CatAbs, the stability of the prodrugs against natural enzymes, and the applicability of abzymes for a wide range of prodrugs [21,24].
Moreover, Ab-proteases can be programmed or/and re-programmed to suit the needs of the body metabolism or could be designed for the development of principally new catalysts with no natural counterparts. And the next important step in the direction of the innovation-based approach should be their early adoption in clinics for future medical interventions!
In this sense, neurodegenerative diseases are promisingly suited models for PPM because of the rapidly expanding Hi-Tech innovations and translational resources including ABZYMES technologies and the development of biomarkers and the potential modifying targeted treatments. In this context, the development in neurology as a PPM approach could represent an excellent possibility to identify subclinical stages of disease, make adequate differential diagnosis (including susceptibility related, screening and monitoring, predictive, prognostic manipulations) and provide timely and optimal treatments instead of the traditional treatments which are utilized at any stages of the disease (Figure 19) [25].
Figure 19: Targeted Ab proteases as applicable to securing the needs of the daily clinical practice.
Note*: Ab, antibody.
With advances in our understanding of Ab-protease functions and properties coupled with improvements in immune and protein engineering, we can expect that Ab protease based diagnostic tools, therapeutics and theragnostic will gain regulatory approval and make significant contributions in healthcare in the near term.
Conclusion
We are experiencing a Renaissance primarily driven by next generation biotechnologies. In this sense, neurodegenerative diseases being inflammatory demyelinating disorders of the central nervous system (CNS), are being triggered by a complex interplay between genetic and environmental factors, in which the precise molecular pathogenesis remains to be comprehensively characterized, are promisingly suited models for PPM because of the rapidly expanding Hi-Tech innovations and translational resources including AB-ZYMES technologies and the development of biomarkers and the potential modifying treatments. In this context, the development in neurology of a PPM approach could represent an excellent possibility to identify subclinical stages of disease, make adequate differential diagnosis and provide timely and optimal treatments instead of the traditional treatments which are utilized at later stage of disease [25].
At this moment, highly efficacious treatments of autoimmune disorders carry serious potential risks, which need to be compared with weighing up the risk of treatment with the risk of irreversible disability. So, there is therefore an unmet need for a biomarker or a panel of biomarkers which can be employed pre-early in the disease (MS, for instance) to identify those at greatest risk of future disability. New technologies and techniques have the potential to address this unmet need but require careful analysis in large cohorts, whilst exploring the potential role of next generation biomarkers in the pre-early relapsing remitting MS.
Auto-Abs are really the key agents involved in the destruction and degeneration of myelin in the CNS (in MS, predominantly) and expansion of autoimmune responses, and pro-inflammatory aggression. And on the basis of the ample data in conventional Abs proteases and Abs with functional reserve in diagnosis of minor lesions occurring in MS patients as well as differentiated diagnosis, we are aimed to identify and thus to pre-select the crucial antigenic targets for autoimmune attack and their prospects as a valuable tool for detecting of dormant pathological processes in the myelin sheath at both clinical and subclinical stages. Meanwhile, as it can be inferred from above mentioned material, Ab-proteases by virtue of its peculiar and bi functional properties, circulate in peripheral blood for years before clinical manifestation. Subsequently, using Ab-proteases as biomarkers, we can define prospective for further progression of current premorbid state to the stages of profound clinical symptoms of MS and blockade it by time-lapse introducing of modern (including abzyme-driven upgraded) predictive and preventive therapeutic protocols.
So, serological assay based on the identification of highly specific immune biomarkers and monitoring of their spectra are fundamental principles of PPM and PPM-related neurological practice. Further search for prospective biomolecules (like, Ab-proteases, for instance) and description of their potential role regarding the principles of subclinical diagnosis is especially important for their capable application and impact in clinical and subclinical neurology related practice [26]. Further studies on Ab-mediated MBP degradation and other targeted Ab-mediated proteolysis may provide biomarkers of the next step generations and thus supplementary tools for assessing the disease progression and predicting disability of the patients and persons at risks. In this context, the complex etiology and multiple pathogenesis of neurodegenerative diseases call for a system level understanding of the currently available biomarkers and the study of new biomarkers via network-based modelling of heterogeneous data types. The latter will provide key insights to fully understand the network degeneration hypothesis (disease starts in specific network areas and progressively spreads to connected areas of the initial loci-networks) with a potential impact for the pre-early diagnosis and diseasemodifying targeted treatments [27].
The present viewpoints mentioned in the pioneering publications, highlight the potential of network-based approaches as complementary methods for disease biomarkers to better predict disease course and monitor treatment effects [28]. We believe that those findings may provide a framework for future studies with the aim to bridge the gap between molecular biomarkers and symptomatology. In this sense, the above mentioned promising tools are needed to secure artificial or edited Ab-proteases as unique translational probes to diagnose, to monitor, to control and to treat and rehabilitate MS patients at clinical stages and to prevent the disorder at subclinical stages in persons at risks to secure the efficacy of regenerative manipulations.
References
- TA Bodrova, DS Kostyushev, EN Antonova, Sh Slavin, DA Gnatenko, et al. (2012) Introduction into PPPM as a new paradigm of public health service: an integrative view, EPMA Journal 3(16): 3-16.
- Julia Maroto García, Ana Martínez Escribano, Virginia Delgado-Gil, Minerva Mañez, Carmen Mugueta, et al. (2023) Biochemical biomarkers for multiple sclerosis. Clinica Chimica Acta 548: 117471.
- Ameneh Jafari, Amirhesam Babajani, Mostafa Rezaei-Tavirani (2021) Multiple Sclerosis Biomarker Discover-ies by Proteomics and Metabolomics Approaches. Biomarker Insights 16: 1-14.
- Alexander G Gabibov, Natalya A Ponomarenko, Eugenia B Tretyak, Mikhail A Paltsev, Sergey V Suchkov, et al. (2006) Catalytic autoantibodies in clinical autoimmunity and modern medicine 5(5): 324-330.
- Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA Jr, Kurkova IN, et al. (2006) Autoanti-bodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci U S A 103(2): 281-286.
- Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA, Telegin GB, et al. (2006) Catalytic activ-ity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale103:45-50.
- Weber MS, Hemmer B, Cepok S (2011) The role of antibodies in multiple sclerosis. Biochim Biophys Acta 1812(2): 239-245.
- Tramontano A, Janda KD, Lerner RA Catalytic antibodies Science 234: 1566.
- Pollack SJ, Jacobs JW, Schultz PG (1986) Selective chemical catalysis by an antibody. Science 234(4783): 1570-1573.
- Friboulet A, Avalle B, Debat H, Thomas D (1999) A possible role of catalytic antibodies in metabolism. Immunol Today 20(10): 474-475.
- Shuster AM, Gololobov GV, Kvashuk OA, Bogomolova AE, Smirnov IV, et al. (1992) DNA hydrolyzing autoantibodies. Science. 256(5057): 665-667.
- Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, et al. (1989) Catalytic hydrolysis of vasoactive intesti-nal peptide by human autoantibody. Science 244(4909): 1158-1162.
- Bharath Wootlaa, Sébastien Lacroix-Desmazesc, Arthur E Warringtona, Allan J Biebera, Srini V Ka-veric, et al. (2011) Autoantibodies with Enzymatic Properties in Human Autoimmune Diseases. Autoim-mun 37(2): 144-150.
- Cassandra De Marshalla, Eric L Goldwasera, Abhirup Sarkara, George A Godseya, Nimish KAcharyad, et al. (2017) Autoantibodies as diagnostic biomarkers for the detection and subtyping of multiple sclerosis. J Neuroimmunology 309: 51-57.
- Alexander S Malinick, Alexander S Lambert, Daniel D Stuart, Bochao Li, Ellie Puente, et al. (2020) De-tection of Multiple Sclerosis Biomarkers in Serum by Ganglioside Microarrays and Surface Plasmon Resonance Imag-ing. ACS Sens 5(11): 3617-3626.
- Anu Paul, Manuel Comabella, Roopali Gandhi (2019) Biomarkers in Multiple Sclerosis. Cold Spring Harb Perspect Med 9(3): 1-22.
- Yang Xu, Noboru Yamamoto, Kim D Janda (2004) Catalytic antibodies: hapten design strategies and screening methods. Bioorg Med Chem 12(20): 5247-5268.
- Desirazu N Rao Bharath, Wootla Bharath (2007) Catalytic antibodies: Concept and promise. Resonance 12: 6-21.
- Sonia Diasab, Florence Jovicac, Pierre Yves Renard, Fréderic Taranc, Christophe Créminona, et al. (2002) Immunologically driven chemical engineering of antibodies for catalytic activity. J Immunol Methods 269(1-2): 81-98.
- Séverine Padiolleau Lefèvre, Raouia Ben Naya, Melody A Shahsavarian, Alain Friboulet & Bérangère Avalle, et al. (2014) Catalytic antibodies and their applications in biotechnology: state of the art. Biotechnol Lett 36(7): 1369-1379.
- Dimiter S. Dimitrov (2019) From Catalytic Antibodies to Antibody-Drug Conjugates. Cell Chem Biol 26(9): 1200-1201.
- WB Motherwell, MJ Bingham Y Six (2001) Recent progress in the design and synthesis of artificial enzymes. Tet-rahedron, 57(22): 4663-4686.
- Ruth D Mayforth, José Quintáns (1990) Designer and Catalytic Antibodies. The NEW ENGLAND JOURNAl of MEDICINE 323: 173-178.
- SV Suchkov, ZS Alekberova, FN Paleev, TE Naumova, VK Misikov, et al. (2005) Achievements and prospects of clinical abzymology. Vestn Ross Akad Med Nauk (9): 38-43.
- Dmitry Kostyushev, Ivan Tsarev, Dmitry Gnatenko, Mikhail Paltsev, Sergey Suchkov (2011) Myelin-associated serological targets as applicable to diagnostic tools to be used at the preclinical and transient stages of multiple sclerosis progression. Open Journal of Immunology 1(3): 80-86.
- Sarah Jane Martin (2021) NEXT GENERATION BIOMARKERS TO UNDERSTAND EARLY MULTIPLE SCLE-ROSIS. Degree of Doctor of Philosophy Institute of Infection, Immunity and Inflammation College of Medical, Veterinary & Life Sciences University of Glasgow 1-175.
- (2021) Thalis Charalambous. Investigating structural network disruption in multiple sclerosis Thesis presented for the degree of Doctor of Philosophy of the University College London 1-207.
- Maria A Rocca, Paola Valsasina, Alessandro Meani, Elisabetta Pagani, Claudio Cordani, et al. (2021) Network Damage Predicts Clinical Worsening in Multiple Sclerosis. Neurol Neuroimmunol Neu-roinflamm 8(4): 1-12.








 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.