Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluation of Interleukin-1β, Interleukin-6 and Interleukin-10 Profiles in Serum and Surgically Created Wound Exudates of Alloxan-Induced Diabetic Rabbits
*Corresponding author:Ifeadi Chekwube Nkemjika, Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Abuja, Nigeria.
Received: March 20, 2024; Published: March 26, 2024
DOI: 10.34297/AJBSR.2024.21.002908
Abstract
The knowledge of immune-modulatory actions of interleukins could be of immense value in the management of diabetic wounds. This study evaluated the interleukin profiles of diabetic wounded rabbits. Sixteen New Zealand White rabbits of mean weight 2.5±0.7 kg used for this study were divided into four groups: A, B, C and D, of four rabbits each A (non-diabetic and nonwounded) as control, B(diabetic and non-wounded, C (wounded and non-diabetic) and D (wounded and diabetic). Three (3)cm2 skin wounds were created in a standard aseptic procedure while diabetes was induced by administration of alloxan monohydrate. Serum and wound exudate were harvested on days 0,3,7,14,21 and 28 for Interleukin-1β, Interleukine-6 and Interleukine-10 assays. The increases in the serum concentration of Interleukin-1β and Interleukine-6 in the C rabbit group between days 3 and 7 (Interleukin-1β) and between days 3 and 28 (Interleukine-6) were relatively higher than the corresponding day 0 and control values. The decrease in the serum concentration of IL-10 (ng/mL) in B rabbit group from days 7 (36.96±8.7) to 14 (128.12±12.05) were significantly lower (P˂0.05) than the corresponding values in groups A and C rabbits. The values of Interleukine-10 in wound exudate of C rabbit group from days 7(160.57±23.76ng/mL) to 21 (177.30±8.60 ng/mL) were significantly higher (P<0.05) than the corresponding group D values. The evidence shows that, the values of Interleukin-1β and Interleukine-6 are up regulated in the inflammatory phase of wound healing in normoglycaemic and diabetic rabbits while the values of Interleukine-10 is down regulated during wound healing in diabetic rabbits. Therefore, the knowledge of interleukin markers shall be of significant roles in proffering solution for management of normoglycaemic and diabetic wounds.
Keywords: Diabetic wounds, Interleukin-1β, Interleukine-6 and Interleukine-10
Introduction
Cytokines are classes of biologically active low molecular weight proteins with hormone-like and or enzyme-substrate actions that play important roles of modulating the intensity, actions, production and duration of immune and physiologic cells of the body for effective response to the environment [1]. Among various classes of these cytokine is the most abundant class that stimulates immune cell proliferation known as the interleukin family. Interleukins are subsets of cytokines secreted by leukocyte and other non-hematopoietic cells including: helper CD4+, T lymphocytes and monocytes, and acts on another type of leukocyte to produce numerous effects [2,3]. They activate T cells by stimulating the proliferation of antigen-activated T and B cells and by stimulating proliferation and differentiation of B cells and Interferon-gamma (IFN-γ) which activates macrophages, Granulocyte Monocyte Colony-Stimulating Factor (GM-CSF) and consequently stimulate hematopoiesis [4]. Interleukins play important roles in the complex signaling network of activities involved in wound healing [1]. The multiple factors that influence macrophage phenotype and other cells expressed in wounds are partly determined by the relative balance between the pro-inflammatory and anti-inflammatory stimuli present in the wound environment [5].
Wound healing is a fundamental, dynamic, coordinated, interdependent and overlapping cellular and immunologic response of tissue to injury [6-8]. It is essential to prevent pathogenic invasion of damaged tissues and to reform the affected tissues partially or completely [7,8]. Diabetes is a metabolic and heterogeneous disorder characterized primarily by hyperglycemia due to deficiency of insulin or insensitivity of insulin receptors for normal processes of glucose metabolism in the body [9,10]. The hyperglycemia is manifested as glycosuria, hyperlipidemia, polyuria and polydipsia [10,11]. Diabetes has remained one of the leading causes of death, illness and economic losses in the world [12]. It is associated with delayed and chronic wound complication that has continued to demand medical attention and poses great challenges to clinicians [13]. Interleukin-1β(IL-1β) is one of the pro-inflammatory cytokines of the interleukin family that is regularly detected in wounds [14]. It is produced by the monocytes, macrophages and the B-cells, and it exerts its effect on various cells of the body [15]. IL-1β stimulates inflammation by increasing mobilization of leukocytes from the bone marrow and secretion of acute-phase proteins from the liver while facilitating proliferation and maturation of other cells [4]. Interleukin-6 (IL-6) has both pro and anti-inflammatory effects and has also been detected at a higher quantity in a chronic wound [16,17]. It plays an active role in the production of acute phase protein and stimulates the production of cellular and humoral immune responses such as B-cell differentiation, T-cell and immunoglobin G secretion. In acute phase, IL-6 plays the dual roles of suppressing the level of pro-inflammatory cytokines while potentiating the anti-inflammatory cytokines [18,19].
Interleukin-10 (IL-10) is one of the most important anti-inflammatory cytokines in wound healing. It is produced and expressed in wound sites by keratinocytes of wound epidermis and infiltrating monocyte cells [20]. IL-10 inhibits the infiltration of macrophages and terminates other inflammatory responses while regulating the differentiation of various immune cells, keratinocytes and other endothelial cells. It is considered anti-fibrotic because of its anti-inflammatory activity and as an inhibitor of Tumour Necrotic Factor (TNF-α). Researchers have focused on understanding the functions of interleukin family of cytokine in wound healing for effective management of wounds [21]. The interleukin profile of animals may vary not only according to species, sex, age, breeds but also based on the health status of the animals and environment where they are located, and this has made it a subject of interest in different fields of studies [16,17,22]. Few reports are available on the interleukin profile of wounded diabetic rabbits [23]. Understanding the immune-modulatory actions of interleukins and their variation in diabetic wound complications, no doubt shall give insight towards the study and management of the wounds [24,25]. It is expected that interleukin markers can be of significant roles in the study of normoglycaemic and diabetic wounds and in proffering solution to the management of the wound [1]. This study therefore, aimed at establishing the IL-1β, IL-6 and IL-10 profiles in serum and surgically created wound exudates of alloxan-induced diabetic rabbits.
Materials and Methods
Ethical clearance was obtained for this study from Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC), (Reference number: ABUCAUC/2019/028). This study was carried out in the Department of Veterinary Surgery and Radiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Kaduna State, Nigeria. Sixteen adult New Zealand White (NZW) rabbits of both sexes, aged 120±0.4 months and weighing 2.5±0.7kg were used for this study. They were procured from the National Animal Production and Research Institute (NAPRI), Zaria, Kaduna State, Nigeria. The rabbits were kept in intensive management system in separate cages and fed morning and evening daily with combined poultry grower feeds, finisher feeds (Grand Cereals Limited (R), Jos, Plateau State) and miller’s bran that were mixed in equal proportion in order to regulate the blood glucose level of the hyperglycaemic groups. Daucus carota subsp. sativus leaves was given to the rabbits twice weekly to avoid gastro-intestinal impaction and constipation while water was given ad-libitum throughout the experimental period. The rabbits were conditioned to the laboratory for two weeks before the commencement of the experiment. During this period, they were administered with anthelmintic Ivermectin (Hebei, Kexing pharmaceutical, China) prophylaxis at dosage of 0.4mg/kg start, and repeated after two weeks to prevent mites and other internal and external parasites. The rabbits were preliminarily clinically evaluated using body condition scores, vital and haematological parameters and adjudged apparently healthy before commencement of the study.
Experimental Protocols
The rabbits were divided into 4 groups (A, B, C and D) of 4 rabbits each comprising of two males and two females in each group. Group A (control): No Diabetes was induced and No Wound was created (NDNW); Group B: Diabetes was induced in this group, but No Wound was created (DNW); Group C: Wound was created, but Diabetes was Not induced (WND) and Group D: Wound was created and Diabetes was induced (WD), respectively.
Induction and Confirmation of Diabetes
Diabetes was induced by intravenous administration of 200mg/kg of Alloxan monohydrate (SIGMA-aldrich, UK) through the marginal ear vein at two occasions (100mg/kg each) at 72 hours intervals [12,26]. Using a glucometer (ACCU-CHEK (R), Roche, Mannheim, Germany), diabetes was confirmed by rise in blood glucose level above normal value of NZW rabbits (150g/dl), to constant values of 250-350mg/dl between day 3 and 28 post alloxan induction [12].
Wound Creation
The dorsum of each of the four rabbits in the NDW (group C) and WD (group D) were prepared aseptically following standard operating procedure. The rabbits were anaesthetized by intramuscular injection of xylazine hydrochloride (Biovetaa.s(R), Czech Republic) at 7mg/kg and Ketamine Hydrochloride (Laborate pharmaceutical, India at 50 mg/kg [27,28]. A full-thickness skin wounds of 3 cm2 was created at the dorsum of each of the rabbits using a template designed from x-ray film [29]. The wounds were bandaged with sterile gauze and re-dressed only on the days of sample collections (days 0,3,7,14,21 and 28) [30].
Serum Sampling
Two millilitres of blood was collected from the ear vein of each of the rabbits on day 0 (before wound creation) and on days 3, 7, 14, 21 and 28 post-surgery [30]. The blood was dispensed into a sterile non-EDTA plastic test tube and allowed to clot at room temperature for 1 hr. The serum was then separated by centrifugation at 2000g for 10 min, after which the serum was harvested and preserved frozen at-200C until needed for analysis [31]. The serum was used for (interleukin) assays; interleukin-1 beta (IL-1β), interleukin- 6 (IL-6) and interleukin-10 (IL-10).
Wound Wash Sampling
Half (0.5) a millilitre of normal saline was used to moisten the wounds and the wound effluent collected using a sterile swab. The swab was washed in sample bottles containing 2ml of normal saline and centrifuged at 4, 500xg for 15 minutes and the sediment was collected in a sterile container [32,33]. The sediment was preserved at-200C in NAPRI Biotechnology Research Laboratory ABU Zaria, Nigeria until required for interleukin assay; interleukin 1 beta (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) analysis.
Interleukins Evaluation
The inflammatory cytokines; interleukins-1β (IL-1β), interleukins-6 (IL-6) and interleukins-10 (IL-10) were evaluated in the serum and wound exudate using the Elabscience Quantitative Cytokine Rabbit Enzyme-Linked Immunosorbent Assay (ELISA) Kits with preset minimum detectable levels of the cytokines as described by the manufacturers [34]. Cytokine (interleukins (IL-1β), interleukins-6 (IL-6) and interleukins-10 (IL-10)) rabbits ELISA Kit used was an in-vitro enzyme-linked immunosorbent assay for the quantitative measurement of rabbit cytokines (interleukins) in serum, plasma, exudates and cell culture supernatants. This assay employed a 96-well plate, tested and coated with an antibody specific for the rabbit cytokine. Standards and samples were pipetted into the wells and the cytokine (interleukins) present in a sample was bound to the wells by the immobilized antibody. The wells were washed and biotinylated and an anti-rabbit cytokine antibody was added. After washing away unbound biotinylated antibody, Horseradish Peroxidase (HRP)-conjugated streptavidin was pipetted to the wells. The wells were again washed in, 3, 3', 5', 5'-Tetra Methyl Benzidine (TMB) substrate solution and were then added to the wells where colours of different intensity developed in proportion to the amount of cytokine (interleukins) bound. The Stop Solution changed the colour from blue to yellow, and the intensity of the colour was measured at 450 nm.
Data Analysis
The data obtained from serum and wound exudate interleukin assay, were expressed as mean±Standard Error of Mean (SEM), and subjected to statistical analysis with Graph-Pad Prism version 5.03. The data of serum interleukins were analysed using repeated One-Way Analysis of Variance (ANOVA) while the data of wound exudate interleukins were analysed using Students’ T. Test, and Bonferroni post hoc. Values of P≤0.05 were considered significant. The results obtained were presented in tables and figures.
Results
Interleukin-1ß, Interleukin-6 and Interleukin-10 in the Serum
The mean±SEM peak serum IL-1β concentration were 239.92±5.78 in group B; 232.11±11.18 in group C and 239.21±5.91 in group D; all of which were observed on psd 3. These peak values decreased to their lowest concentration of 229.21±7.81 in group B, 219.42±18.44 in group C and 228.56±12.73 in group D (psd 21, 28 and 28) respectively. These peak values were also relatively higher than their corresponding day 0 values (229.94±8.85, 229.2±6.71 and 215.67±4.83) for groups B, C and D respectively (Table 1). The mean±SEM peak serum IL-6 concentration were 84.64±13.79 in group B; 81.88±12.49 in group C and 91.01±15.74 in group D; (psd 3,7and3) respectively. These peak values decreased to their lowest concentration of 66.10±5.72 in group B, 62.51±8.68 in group C and 76.08±15.66 in group D (psd 21, 28 and 21) respectively. These peak values were also relatively higher than their corresponding day 0 values (Table 2). However, these changes in the values of IL-1β and IL-6 throughout the post-surgery days were not statistically significant (P>0.05) between and across the group. The mean±SEM peak serum IL-10 concentration were 228.47±56.03 in group B and 215.10±43.68 in group D all of which on day 0 while the mean±SEM peak serum IL-10 concentration in group C increased from psd 3 (196.93±24.96) and peaked on psd 14 (225.12±31.57). These peak values in these groups decreased to their lowest concentration of 128.12±12.05 in group B; 183.39±29.37 in group C and 172.63±19.74 in group D (psd 14, 28 and 21) respectively. The value of IL-10 in group C from psd 3 (196.93±24.96 to 28 (183.39±29.37) were significantly higher (P˂0.05) than groups A and B (158.30±18.28 to 154.93±11.81 and 143.22±8.68 to 156.89±15.17) respectively (Table 3). Also, the mean IL-10 value in group C from psd 7 (206.64±47.79) to 14 (174.01±14.39) were significantly higher (P˂0.05) than the corresponding values in group D (140.44±10.29 to 174.01±14.39).

Table 1: The Mean Serum Interleukin-1β (ng /mL) recorded in the Different Experimental Rabbit Groups Post-Operatively.
Note*: Values are significantly different at P<0.05. Group A=Non-Diabetic and Non-Wounded (NDNW); Group B=Diabetic and Non-Wounded (DNW); Group C=Wounded and Non-Diabetic (WND); Group D=Wounded and Diabetic (WD).

Table 2: The Mean Serum Interleukin-6 (ng/mL) recorded in the Different Experimental Rabbit Groups Post-Operatively.
Note*: Values are significantly different at P<0.05. Group A=Non-Diabetic and Non-Wounded (NDNW); Group B=Diabetic and Non-Wounded (DNW); Group C=Wounded and Non-Diabetic (WND); Group D=Wounded and Diabetic (WD).
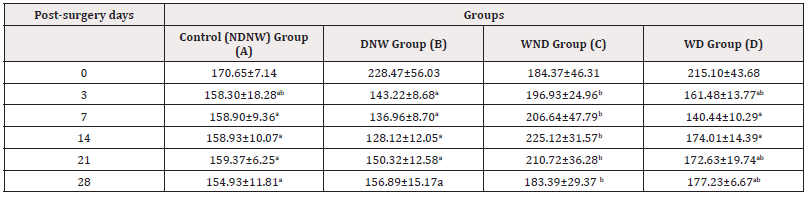
Table 3: The Mean Serum Interleukin-10(ng/mL) recorded in the Different Experimental Rabbit Groups Post-Operatively.
Note*: Values with the different superscript alphabets a,b,c are significantly different at P<0.05. Group A=Non-Diabetic and Non-Wounded (NDNW); Group B=Diabetic and Non-Wounded (DNW); Group C=Wounded and Non-Diabetic (WND); Group D=Wounded and Diabetic (WD).
Interleukin-1ß, Interleukin-6 and Interleukin-10 in Wound Exudates
The values of Interleukin-1β observed in wound exudate (WE) in group C was low on psd 3 (217.82±7.06), increased slightly on psd 7 (219.95±7.02) and subsequently declined on psd 14 (215.86±9.26) and 21 (212.89±14.46). On the other hands the values of IL-1β observed in WE in group D was highest on psd 3 (227.85±12.91), declined slightly on psd 7 (224.22±7.67) and 14 (222.92±16.18) but subsequently rose again on psd 21 (225.60±7.45) (Figure 1). The values of IL-1β observed in WE in group D appeared relatively higher than the value observed in group C throughout the study. However, there was no significant difference (P>0.05) between the values across the groups. The values of IL-6 observed in the wound exudate (WE) in group C was low on psd 3 (62.21±3.28), increased slightly on psd 7 (71.8±5.54) and remained higher on psd 21 (74.9±10.36). While the values of IL-6 observed in WE in group D rabbits was high on psd 3 (79.71±13.85), peaked on psd 7 (82.81±15.95) but declined progressively up to psd 21 (73.95±9.66). No wound exudate was collected on psd 28 (Figure 2). The values of IL-6 observed in wound exudate (WE) in group D appeared relatively higher than the values observed in the group C rabbits from psd 3 to 14. However, there was no significant difference (P>0.05) between the values across the groups. The values of IL-10 observed in WE in group C rabbits was low on psd 3 (149.00±9.34), peaked on psd 14 (188.79±6.17) and started declining on psd 21 (177.30±8.60). On the other hand, the values of IL-10 observed in WE in group D rabbits was high on psd 3 (153.33±14.31), decreased constantly to the lowest level on psd 14 (129.64±8.78) and subsequently peaked on psd 21 (155.82±4.70) (Figure 3). The value of IL-10 observed in the wound fluid (WE) of the rabbits in group (C) was significantly higher (P˂0.05) than the values observed in group D rabbits from psd 14 to 21.
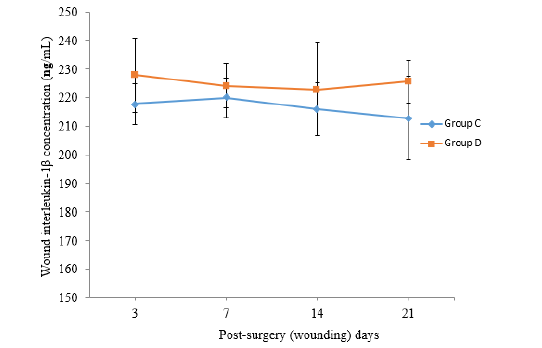
Figure 1: The Mean Wound Exudates Interleukin-1β (ng /mL) recorded in the Wounded Experimental Rabbit Groups (C and D).
Note*: Group C = Non-diabetic and Wounded (WND); Group D = Diabetic and Wounded (WD).
Discussion
The transient increase in the value of serum IL-1β on psd 3 in groups B, C and D might have resulted from acute inflammation which released IL-1β and other acute-phase protein from the liver. IL-1β was better expressed in this early stage of wounds probably because, it is beneficial at the acute phase of wound healing, as it secretes and regulates other proinflammatory mediators, activates neutrophils and subsequently, contributes to local inflammatory response [35-37]. reported a transient increase of serum IL-1β following musculoskeletal trauma. Downregulation of this serum IL-1β at the repair phase of the wound observed in group C (from psd 7) indicated progressive wound healing [36]. Observed downregulation of IL-1β in progressive wound healing from post-wound day 14 to 21 in rats. The prolonged upregulation of serum IL-1β and IL-6 with higher value in the WD group (up to psd 28) when compared with the DNW group (up to psd 14) might have resulted from combined skin wound and hyperglycaemia of diabetes which have severe pathologic implications that might have prolonged the inflammation and other preparatory phases of wound healing in the WD group. There is a positive co-relation between prolonged duration of inflammation and upregulation of IL-1β [36,38,39]. Also, Interleukin-6 is the principal regulator of most acute-phase protein genes and regulates local and systemic inflammatory responses, including the synthesis of hepatic acute-phase reactants like C-reactive protein in both hyperglycaemic and normoglycaemic wounds [38,39]. In addition, it has been documented that alloxan-induced diabetic rabbits have displayed obvious hyperglycaemia, delayed wound healing and significantly higher wound expression of IL-6 [40,41]. It has also been observed that regulation in circulating IL-6 level could be a key tool in the field of diabetic wound healing, and that diabetic insulin resistance β-cell inflammation correlated with upregulation of IL-6 [42,43].
The mean value of serum IL-6 was also relatively higher in non-alloxan group C from psd 3 to 21 when compared with the control. Such upregulation was possible because, in the acute phase of wound healing, IL-6 plays the dual roles of suppressing the level of pro-inflammatory cytokines while potentiating the anti-inflammatory cytokines [18]. IL-6 has a reputation for dictating the transition from inflammatory to repay phase in wound healing as it has stimulatory effects on T and B cells for cellular and humoral immune responses, while maintaining its inhibitory role on TNF and IL-1β production by macrophages [19]. Transient increase in the level of IL-6 from post wound days 2 to 6 following surgery have been reported by earlier researchers [37, 44, 45, 46]. The mean value of serum IL-10 observed in group C was significantly higher (P<0.05) than the control and B group (from psd 3 to 28) and D group (psd 7 and 14). The significant increase in the value of IL-10 in normal wound healing on psd 3 was in line with the report of previous researchers [47,48]. IL-10 is a known anti-inflammatory cytokine that should ideally be upregulated at the repair and maturation phase of a normal wound healing. IL-10 detection in abundance during the repair and late stage of wound healing in this study was probably due to its role in inhibiting the proinflammatory cytokines [49].
It was possible that Increased IL-10 reduced inflammation and created environment conducive for regenerative wound healing as alluded by [50]. The mean values of serum IL-10 detected in rabbits in groups B and D from post-surgery days 3 to 28 were relatively lower than the pre-surgery values. It might be argued that effects of diabetes in the body system has deleterious effect in the production and detection of IL-10 in the serum [51]. noted that there is association between obesity, type-2 diabetes and low circulating levels of IL-10. The low level or total absence of IL-10 have been reported in isolated macrophages and keratinocytes from diabetic mice, rats and endothelial cells at the wound margins of human diabetic foot ulcers [43,52,53,37] also reported that IL-10 decreased less than the pre-surgery values following musculoskeletal surgery and, [54] observed the tendency of increase in IL-10 levels in type -2 diabetic patients after they obtained adequate metabolic control. In contrast, over expression of IL-10 is associated with intense inflammatory phase, delayed epithelialization, prolonged and disorganized granulation, impaired angiogenesis and delayed healing [55].
The mean values of IL-1ß and IL-6, expressed in diabetic wound exudate (WE) (group D) were relatively higher than the values expressed in non-diabetic WE (group C). This could be due do prolonged retention of inflammatory mediators in delayed wound healing of diabetic wound as seen in group D [56]. stated that wounds with a high degree of inflammation contained increased level of IL-1ß and IL-6, and that wound inflammation may be correlated with total protein leakage absorbed into the wound dressing. The decline in the mean values of IL-1β in WE in the group C from psd 7 might be indicative of faster and progressive wound healing at a shorter duration of time in normoglycaemic wounds as against the hyperglycaemic wounds. In contrasts, the high value of IL-1β in the WE of rabbits in the WD group for the period of 21 days might be attributed to prolonged inflammatory response following delayed wound healing of these diabetic rabbits. The peak IL-1β in this WE of WD group might also be associated with high serum values of 1L-1β in this group as against the values recorded in the normoglycaenic wound (group C) as observed in this study. The mean values of IL-6 in the WE of groups C and D rabbits reached their peak level on psd 7 and the values remained high in subsequent days in group C. It was probable that IL-6 continues its mediation role between the preparatory and maturation phases of wound healing of normoglycaemic wound, where it remain at high values. The cause of the slight decline in the values of IL-6 in the WD group from psd 7 is not clear and also not in consonance with the serum values of IL-6 observed in this study, and is therefore subject for further investigation.
The values of IL-10 in wound exudate of the WND group was significantly higher (P<0.05) than the values in the WD group from psd 14 to 21 while values of IL-10 in wound exudate of the group D rabbits decreased progressively and was lowest on psd 14. Diabetes could have probably caused expression of lower values of IL-10 from the wound exudate. The positive co-relation between diabetes and low circulating levels of IL-10 is also a factor in this finding. The low level or total absence of IL-10 have been reported in isolated macrophages and keratinocytes from diabetic mice, rat and humans [52,53]. This might also be correlated with the findings in serum IL-10 in group D which was lower than the serum interleukin in other groups [51]. However, this finding is contrary to the study of [57] who reported over expression of IL-10 associated with delayed wound healing, although his report did not specify the fluid (blood, serum or wound fluid) used for his studies.
Conclusion
It was concluded that the values of IL-1β and 1L-6 are upregulated in the inflammatory phase of wound healing in normoglycaemic and diabetic rabbits while the values of IL-10 is downregulated during wound healing in diabetic NZW rabbits. It is therefore, imperative that clinicians take cognisance of the variation in the interleukin levels in serum and wound exudate of patients as such knowledge will contribute immensely in the efficient management of wound and other clinical challenges.
Acknowledgment
Faculty of Veterinary Medicine, University of Abuja, Nigeria; Faculty of Veterinary Medicine, ABU, Zaria.
Conflict of Interest
None.
References
- Barrientos S, Stojadinnovic O, Golinko MS, Brem H, Tomic Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair and Regeneration 16(5): 585-560.
- Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, et al. (2011) Interleukins, from 1 to 37, and interferon-gamma: Receptors, functions, and roles in diseases. J Allergy Clin Immunol 127(3): 701721.
- Abbas AK, Lichtman AH, Pillai S (2015) Cellular and Molecular Immunology (8 ed.). Elsevier Saunders, Philadelphia, USA: P. 535.
- Dinarello CA (2005) Blocking IL-1 in systemic inflammation. J Exp Med 201(9): 1355-1359.
- Mirza RE, Fang MM, Ennis WJ, Koh TJ (2013) Blocking interleukin-1β induces healing associated wound macrophage phenotype and improves healing in Type-2 diabetes. Diabetes 62(7): 2579-2587.
- Hosgood G (2002) Wound repair and specific tissue response to injury. In: Slatter, D (3rd pp. 66–86). Textbook of small animal Surgery, Philadelphia; Saunders Elsevier Sciences.
- Schultz GS, Chin GA, Moldawer L, Diegelmann RF (2011) Principles of Wound Healing. In: Mechanisms of Vascular Disease (A Reference Book for Vascular Specialists): by Robert Fitridge and Matthew Thompson. University of Adelaide Press.
- Sharma AK, Sunder V, Yashavarddhan MH, Shukla SK (2017) Wound healing: current understanding and future prospect. International Journal of Drug discovery 8(1): 240-246.
- Pradham L, Nabzddyk CS, Lorgefo FW, Veves A, Sarada Kuchibhotla, et al. (2013) Expression of neuropeptide and Cytokines in a rabbit model of diabetic neuroishemic wound healing. J Vasc Surg 58(3): 766-775.
- Sharma R, V Dave, Sharma S, Jain P, Yadav S (2013). Experimental Models on Diabetes: a comprehensive review. IJAPS 4: 01-08.
- Nagata M, Suzuki W, Ilzuka S, Tabuchi M, Maruyama H, et al. (2006) Type-2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp Anim 55(2): 109-115.
- Wang J, Wan R, Mo Y, Zhang Q, Sherwood LC, et al. (2010) Creating a long-term diabetic rabbit model. Exp Diabetes Res 2010: 289614.
- Hassan AZ, Amber EI, Awasum CA, Remi Adewumi BD, Ayila AS, et al. (2002) A review of wound healing. Nigerian Veterinary Journal 23(2): 1-14.
- Cannon JG (2000). Inflammatory cytokines in nonpathological states. News Physiol Sci 15: 298-303.
- Kupper TS, Deitch EA, Baker CC, Wong WC (1986) The human burn wound as a primary source of interleukin-1activity. Surgery 100(2): 409-415.
- Grellner W, Georg T, Wilske J (2000) Quantitative analysis of proinflammatory cytokines (IL-1 beta, IL-6 and TNF-alpha) in human skin wounds. Forensic Sci Int 113(1-3): 251-264.
- Di Vita G, Patti R, Dagostino P, Caruso G, Arcara M, et al. (2006) Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair, Wound Repair Regen 14: 259-265.
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, et al. (1998) IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101(2): 311-320.
- Kaplanski G, Marin V, Montero Julian F, Mantovani A, Farnarier C (2003) IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24(1): 25-29.
- Liechty KW, Kim HB, Adzick NS, Crombleholme TM (2000) Fetal wound repairresults in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 35(6): 866-872.
- Diane MC, Erik ZU, Patrick H, Francis K, Martin CR (1994) Determination of endogenous cytokine in chronic wounds. Ann Surg 219(6): 688-692.
- Vayrynen O (2003) Proinflammatory cytokines modify the expression of surfactant proteins: study in perinatal rabbit lung (Academic Dissertation, Faculty of Medicine, University of Oulu). Oulu University Press, Oulu, Finland.
- Johnson JM, Orrgren M, Auster M, FW LoGerfo, LK Pradhan Nabzdyk, et al. (2016) Cytokines and Neuropeptide Receptors in a Neuroischemic Rabbit Model of Wound Healing. Academic Surgical Congress.
- Brauchle M, Angermeyer K, Hubner G, Werner S (1994) Induction of keratinocyte.
- Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, et al. (2006) Cytokine expression profile over time in severely burned pediatric patients. Shock 26: 13-9.
- Sun WT, Lei CL, Bi CC, Chen ZL, Zhang L (2010) Effect of alloxan time administer drug on establishing diabetic rabbit model. Int J Olphthalmol 3(3): 200-202.
- Flecknell PA, Anna Meredith (2000) Anaesthesia In: Manual of Rabbit Medicine and Surgery. BSAVA 103-116.
- Martin M, Kirsipuu V (2016) Rabbit anaesthesia. Cornell University Institutional Animal Care and Use Committee 1-6.
- Kilic S, Timurkaan N, Unsaldi S, Gunay C, Stek O, et al. (2002) Comparison of effects of some wound healing materials on full thickness skin wounds in rabbits. Turkish Journal of Veterinary and Animal Sciences 26(2): 263-272.
- Onah JA, Aba PE, Eze CA, Ukweze CO, Odo RI (2014) Blood and serum biochemistry changes following peritoneum sutured and not-sutured techniques of laparotomy in omentopexed West African Dwarf (WAD) goats. Journal of Animal Research International 11(1): 1881-1888.
- Zuhoor KA (2013) Proinflammatory cytokines dynamics at operation site and serum after breast surgery. Arch Clin Exp Surg 2(3): 161-169.
- Cheesbrough M (2000) District laboratory practice manual in tropical countries (part 2). Cambridge University Press, London Pp: 178-179.
- Ambrosch A, Lobmann R, Pott A, Preissler J (2008) Interleukin-6 concentrations in wound fluids rather than serological markers are useful in assessing bacterial triggers of ulcer inflammation. Int Wound J 5(1): 99-106.
- Elabscience (2016) Rabbit IL-6 (Interleukin 6) ELISA kit: cat no: E-EL-RB0014. Elabscience Biothechnology Company Limited Pp: 1-11.
- Giannoudis PV (2003) Current concepts of the inflammatory response after major trauma: an update. Injury 34(6): 397-404.
- Changyao LV, Jing W, Hongbo H (2017) Experimental study on the expression of IL-1𝛽 and BFGF in wound healing process of rabbit cutaneous infective wound in Liu He-Dan. Evid Based Complement Alternat Med: 7230178.
- Reikeras O, Borgen P, Reseland JE (2014) Changes in serum cytokines in response to musculoskeletal surgical trauma. BMC Res Notes 7: 128.
- Jager J, Gremeaux T, Cormont M, Yannick Le Marchand Brustel, Jean Francois Tanti (2007) Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148(1): 241-251.
- Oncul O, Yildiz S, Gurer US, Yeniiz E, Qyrdedi T, et al. (2007) Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J Infect 54: 250-256.
- Pradhan L, Cai X, Wu S, Andersen ND, Martin M, et al. (2011) Gene expression of proinflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res 167(2): 336-342.
- Agyare C, Osafo N, Boakye YD (2018) Biomarkers of wound healing. Intech Open Science P: 23-29.
- Alkanani AK, Rewers M, Dong F, Waugh K, Peter A Gottlieb, et al. (2012) Dysregulated Toll-like receptor-induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type-1 diabetes. Diabetes 61(10): 2525-2533.
- DeClue CE, Shornick LP (2015) The cytokine milieu of diabetic wounds. Diabetes Management 5(6): 525-537.
- Taniguchi T, Koido Y, Aiboshi J, T Yamashita, S Suzaki, et al. (1999) The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. Am J Emerg Med 17(6): 548-51.
- Weigelt C, Rose B, Poschen U, Ziegler D, Gerd Friese, et al. (2009) Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care 32(8): 1491-1496.
- AlGaithy ZK (2013) Proinflammatory cytokines dynamics at the operation site andserum after breast surgery. Archives of experimental surgery 2: 161-169.
- Ohshima T, Sato Y (1998) Time-dependent expression of interleukin-10 (IL-10) mRNA during the early phase of skin wound healing as a possible indicator of wound vitality. Int J Legal Med 111: 251-255.
- Giannoudis PV, Smith RM, Perry SL, AJ Windsor, RA Dickson, et al. (2000) Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med 26: 1076-1081.
- Giannoudis P V (2003) Current concepts of the inflammatory response after major trauma: an update. Injury- International Journal for the Care of the Injured 34: 397-404.
- Peranteau WH, Zhang L, Muvarak N, Andrea T Badillo, Antoneta Radu, et al. (2008) IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 128(7): 1852-1860.
- Van Exel E, Gussekloo J, de Craen AJM, Frolich M, Bootsma vander Wiel A, et al. (2002) Westendorp RGJ. Low production capacity of interleukin-10 is associated with the metabolic syndrome and type-2 diabetes: the Leiden85-plus study. Diabetes 51(4): 1088-1092.
- Galkowska H, Wojewodzka U, Olszewski W (2006)Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair regen 14(5): 558-565.
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, et al. (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 5(3): e9539.
- Foss Freitas MC, Foss NT, Donadi EA, Foss MC (2007) Effect of metabolic control on interferon-γ and interleukin-10 production by peripheral blood mononuclear cells from type 1 and type 2 diabetic patients. Braz J Med Biol Researc 40(5): 671-677.
- Lundberg JE, Roth TP, Dunn RM, Doyle JW (1998) Comparison of IL-10 levels in chronic venous insufficiency ulcers and autologous donor tissue. Arch Dermatol Res 290(12): 669-673.
- Saleh K, Strömdahl AC, Riesbeck K, Schmidtchen A (2019) Inflammation biomarkers and correlation to wound status after full thickness skingrafting. Fron Med 6: 159.
- Lundberg JE, Roth TP, Dunn RM, Doyle JW (1998) Comparison of IL-10 levels in chronic venous insufficiency ulcers and autologous donor tissue. Arch Dermatol Res 290: 669-673.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.