Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Frequency and Risk Factors for Nasopharyngeal Carriage of Streptococcus Pneumonia in Children Under 5 Years of Age Not Vaccinated with PCV13, in the Daïra of Sidi M’Hamed Algiers
*Corresponding author:Malika Keddari Hamdani, Pediatrics Department, CHU Mustapha Algiers, Algeria.
Received: September 15, 2023; Published: February 02, 2024
DOI: 10.34297/AJBSR.2024.21.002843
Abstract
Introduction: Information on nasopharyngeal carriage (PNP) of Streptococcus pneumoniae prior to the introduction of Pneumococcal Conjugate Vaccine (PCV) is essential to monitor the impact of vaccination. PCV13-valent (PCV13) was officially introduced into the national vaccination program in Algeria on June 24, 2016.
Objectives: To determine the prevalence of Streptococcus pneumoniae (SP) carriage, the distribution of serotypes and resistance to antibiotics in asymptomatic children under 5 years old in the daïra of Sidi M’Hamed, recruited at the pediatric department of CHU Mustapha Pasha in Algiers before the introduction of PCV13.
Material and Method: We carried out a cross-sectional prospective study from September 19, 2016 to September 19, 2017. A single sample was taken and analyzed for each child, presenting to the CHU Mustapha vaccination center. After a normal clinical examination, a nasopharyngeal sample is taken from children not vaccinated against pneumococcus as well as accompanying siblings under 5 years not vaccinated against pneumococcus.
Results: Of the 650 samples taken, we identified 178 positive samples, i.e., a carrier rate of SP of 27.38%. Carrying was highest 36.9% at age (6-11months). Age, siblings, household size, mean NSE, and cold season were significantly associated with carrying SP (p<0.05) of the 178 strains isolated, we serotyped 172 strains and excluded 6 strains because they were not viable. We obtained by multiplex PCR and the capsule swelling technique 237 isolates divided into Vaccine Serotypes (SV) 150/237 (63.29%), and nonvaccine serotypes (SNV) 87/237 (36.70%). Among the 172 strains of pneumococcus isolated we have 11 vaccine serotypes (SV) of the PCV13 type (6A/B, 19F, 23F, 14,18C, 1, 4, 19A, 7F, 9V, 3) and 17 non-vaccine serotypes (SNV) (15A/F, 11A, 23, 19, 15B, 15, 6,10A, 17.8, 18, 9, 11, 6C/D, H, C, NT). For non-vaccine serotypes/serogroups, the most common are 15A/F (11.05%), 23 (7.55%), 11A (6.97%), 19 (4.65%), 15B (4.07), 6 (2.90), 15.10A (2.32% each), 17 (1.74), 6CD (1.74) 18 and 8 (1.62 each), 11.9, C, H and NT (0.58 each) for a total of 87/237 isolates (36.70%). 15A/F is in the majority in the age group (0 -23 months). For non-vaccine serotypes/ serogroups, the most common are 15A/F (11.05%), 23 (7.55%), 11A (6.97%), 19 (4.65%), 15B (4, 07), 6 (2.90), 15.10A (2.32% each), 17 (1.74), 6CD (1.74) 18 and 8 (1.62 each), 11.9, C, H and NT (0.58 each) for a total of 87/237 isolates (36.70%). 15A/F is in the majority in the age group (1-23months).
Conclusion: Our study identified a carrier prevalence of 27.38%, the vaccination coverage of the PCV13 serotypes is high (63.29%), the PSDP rate is 82.58%. The introduction of PCV13 in Algeria is likely to have an impact on the transmission of pneumococcus in children, especially <5years, older people, and on TBT resistance.
Keywords: Pneumococcus (Streptococcus pneumoniae), Serotypes, Resistance (PSDP), Vaccine
Introduction
Streptococcus pneumoniae is the leading cause of death in children and the elderly, particularly in developing countries, and constitutes a public health problem worldwide [1,2]. The World Health Organization (WHO) estimates that, each year, approximately 1.6 million people, including 1 million children under the age of 5, die from infections caused by pneumococcus [3]. In developing countries, the case fatality rate for Invasive Pneumococcal Disease (IPD) can be up to 20% for septicemia and 50% for meningitis.
Acute Community-Acquired Pneumonia (CAP) represents a significant morbidity among children in developing countries as well as industrialized countries [4], 20 to 60% of cases of CAP observed in children are linked to pneumococcus, but only a small proportion is proven by blood culture or pleural sample [5]. UNICEF and WHO have highlighted pneumonia as the forgotten killer of [children]. Worldwide, 13% of deaths in children under 5years of age are caused by pneumonia, with a mortality of 10% in cases of bacteremia pneumococcal pneumonia. Streptococcus Pneumoniae (SP) is particularly dangerous in children under 5years of age, immunocompromised subjects and the elderly, who constitute groups in which the incidence of the disease is more than 10 times higher than that observed in healthy subjects [6,7].
There are more than 95 distinct serotypes [7,8], based on differences in the composition of the polysaccharide capsule which plays a major role in the virulence of the bacteria [9,10]. Carriage always precedes infection and IPD, it is correlated with the young age of the child and the peak incidence is before the age of 2 years [11].
The introduction of anti-pneumococcal conjugate vaccines (PCV7, PCV10, and PCV13) in several countries was followed by a clear decline in serious pneumococcal infections due to vaccine serotypes, penicillin resistance and nasopharyngeal carriage (PR) asymptomatic. In Algeria, nasopharyngeal carriage studies (NPC) are less well documented; only 2 studies carried out over a period of 3 months are available [12,13]. In Algeria, the PCV13 vaccine is introduced into the vaccination schedule from June 24 on the cohort of children born from April 24, 2016. This choice was made by essentially taking into account the results of studies carried out on IIP. Given these data, we proposed to conduct a prospective investigation to evaluate nasopharyngeal carriage in asymptomatic children under 5 years of age and not vaccinated with an anti-pneumococcal vaccine.
Material and Methods
Target population
Inclusion Criteria: This is a descriptive, prospective, cross-sectional study involving children aged 1 to 59months, recruited at the vaccination center of the pediatric department of Mustapha University Hospital, asymptomatic, having not received pneumococcal vaccine, presenting for other vaccinations as well as accompanying siblings aged under 5 years. All children were assessed and subjected to a questioning and a clinical examination to verify the absence of symptoms.
Non-Inclusion Criteria: Newborns (<28days) and children aged over 5years, any clinical sign suggestive of infection, any child having received antibiotics 7days before sampling, having received an anti-pneumococcal vaccination and parental refusal. After parental consent, clinical and epidemiological data were collected using an individual questionnaire, including the search for information related to the objectives of the study. The nasopharyngeal secretions were collected by the investigating doctor from both nostrils using a sterile swab.
Bacterial Identification
Each sample was cultured in the bacteriology laboratory of Mustapha University Hospital within hours of collection (<2hours). The analysis of the sample and the identification of pneumococci was carried out on three criteria: The microscopic appearance on Gram staining and the macroscopic appearance of the colonies on the petri dish, presence of αhemolysis, sensitivity to optochin and the bile lysis test. A quality control by the strain (ATCC 49619) was done to see if our diagnostic steps are valid.
Determination of Antibiotic Susceptibility
First carried out by a standard antibiogram for all our strains then determination of the MICs. The detection of pneumococci with reduced sensitivity to penicillin G (PSDP) is carried out with a 5µg oxacillin disk (OXA-5) according to the CA-SFM method (antibiogram committee of the French microbiology society and by CLSI 2014 standards. The determination of the Minimum Inhibitory Concentration (MIC) in our work was done by 2 methods: Walk-Away (WK) and E-test. The E-test technique was carried out for MICs>1µg/ml in WC.
Determination of Serogroups and Serotypes
Serotyping is carried out according to the recommendations of the Statens Sérum Institut in Copenhagen by:
PCR: Carried out as first intention for all strains.
Capsule Swelling or NEUFELD Reaction: All strains not identified by PCR underwent capsule swelling.
Statistical Analysis
The Excel EPI data software was used to collect the responses to the questionnaire and their statistical analysis for all the tests, a significance threshold of 5% was retained (p<0.05).
Results
from 2016-2017, 3104 children presented to the PMI for vaccination. Among them, 650 met the inclusion criteria. We excluded children who received a PCV 13 vaccine and children with acute respiratory symptoms. Streptococcus pneumoniae was isolated in 178 children among the 650 sampled, or in 27.38% of cases.
Risk Factors for Pneumoniae Colonization
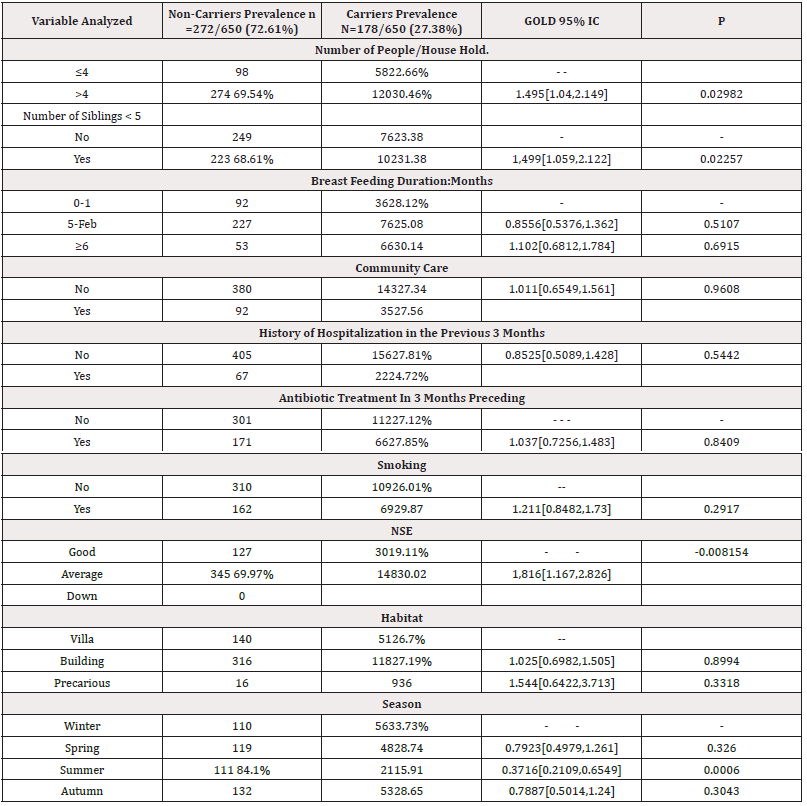
The univariate analysis found 5 risk factors with a significant difference (p<0.05): Age 6-11 months, the number of people living under the same roof >4, the presence of at least one brother or sister, the average socio-economic level of the parents represents a risk factor for Carrying, the season summer, particularly the month of August, represents a lower risk of Portage.
Distribution Of Serotypes Involved in Asymptomatic Carriage of Children Under 5 Years of Age by PCR
We noted a number of positives of 178 including 6 Non-Viable Strains (NV) which were excluded. Which gives a number of serotyped strains: 172. The total isolates: SV (150) +SNV (87) =237 serotypes. Among the 172 strains of pneumococcus isolated we have 11 vaccine serotypes (SV) among the 13 of PCV13. The most frequently identified PCV13 vaccine serotypes are in order of frequency (Table 1) with a total of 150/237 isolates or 63.29%. We were unable to determine PCV7 and PCV10 vaccination coverage due to the absence of serotypes 6B and 6A. On the other hand, PCV13 vaccination coverage is 63.29% because serotype 6A/B could be 6A or 6B, both are included in PCV13. non-vaccine serotypes / serogroups, represent a total of 87/237 isolates 36.70%. The most common are 15A/F,23,11A, 19,15B,6 ,15,10A and 17. In total: 650 samples: 172 serotyped strains (6 excluded because not viable) 237 serotypes or isolates: 150 vaccine serotypes + 87 non-vaccine serotypes (Table 1 and Figure 1).
All PCV13 vaccine serotypes are present before the age of 6 months with predominance of 6A/B, 19F,14,23F,1,4,19A and 18C at rates (31.6%, 18.4%, 13.2%, 11.8%, 5.3%,5.3%,2.6%) respectively. The 7F, 3 and 9V (1.31% each). From 6-11 months, the 6A/B is the majority (43.33%) followed by the 23F and 19F (10% each) then 4, 9V 19A and 14 (3.33%) each (Figure 2).
Frequency of Carriage with Multiple Serotypes
Carriage with a single serotype is the most frequent 113/172 (65.7%), followed by carriage with two serotypes 53/172 (30.81%) and with three serotypes in 6/172(3.49%). 237 serotypes or isolates: Carriage of one serotype 65.7%, 2 serotypes (30.8%), Carriage of 3serotypes (3.5%) (Figure 3).
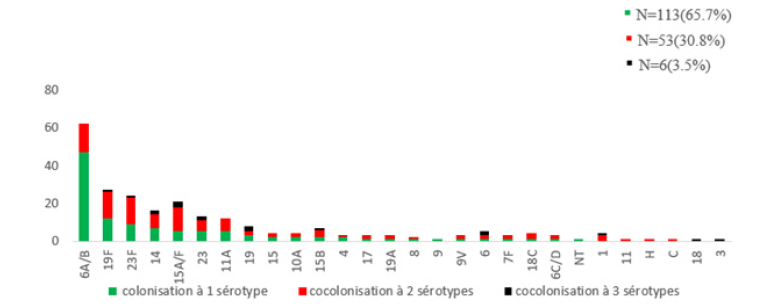
Figure 3: Distribution of carriage according to the number of serotypes (carriage to one serotype or several).
Susceptibility Profile of MS Strains to Beta-Lactams and Other Antibiotics According to CLSI 2016
Of the 178 isolated MS strains, 82.58% (147/178) have reduced sensitivity to penicillin G, including 67.98% with intermediate sensitivity (MIC>0.125-1mg/l) and 14.61% with high level resistance (MIC≥2mg/l). (Figures 4-6).
We observe that vaccine serotypes are the most resistant to penicillin G compared to non-vaccine serotypes in number and level. Serotypes 3, 18, 9 and C are sensitive to penicillin G. Serotype 1, generally sensitive, has intermediate sensitivity. This could be explained by cocolonization with a resistant strain. In fact, serotype 1 is present in cocolonization with 2 and 3 serotypes.
Risk factors for PSDP
Univariate analysis showed four parameters strongly associated with PSDP in a statistically significant manner (p<0.05): Age 18-23 months, presence of siblings (<5years), the notion of AKI (nasopharyngitis, tonsillitis) and bronchiolitis in the three months preceding the sample.
Discussion
The RP Carrying Frequency of SP
The frequency of carriage found in our study is close to that reported by two studies carried out in Algiers and Blida in 2012 and 2014 over a period of 3 months [12,13]. Comparison of different studies carried out in Algeria on asymptomatic PNP Table 2 shows fluctuating but relatively stable frequency of carriage. Vaccination coverage is practically the same in 2012, 2014 and 2016, however the PSDP rate has significantly increased (69.4% in 2012 versus 82.58% in 2016). Worldwide, there are numerous surveys on the prevalence of RP of SP carriage and find rates that vary greatly from one country to another. In Egypt, in a study carried out in Alexandria by Elnawawy, et al on 600 asymptomatic children aged 2 months to 5 years, 29.2% of children were carriers of MS [14]. In India, 2 studies carried out 5 years apart in children aged under 5 years found SP carriage at 27.9% and 28.0% [15]. In Morocco, Bouskraoui, et al., [16] reported a prevalence of MS PRP of 45.8% in 660 children aged less than 2 years. The Charvériat study in New Caledonia showed that 52% of children under 2 years old were carriers of pneumococcus [17]. It is clear that globally, the prevalence of pneumococcal NP carriage varies between countries and regions [18]. Data from young children conducted by Adegbola, et al, before the introduction of PCVs showed a combined prevalence of 64.8% in low-income countries and 47.8% in lower-middle-income countries [19]. These considerable fluctuations in carriage estimates from one country to another can be explained by methodological differences between studies, geography, climate, season, local genotype prevalence, a variety of factors related to host, as well as the external environment. Indeed, living conditions (overcrowding and promiscuity at home or in daycare centers) are known to intensify the transmission of pneumococcus [18] (Tables 2,3).
Carrying Risk Factors
Several meta-analyses have proven that certain factors are strongly linked to nasopharyngeal carriage. In our work, the frequency of carrying is significantly higher among infants aged 6 to 11months (36.9%) (p=0.02763), compared to those aged 0-5 months (24.76%). In infants aged 12 to 23months, the prevalence of carriage is 29.89% and 20.9% in those over 24months. We observe an increase in carriage with increasing age from the 1-5month age group. Then a decrease from the age of 12 months. In Morocco, the Bouskraoui investigation [16]. The distribution of carriage varies with age, which appears to be a major determinant of the prevalence of carriage. In Indonesia, Murad, et al carried out a beautiful study whose objective was to evaluate the dynamics of carriage on a total of 1574 samples taken, the overall prevalence of pneumococcal carriage was 22.0% at the time of recruitment and increased to 68.4% at age 12 months [20].
In Total
The analytical study of our series made it possible to identify 5 carriage risk factors (p<0.05): Age 6-11 months, number of people living under the same roof >4, presence of at least one brother or sister, average socio-economic level of parents, summer season, particularly the month of August represents a lower risk of carry.
In Comparison with Previous Studies
The SVs isolated in our study are close to those isolated in national studies on asymptomatic carriage in Algeria (Figure 7,8). In fact, the predominant serotypes in 2014 were 19F (13%), 6B=13%, 14, 23F, 19A, and 11A, with vaccination coverage at 61% PCV13 [12]. In 2012 the most frequently reported in asymptomatic carriage were 6A/B, 19F, 14, 23F, 19A, 15A/F, 15B/C, 3A, 11A/D [13]. Serotypes, 6A/B, 19F, 23F, 14,1 and 15A/F are increasing in our study PCV13 vaccine serotypes are the majority in all three studies. The number of serotypes, 6A/B, 19F, 23F,14, and serotype 1 increased in 2016 compared to 2012. Serotypes 19F and 23F are emerging in 2016. Certain serotypes are observed exclusively in 2016: 18C, 7F, and 3. The vaccine serotypes isolated in our study are those isolated in IIP infections in Algeria [21-25,13], and among them some are responsible for deaths. Among the 220 strains isolated from IIP in the most recent Algerian work [13], 15A/F is one of the emerging serotypes in 2016 in other countries [like] us.

Figure 7: Comparison of vaccine serotypes from previous surveys.
Note*: (Ziane 2012, Oukid 2014, Keddari 2016) a) ▪ N=102/318 (2012) Ziane b) ▪ N=67/567 (2014) Oukid c) □ N=178/650 (2016) Keddari
Frequency of Multiple Serotype Carriage
The existence of several serotypes or serogroups promotes the exchange of genetic material between SPs and therefore resistance to antibiotics by capsular switch [26]. Cocolonization with 5 serotypes has been reported [27]. The study of cocolonization must be supplemented by sequence typing (sequencing or study of clones). Indeed, this study demonstrated that serotyping alone underestimated the diversity of pneumococci colonizing the nasopharynx [27]. Cocolonization is common in developing countries and in the absence of PCV vaccination. In our series, the distribution of serotypes according to age identified the 11 PCV13 vaccine serotypes in 63.15% in infants aged 1-5 months with a different distribution (Figure 2).
Susceptibility Profile of Pneumococcus Strains to Beta-Lactams and Other Antibiotics according to CLSI 2016
Carriage Of PSDP Strains Represented 147/178 (82.58%) In Our Cohort. This resistance was intermediate in 67.98% (MIC=0.12-1mg/l) and high level in 14.61% (MIC≥2mg/l), according to CLSI 2016. The serotypes affected by this resistance are all PCV13 vaccine serotypes except serotype 3 which remains sensitive to penicillin. Among non-PCV13 serotypes, only 9, 18, and C are not resistant to PéniG. As for resistance to Imipenem, it is very high, of intermediate type (MIC 0.25-0.5mg/l), affecting 64.60% of strains. Beta-lactam resistance was high at 50.71%. In Algeria, several reports showed an increase in antibiotic resistance from 1996 to 2010, particularly among children [28,29]. These data place Algeria among the countries where the rates of resistance to penicillin are the highest [24] (Figures 5,6).
In Morocco, among the strains of S. Pneumoniae, 34.7% had reduced sensitivity to penicillin with 87.1% low level, 12.9% of them were high level (MIC>2mg/L) [30]. Regarding resistance to other ATBs, we report rates of 69.66%, 59.55%, 64.04%, and 55.06%, for erythromycin, clindamycin, tetracyclines, and cotrimoxazole, respectively. No resistance to vancomycin was recorded in our study. Our results are generally in agreement with literature data. National studies have reported a rate of PSDP strains that remains high among pediatric strains isolated during invasive infections [12,13,24,28]. In comparison with the previous study on carriage in 2012, we observed an increase in the PSDP rate which went from 69.4% in 2012 to 82.58% in 2016, however the PCV13 vaccination coverage remained stable from 2012 to 2016 (63.5%). The serotypes increasing in 2016 are 6A/B, 19F, 23F, 14, and serotype 1. 19F, 23F and 15A/F are emerging and bearers of resistance.
Regarding other beta-lactams, resistance increased from 6.5% to 23.59, from 17.6% to 38.76 and from 39.2 to 64.60% for amoxicillin, cefotaxime and imipenem respectively. As for other ATBs, erythromycin, clindamycin tetracycline cotrimoxazole, resistance levels increased sharply in 2016. No resistance to vancomycin was reported in 2012 or 2016. The emergence of PSDP strains and strains resistant to other ATBs is alarming. It is generally attributed to antibiotic selection pressure on bacteria present in nasopharyngeal carriage. This exposes to major therapeutic problems complicating the management of invasive pneumococcal infections. This selection pressure is related to the inappropriate use in quantity or duration of antibiotics and the absence of vaccination with PCVs.
Risk factors for carrying PSDP
In this work, we observed 5 factors increasing this risk. Thus, the age group located between (18-23months), the existence of siblings of at least one member, hospitalization, the notion of bronchiolitis and ARI (angina and nasopharyngitis) in the three-month preceding the collection were significantly correlated with carriage (p=0.0071). What has been approved by several authors, even after the era of PCV vaccination, these factors remain correlated with carriage [31]. They are generally the same as those already known in port [32].
Conclusion
Our study carried out over a year concerning asymptomatic children aged 1-59 months, made it possible to determine the PNP rate of S pneumoniae in the Daïra of Sidi m'Hamed in Algiers which is 27.38% as well as the factors of risk favoring this carriage, the distribution of serotypes and the study of antibiotic resistance. The PSDP rate remains high compared to previous national studies. This study carried out in the pre-vaccination period will serve as a basis of comparison for future PNP surveys after the implementation of pneumococcal vaccination and the achievement of good vaccination coverage. Monitoring NP carriage is important and can provide early relevant information on the effects of vaccines, particularly to identify serotypes that may substantially contribute to pneumococcal disease post-vaccination. This is why monitoring of this carriage is necessary to monitor the evolution of circulating serotypes and their sensitivity to antibiotics.
Acknowledgement
None.
Conflict of Interest
The authors have no conflict of interest in relation to this article.
References
- GBD2015 LRI Collaborators (2017) Estimates of the global, regional, and national morbidity, mortality, and a etiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17(11): 1133-1161.
- Walker CLF, Rudan I, Liu L, Harish Nair, Evropi Theodoratou, et al. (2013) Global burden of childhood pneumonia and diarrhea. Lancet 381(9875): 1405-1416.
- (2007) Pneumococcal conjugate vaccine for childhood immunization. WHO positions paper. wkly Epidemiol Rec 82(12): 93-104.
- Igor Rudan, Katherine LO Brien, Harish Nair, Li Liu, Evropi Theodoratou, et al. (2013) Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 3(1): 010401.
- Bartlett JG (2011) Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 52(Suppl 4): 296-304.
- UNICEF (2006) Pneumonia the forgotten killer of the children pp. 1-42.
- Cohen N Ouldali, E Varon, C Levy (2019) A germ and its prevention. Pneumococcus, Pediatric realities: 233.
- Bogaert D (2011) Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. 6(2): e17035.
- T Goulenok (2014) Pneumococcal vaccination in adults: how to improve vaccination coverage. Journal of Anti-infectives 89: 10.
- PO Lang (2012) Anti-pneumococcal vaccination: current situation and perspectives, NPG Neurology - Psychiatry - Geriatrics 12: 111-120.
- Deirdre A Collins, Anke Hoskins, Jacinta Bowman, Jade Jones, Natalie A Stemberger, et al. (2013) High Nasopharyngeal Carriage of Non-Vaccine Serotypes in Western Australian Aboriginal People Following 10 Years of Pneumococcal Conjugate Vaccination 8(12): e82280.
- S Oukid, H Tali Maamar, L Laliam, A Belboul, B Boutareg, et al. (2015) Streptococcus pneumoniae carriage and frequency of serotype: in children less than 25 months: a preliminary study in Algeria, 33nd Annual Meeting of the European Society for Peadiatric Infectious Diseases Leipzig.
- H Ziane (2015) Microbiology department chu Mustapha Algiers, thesis for obtaining the degree of doctor in medical sciences. Supported in 2015: Streptococcus Pneumoniae: Antibiotic resistance, frequency of circulating serotypes and main genotypes involved in invasive infections and nasopharyngeal carriage.
- Ahmed A El Nawawy, Soad F Hafez, Marwa A Meheissen, Nehal M Shahtout, Essam E Mohammed, et al. (2015) Nasopharyngeal Carriage, Capsular and Molecular Serotyping and Antimicrobial Susceptibility of Streptococcus pneumoniae among Asymptomatic Healthy Children in Egypt. Journal of Tropical Pediatrics 61(6): 455-463.
- KL Ravi Kumar, Vandana Ashok, Feroze Ganaie, AC Ramesh (2014) Nasopharyngeal Carriage, antibiogram & serotype distribution of Streptococcus pneumoniae among healthy under five children. Indian journal of medical research 140(2): 216-220.
- M Bouskraoui Soraa, K Zahlane, L Arsalane, C Doit, P Mariani, et al. (2011) Study of nasopharyngeal carriage of Streptococcus pneumoniae and its sensitivity to antibiotics in healthy children aged less than 2 years in the Marrakech region (Morocco). Archives de Pédiatrie 18: 1265-1270.
- MA Charvériat, M Chomarat, M Watson, B Garin (2005) Study of nasopharyngeal carriage of Streptococcus pneumoniae in healthy children aged 2 to 24 months in New Caledonia. Med Mal Infect 35(10): 500-506.
- Miwako Kobayashi, Laura M Conklin, Godfrey Bigogo, Geofrey Jagero, Lee Hampton, et al. (2017) Pneumococcal carriage and antibiotic susceptibility patterns from two cross-sectional colonization surveys among children aged <5 years prior to the introduction of 10-valent pneumococcal conjugate vaccine-Kenya, 2009–2010. BMC Infect Dis 17(1): 25.
- Richard A Adegbola, Rodrigo DeAntonio, Philip C Hill, Anna Roca, Effua Usuf, et al. (2014) Greenwood. Carriage of Streptococcus pneumoniae and Other Respiratory Bacterial Pathogens in Low and Lower-Middle Income Countries: A Systematic Review and Meta-Analysis. PLOS ONE 9(8).
- Murad Chrysanti, Eileen M Dunne, Sunaryati Sudigdoadi, Eddy Fadlyana, Rodman Tarigan, et al. (2019) Pneumococcal carriage, density, and co-colonization dynamics: A longitudinal study in Indonesian infants. Int J Infect Dis 86: 73-81.
- N Ramdani Bouguessa (2001) Streptococcus pneumoniae: resistance to penicillin and serotypes in children. Doctor of Medical Sciences thesis 2001. Unidversity of Medicine of Algiers, Algiers, Algeria.
- H Tali Maamar, R Laliam, C Bentchouala, D Touati, K Sababou, et al. (2012) Serotyping and antibiotic susceptibility of Streptococcus pneumoniae strains isolated in Algeria from 2001 to 2010. Med Mal Infect 42(2): 59-65.
- Abla Hecini Hannachi (2014) Thesis With a view to obtaining a DOCTORATE IN SCIENCE degree in Applied Microbiology, Streptococcus pneumoniae in invasive infections: identification, antibiotic resistance and serotyping.
- N Ramdani Bouguessa, H Ziane, S Bekhoucha, Z Guechi, A Azzam, et al. (2015) Evolution of antimicrobial resistance and serotype distribution of Streptococcus pneumoniae isolated from children with invasive and noninvasive pneumococcal diseases in Algeria from 2005 to 2012. New Microbes and New Infections 6: 42-48.
- Melody Kasher, Hector Roizin, Adi Cohen, Hanaa Jaber, Sharon Mikhailov, et al. (2020) The impact of PCV7/13 on the distribution of carried pneumococcal serotypes and on pilus prevalence; 14 years of repeated cross-sectional surveillance. Vaccine 38(19): 3591-3599.
- C Wintenberger (2010) Pneumococcus in 2010: from genomics to the clinic Proceedings of Congress. Medicine and infectious diseases 40: 605-609.
- Marcus HY Leung a, Ndekya M Oriyo, Stephen H Gillespie, Bambos M Charalambous (2011) The adaptive potential during nasopharyngeal colonization of Streptococcus Pneumoniae. Infect Genet Evol 11(8): 1989-1995.
- H Tali Maamar Laliam, C Bentchouala, D Touati, K Sababou, S Azrou, et al. (2012) Serotyping and antibiotic susceptibility of Streptococcus pneumonia strains isolated in Algeria from 2001 to 2010. Med Mal Infect 42(2): 59-65.
- Benouda, S Ben Redjeb, A Hammami, S Sibille, M Tazir, (2009) Antimicrobial resistance of respiratory pathogens in North African countries. J Chemother 21(6): 627-632.
- N Elmdaghri, M Benbachir J Najib, H Belabbes (2009) Invasive pneumococcal infections in children in Morocco: antibiotic resistance and fluctuation of responsible serotypes before introduction of conjugated vaccines. French-speaking laboratory review - supplement to n°
- P Chavanet, A Atale, S Mahy, C Neuwirth, E Varon, et al. (2011) Nasopharyngeal carriage, sensitivities and serotypes of Streptococcus Pneumoniae and Haemophilus influenzae in nursery children Medicine and infectious diseases 41: 307-317.
- J Raymond, R Cohen, F Moulin, D Gendrel P, P Berche, et al. (2002) Factors influencing the carriage of Streptococcus pneumoniae, Med Ma and Infect 32(1): 13-200.











 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.