Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Improvements in Diabetic Neuropathy with Topical Human Mesenchymal Stem Cell Conditioned Media
*Corresponding author: Jonathan RT Lakey, Department of Surgery, University of California Irvine, Irvine, CA 92868, USA.
Received: December 21, 2024; Published: January 03, 2024
DOI: 10.34297/AJBSR.2024.21.002792
Abstract
Chronic hyperglycemia in both type 1 and type 2 diabetes commonly leads to microvascular diseases of the eyes, kidneys, and peripheral nerves. Diabetic peripheral neuropathy (DN) commonly affects the lower limbs first and can result in nerve damage leading to gradual onset of foot pain, tingling, numbness, muscle weakness, and extreme sensitivity to touch. Mesenchymal stem cells (MSCs) have been shown to have anti-inflammatory, neurotropic, neuroprotective, and angiogenic effects through paracrine signaling. From this pilot study, we propose a potential method of reversing DN-associated nerve damage using mesenchymal stem cell secretion product-conditioned cream applied directly to affected areas.
Adult type 2 diabetic patients (T2D) with progressive neuropathy and non-type 2 diabetic patients (non-T2D), mean age 52.5 years, were invited to participate in this pilot study where they were provided a vial of newly-formulated Neurocream, an MSC mediaconditioned cream (MSCM-conditioned cream) to apply twice daily to the soles of their feet after brief micro needling.
Individuals in the T2D group experienced an average decrease in all symptoms of approximately 50% at 30 days, and individuals in the non-T2D group experienced a 57% decrease in swelling, a 40% reduction in foot pain, and no change in reported tingling after 30 days of Neurocream use. All participants reported improved sleep.
Our study indicates that MSC-conditioned media could be a novel alternative to current diabetic peripheral neuropathy treatments (medications, topical anesthetics, surgery). This method may also reverse existing nerve damage in addition to providing relief from severe DN symptoms.
Keywords: Stem cell therapy, Pain, Neuropathy, Type 2 diabetes
Case Report
Diabetes is a chronic condition that affects the body’s ability to utilize glucose for conversion to energy. The two most prevalent forms of diabetes are type one and type two. In type one diabetes (T1D), an individual’s pancreatic β cells are targeted and destroyed by the host’s immune system. The result is an absence of insulin secretion by the pancreas, impaired glucose uptake in insulin-sensitive tissues, and a resulting hyperglycemia [1]. Type two diabetes (T2D) also results in impaired glucose uptake in tissues, however, its progression is explained through genetic, as well as metabolic, and environmental risk factors [1,2]. While factors like ethnicity, age, and family history of diabetes are non-modifiable, obesity and lifestyle can be modified, and they represent the strongest risk factors for the development and progression of T2D [1]. Together type one and type two diabetes affect approximately 10% of the US population (37 million individuals) making diabetes the sixth most common chronic disease and cause of death in the united states [3,4]. Of the total number of diabetics in the united states, approximately 90-95% of cases are type 2 [5]. The large prevalence of cases results in a total cost of approximately $327 billion, or nearly 10% of total healthcare spending in the US each year [3].
Diabetic Peripheral Neuropathy
Chronic hyperglycemia in both T1D and T2D commonly lead to microvascular diseases of the eyes, kidneys, and peripheral nerves [6]. Diabetic peripheral neuropathy (DN) commonly affects the lower limbs first and can result in nerve damage leading to gradual onset of foot pain, tingling, numbness, muscle weakness, extreme sensitivity to touch, and heat intolerance among other symptoms [7].
While intense research has been conducted in recent years into the development and progression of DN, there is no consensus about its pathophysiology. Some studies have shown that increased systemic iron levels are associated with increased risk of developing diabetes [8,9]. This led the authors to conclude that high iron levels might be associated with the progression and severity of diabetes symptoms. Yet, Baum et al. demonstrated the opposite in an animal model; low iron levels were associated with increased inflammation and nerve fiber degeneration. Additionally, they found that obesity and dyslipidemia were associated with increased peripheral nerve inflammation [10]. In their model, chronic hyperglycemia leads to an increase in reactive oxygen species (ROS) in tissues including peripheral nerves. Increased ROS presence upregulates transcription factors like NFκB which ultimately produces a cocktail of pro-inflammatory cytokines: IL-1β, IL-2, IL-8, IL-6, TNF-α, CCL2, and CXCL1 [10]. Chronic inflammation of peripheral nerves leads to their destruction and ultimately the emergence of symptoms associated with DN.
Under physiologic conditions, Schwann cells (SCs) play a central role in the survival and repair of peripheral neurons. They secrete various neuroprotective factors that provide neural support against reactive oxygen species (ROS), and inflammation in pathological as well as physiologic states [11,12]. Both peripheral neurons (PN) and Schwann cells are sensitive to excessive ROS levels like those seen in T2D. Therefore, T2D could lead to a state of enhanced PN death both directly from ROS, and indirectly from SC death [13]. De Gregorio et al. demonstrated a complete reversal of the impaired neuron regeneration phenotype in distal root ganglion (DRG) neurons in diabetic mice as well as a significant reduction in apoptosis in both DRG neurons and SCs in vivo using mesenchymal stem cell-conditioned media [13].
Current Treatments for Peripheral Neuropathy
Peripheral neuropathy is a serious complication of diabetes and is associated with increased all-cause mortality and morbidities such as foot ulcers, poor wound healing, local and systemic infection, limb amputation, and painful neuropathic symptoms [14]. DN is also typically diagnosed during later stages when disease progression has already led to irreversible nerve damage. Current first-line treatments are geared toward improving the painful symptoms of DN. In addition to careful diabetes management, analgesics, anti-seizure medications like pregabalin, certain antidepressants like duloxetine and amitriptyline, and topical agents containing lidocaine all work to treat DN by reducing associated pain [7]. While there is a wide array of pharmacological therapies available, currently, there are no FDA-approved treatments to reverse the damage caused by DN.
Mesenchymal Stem Cells
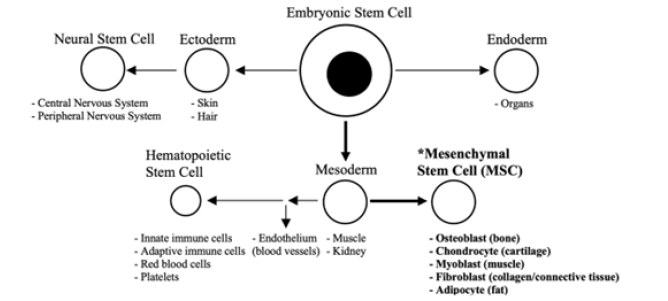
Figure 1: Schematic depicting the basic relationship between the three embryonic layers and major stem cell types including mesenchymal stem cells (MSCs). Figure generated in Microsoft Word®.
Mesenchymal stem cells (MSCs) are a unique type of adult stem cell in that they have the ability not only to differentiate into their own mesodermal lineage to give rise to new bone, cartilage, muscle, connective tissues, and fat, but also into endodermal and ectodermal lineages as well [15]. (Figure 1) provides a schematic of the relationship between embryonic layers and the major stem cell types. Bone marrow MSCs, a subtype of mesenchymal stem cell, migrate to sites of tissue damage where they differentiate into cell subtypes involved in tissue repair [15]. And, since they can be isolated from bone marrow aspirate and expanded to large numbers in culture, these MSCs make an ideal cell type for use in healing and regenerative medicine applications [16].
MSCs are recruited to different locations in the periphery by chemokine release from sites of injury. Stromal derived factor-1 (SDF-1) is an example of such a chemokine, and its ability to attract BMSCs is well characterized [17]. Osteopontin (OPN) secreted by several cell types, has been shown to attract BMSCs to sites of active bone remodeling, vascularization, cell regeneration, and inflammation [17,18]. Other central factors involved in MSC migration and tissue repair include: basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF)-A, hepatocyte growth factor (HGF) platelet-derived growth factor (PDGF), and transforming growth factor (TGF-β) [17]. (Table 1) depicts these main repair-signaling molecules and the cell types that secrete them.
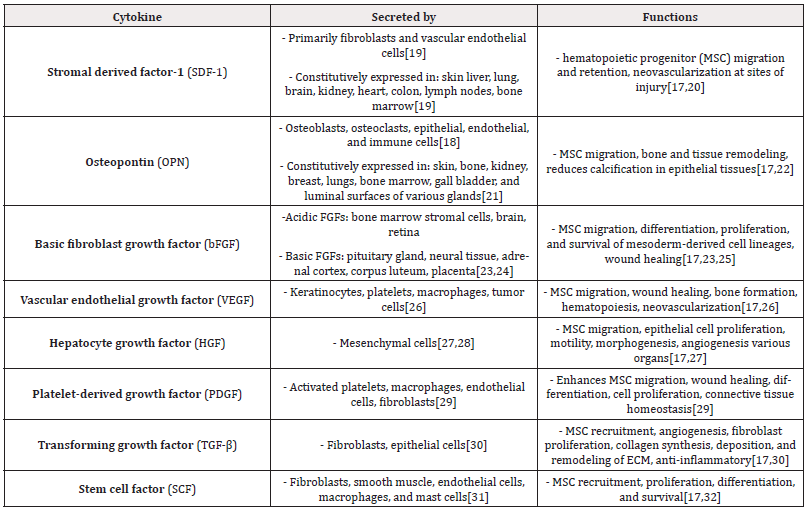
Table 1: Centrally-involved cytokines and factors involved in mesenchymal stem cell (MSC) migration and tissue repair.
Mesenchymal Stem Cells and Wound Healing
Once at the site of injury, mesenchymal stem cells initiate the healing process by several mechanisms: directed differentiation, fusion with local cells, microvesicle/exosome release, and paracrine signaling [33]. Stem cell-derived microvesicles have been shown to transfer mRNA, microRNA, and proteins to adjacent cells. It is thought that this horizontal gene transfer may help inhibit apoptosis and promote cell cycle entry thereby inducing replication of already differentiated cells [34]. Given the local function of MSC-derived microvesicles on target cells, their release may be considered a part of the paracrine-directed healing response.
Paracrine signaling has been shown to be the main mechanism by which MSCs direct wound healing [33]. As previously discussed, bFGF, VEGF, HGF, PDGF, TGF-β, OPN, and SDF-1 are all potent paracrine factors secreted by BMSCs at sites of tissue injury that direct and enhance wound healing. While each factor has a predominant role (Table 1), they are not secreted in isolation, rather, they are released in combinations and at varying concentrations based on the tissue damage site [17]. Other stem cell-derived factors and cytokines involved in inflammation and healing not previously discussed are listed in (Table 2).

Table 2: Stem cell-derived cytokines and growth factors involved in inflammation, healing, and neuron survival and growth.
Use of MSC-conditioned Media to Decrease Neuronal Inflammation and Increase Axon Repair
MSC engraftment studies have demonstrated enhanced wound healing outcomes as well as nerve regeneration, however, this method comes with the risk of developing teratomas [17,45]. Instead, MSC-conditioned media containing growth factors and chemokines is an attractive alternative. BMSCs secrete neurotrophic factors like: nerve growth factor (NGF), glial-cell-line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and ciliary neurotrophic factor (CNTF) all of which promote nerve survival and growth [45].
Furthermore, MSCs have been shown to have anti-inflammatory, neurotropic, neuroprotective, and angiogenic effects due to release of tumor necrosis factor β1 (TNF), interleukin 13, 18, ciliary neurotrophic factor (CNTF), and neurotrophin 3 factor (NT3) in addition to release of: BDNF, NGF, bFGF, CNTF [36]. Release of VEGF, IGF, PDGF, IL-6, IL-8, TGF-β, and HGF from BMSCs promotes angiogenesis and enhances healing at sites of injury. Last, MSCs can promote nerve regeneration by inhibiting inflammation and apoptotic pathways [36]. From this pilot study, we propose a potential method of reversing DN-associated nerve damage using mesenchymal stem cell secretion product-conditioned cream applied directly to affected areas.
Materials and Methods
Stem Cell Media-conditioned Cream
Following culture, the stem cell-conditioned media was collected and frozen in sterile bottles at -80oC. Bottles of conditioned media were sent in temperature-monitored boxes in dry ice to our formulator where the media was blended with polysorbate 80 using overhead mixer and Heidolph Overhead Stirrer Impeller BR 12 Pivoting-Blade at a 30o angle for 1 hour at 400 rpm.
The stem cell media and sorbate blend was slowly added to a cream base and stirred at 600rpm with a straight blade impeller. The blended formulation was left to rest at room temperature for 2 hours. The blended cream was either transferred to an aseptic filler hopper for bottling in airless containers or to climate-controlled storage at 20oC until filled. Samples were labeled and shipped using standard methods as the formulation is stable after filling. Each lot of MSC-conditioned media vials was labelled with a production date and unique lot number. Samples from each lot were subjected to internal quality control including aerobic, anaerobic microbiology (48 hr and 7-day review). One vial from each lot was archived while the remaining vials were stored at 4ºC until they were distributed. Each vial was shipped with use instructions. Needle rollers were advised to be used with caution and as per the instructions provided by the manufacturer.
Other Ingredients in Neurocream
Organic aloe leaf juice, SCM conditioned media, Glycerin, DMAE Bitartrate, Meadowfoam Seed Oil, Emulsifying Wax, MSM, Vitamin E, Sunflower Seed Oil, Organic Blue Green Algae Extract, Organic White Willow Bark Extract, Organic Neem Seed Oil, Organic Rosemary Leaf Extract, Organic Sunflower Seed Oil, Organic Alcohol, Xanthan Gum, Tetrasodium Glutamate Diacetate, Organic Pepermint Oil.
Participants/ Pain Score
Adult type 2 diabetic patients (T2D) with progressive neuropathy and non-type 2 diabetic patients (non-T2D), mean age 52.5 years, were invited to participate in this pilot study where they were provided a vial of newly formulated MSC media-conditioned cream (MSCM-conditioned cream). Patients completed a brief survey before and after one month of treatment. All participants in the T2D group reported taking metformin daily, and nobody in either group reported regular use of medications for neuropathy.
Participants applied our MSCM-conditioned cream twice daily to the soles of their feet after brief micro needling (0.25μm).
Neurocream Application Protocol
After bathing and washing the affected area, participants were instructed to spray the 25μm needle roller and feet soles with disinfectant spray. The disinfectant spray was allowed to dry for 1 to 2 minutes, after which, the serum was applied. Participants were then instructed to roll the needle roller gently over the applied serum. Once done, the microneedle roller was rinsed and disinfected.
Statistical Analysis
Quantitative values were presented as mean ± standard deviation. Statistical analysis was conducted in Excel®. An unpaired, two-tailed t-test assuming unequal variance was used to compare differences between the treatment and the control groups at specific time points. A value of p<0.05 was considered statistically significant.
Results
In a pilot trial of three adult type 2 diabetic patients (T2D) and three adult non-type 2 diabetic participants (non-T2D), mean baseline scores for individuals in the type 2 diabetes (T2D) group were 4 (severe) for foot pain, 4 (severe) for tingling in the feet, and 3.3 (high-severe) for foot swelling. Mean baseline scores for individuals in the non-T2D group were 1.67 (slight-moderate) for foot pain, 1 (slight) for tingling in the feet, and 2.3 (moderate-high) for foot swelling. Mean scores after 30 days of twice-daily topical application for individuals in the T2D group were 2 (moderate) for foot pain, 2 (moderate) for tingling in the feet, and 1.67 (slight-moderate) for foot swelling. Mean scores after 30 days of twice-daily topical application for individuals in the non-T2D group were 1 (slight) for foot pain, 1 (slight) for tingling in the feet, and 1 (slight) for foot swelling.
See table (Table 3) for mean baseline and 30-day scores ±SEM. Individuals in the T2D group experienced an average decrease in all symptoms of approximately 50% at 30 days, and individuals in the non-T2D group experienced a 57% decrease in swelling, a 40% reduction in foot pain, and no change in reported tingling after 30 days of Neurocream use. All participants reported improved sleep.
The largest decrease was in the swelling score for the control group (57%), however this group saw no change in tingling from baseline through 30 days. In contrast, the T2D group’s foot pain, tingling, and swelling scores were all decreased by 50% in 30 days. Between groups, foot pain and tingling scores were greater in the T2D group compared to the control group at baseline. At the end of the 30-day trial there was no significant difference in foot pain, tingling, or swelling between groups after using Neurocream for 30 days see (Figure 2). (Table 4) depicts quantitative pain scores from (Table 3) as descriptive measurements (none, mild, moderate, severe, and intolerable).
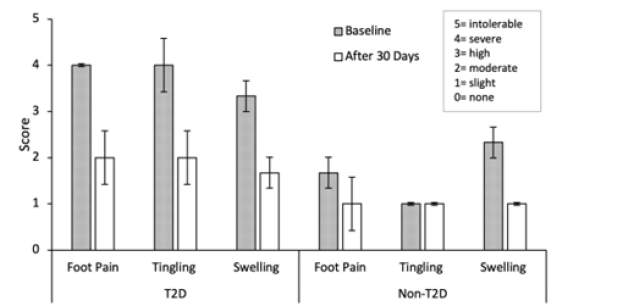
Figure 2: Mean self-reported scores for individuals in the type 2 diabetic group (T2D) and the non-type 2 diabetes group (non-T2D) at baseline (Pre) and after 30 days of daily topical application of Neurocream. Error bars are ±SEM, n=3. Individuals in the T2D group experienced an average decrease in pain score in all symptoms of approximately 50% at 30 days, and individuals in the non-T2D group experienced a 57% decrease in swelling, a 40% reduction in foot pain, and no change in reported tingling after 30 days of daily Neurocream use. Figure generated in Microsoft Excel®..
Discussion
Diabetic peripheral neuropathy (DN) is a complication of diabetes that arises from chronic inflammation which leads to structural and functional changes in peripheral nerves. This occurs most-commonly in the lower extremities and results in increased morbidity and mortality [14]. Stem cell-based therapies represent a potential new type of wound healing treatment, especially for intractable, non-healing pathologies like DN. The use of stem cells to enhance wound healing only began to gain attention around the early 2000s. The number of wound healing studies implementing mesenchymal stem cells (MSCs) has increased appreciably only within the last few years, and several studies have demonstrated MSC’s potential to enhance wound healing and reduce inflammation beyond what occurs with conventional treatment methods [46-50]. The direct application of cells to traumas and pathologies presents several challenges, however. Some of which include: histocompatibility, tumor formation, cell collection, storage and delivery conditions, commercialization of cell-based therapies, and public perception [51,52].
In light of these challenges, and with the knowledge that MSC-directed healing occurs predominantly through paracrine effects, focus has shifted to the development of cell-free therapies like the use of MSC-conditioned media. Studies support the efficacy of this delivery method in numerous tissue types and pathologic states including: keloid scars, dermal trauma, periodontal disease, hypoxia-induced endometrial injury, and diabetic neuropathy [13, 53-56].
Using MSC-conditioned media, we observed a 50% reduction in foot pain, tingling in the feet, and swelling in the diabetic group. This may have been due to the presence of neuroprotective factors like NT3, BDNF, GDNF, and CNTF, as well as anti-inflammatory, anti-apoptotic cytokines like TGF-β, IL-10, IL-13, IL-18, and TNF β from the MSC-conditioned media in Neurocream. Although results were not statistically significant, a 50% reduction in foot pain, from severe to moderate in all three scores, would likely be clinically significant and likely a welcome relief to patients suffering from DN. Similarly, the 40% and 57% reduction in foot pain and swelling scores in the non-diabetic group provide evidence that Neurocream may be of benefit to a broader range of individuals, even those not suffering from diabetes. Although the tingling scores for individuals in the non-diabetic control group remained constant from baseline to 30 days, people in this group reported an average score of 1 (slight) at both time points. This suggests, tingling was not a predominant sensation for these individuals rather than suggesting the MSC-media had no effect on this symptom.
While these results are promising, there were some limitations to our study. First, the small sample size (n=3) could affect reliability. However, this was a pilot study, and the results show that a follow up study with a larger sample would be warranted to validate these results. Second, Neurocream was applied for 30 days which may not have been long enough to elucidate the full effects of the treatment. A longer topical application period may have reduced foot pain, tingling, and swelling further or even eliminated them altogether. Prolonged use of cytokines and growth factors could have had negative effects locally such as increased inflammation or local immunosuppression. Third, the conditioned media was not characterized in this study. Characterization could provide insights into the predominant cytokines/factors involved in DN symptom amelioration. This could allow for development of a cytokine/ growth factor serum without the need for MSC collection and culture. A future study would seek to address these current limitations.
Conclusion
Our study indicates that MSC-conditioned media could be a novel alternative to current diabetic peripheral neuropathy treatments (medications, topical anesthetics, surgery). This method may also reverse existing nerve damage in addition to providing relief from severe DN symptoms. Future prospective studies will further investigate these beneficial effects of Neurocream including novel methods to enhance growth factor penetration into the skin to deliver their healing cargo.
Statement of Research Involving Human Participants
Patients involved in this trial reviewed and signed a standard medical consent.
Conflict of Interest
Funds to this study and its publication came from Global Innovative Health Solutions in Carrollton Georgia.
References
- Galicia Garcia U, Benito Vicente A, Jebari S, Larrea Sebal A, Siddiqi H, et al (2020) Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci 21(17): 6275.
- Committee ADAPP (2022) Standards of Medical Care in Diabetes. Diabetes Care 45: S17-S38.
- (2018) Association AD. Statistics About Diabetes 2018.
- Prevention CfDCa. Chronic Diseases in America.
- (2023) Prevention CfDCa. What is Diabetes?2023.
- Goyal R SM, Jialal I 2023 Type 2 Diabetes.
- (2023) Clinic M. Peripheral Neuropathy.
- Aregbesola AV S, Virtanen JK, Mursu J, Tuomainen TP (2013) Body iron stores and the risk of type 2 diabetes in middle-aged men. Eur J Endocrinol. 169: 247-253.
- Y Zheng, X KL, Y Wang, L Cai (2008) The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin 32(1-2): 135-145.
- Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M (2021) Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)-New Aspects. International Journal of Molecular Sciences 22(19): 10835.
- Li J, Guan R, Pan L (2023) Mechanism of Schwann cells in diabetic peripheral neuropathy: A review. Medicine (Baltimore) 102(1): e32653.
- Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL (2009) Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal 11(3): 425-438.
- De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, et al. (2020) Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 11(1): 168.
- Sloan G, Selvarajah D, Tesfaye S (2021) Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol 17(7): 400-420.
- Kuroda Y, Kitada M, Wakao S, Dezawa M (2011) Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 59(5): 369-378.
- Park CW, Kim KS, Bae S, Son HK, Myung PK, et al (2009) Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2(1): 59-68.
- Fu X, Liu G, Halim A, Ju Y, Luo Q, et al (2019) Mesenchymal Stem Cell Migration and Tissue Repair. Cells 8(8): 784.
- Bastos A, Gomes AVP, Silva GR, Emerenciano M, Ferreira LB, et al (2023) The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int J Mol Sci 24(3): 2942.
- Cun Y, Diao B, Zhang Z, Wang G, Yu J, et al (2021) Role of the stromal cell derived factor‑1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp Ther Med 21(1): 39.
- Deshane J, Chen S, Caballero S, Grochot Przeczek A, Was H, et al (2007) Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med 204(3): 605-618.
- Brown LF, Berse B, Van de Water L, Papadopoulos Sergiou A, Perruzzi CA, et al (1992) Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 3(10): 1169-1180.
- Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. J Cell Commun Signal 3(3-4): 311-322.
- Li X (2018) Chapter 6 - Design and Discovery of FGF/FGFR Inhibitors. In: Li X, ed. Fibroblast Growth Factors. Academic Press 339-383.
- Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G (1987) Structural Characterization and Biological Functions of Fibroblast Growth Factor. Endocrine Reviews 8(2): 95-114.
- Yun YR, Won JE, Jeon E, Lee S, Kang W et al (2010) Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng 218142.
- AM Duffy DB H, JH Harmey (2000-2013) Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. Madame Curie Bioscience Database. Austin (TX): Landes Bioscience.
- Nakamura T, Mizuno S (2010) The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci 86(6): 588-610.
- Kolatsi Joannou M, Moore R, Winyard PJD, Woolf AS (1997) Expression of Hepatocyte Growth Factor/Scatter Factor and Its Receptor, MET, Suggests Roles in Human Embryonic Organogenesis. Pediatric Research 41(5): 657-665.
- Heldin C-H (2003) Platelet-Derived Growth Factor (PDGF). In: Henry HL, Norman AW, eds. Encyclopedia of Hormones. New York 231-237.
- Chaudhury A, Howe PH (2009) The tale of transforming growth factor-beta (TGFbeta) signaling: a soigné enigma. IUBMB Life 61(10): 929-939.
- Frangogiannis NG, Entman ML (2000) Chapter 32 - Mast Cells in Myocardial Ischaemia and Reperfusion. In: Marone G, Lichtenstein LM, Galli SJ, eds. Mast Cells and Basophils. London 507-522.
- Hassan HT, Zander A (1996) Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol 95(3-4): 257-262.
- Pankajakshan D, Agrawal DK (2014) Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J Biomed Technol Res 1(1): 10.
- Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G (2012) Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 27(8): 3037-3042.
- Brennan K, Zheng J (2007) Interleukin 8. In: Enna SJ, Bylund DB, eds. xPharm: The Comprehensive Pharmacology Reference. New York Elsevier 1-4.
- Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, et al (2019) Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy Int J Mol Sci 20(11): 2698.
- Swain SL (2001) Interleukin 18: tipping the balance towards a T helper cell 1 response. J Exp Med. 194(3): F11-14.
- Verrecchia F, Mauviel A (2004) TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal 16(8) 873-880.
- Laron Z (2001) Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol 54(5): 311-316.
- Andres RY (1980) Bradshaw RA. NERVE GROWTH FACTOR. In: Kumar S, ed. Biochemistry of Brain. Pergamon 47: 545-562.
- Kakizawa S (2021) Subchapter 41C - Neurotrophin-3. In: Ando H, Ukena K, Nagata S, eds. Handbook of Hormones (Second Edition). San Diego 483-485.
- Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11(6): 1164-1178.
- Fitzgerald KA, O Neill LAJ, Gearing AJH, Callard RE (2001) GDNF. In: Fitzgerald KA, O'Neill LAJ, Gearing AJH, Callard RE, eds. The Cytokine FactsBook and Webfacts (Second Edition). London 260-266.
- Krüttgen A, Grötzinger J, Kurapkat G, Weis J, Simon R et al (1995) Human ciliary neurotrophic factor: a structure-function analysis. Biochem J 309 (Pt 1) (Pt 1): 215-220.
- Lavorato A, Raimondo S, Boido M, Muratori L, Durante G, et al (2021) Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int J Mol Sci 22(2): 572.
- Mehanna RA, Nabil I, Attia N, Bary AA, Razek KA, et al (2015) The Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Their Conditioned Media Topically Delivered in Fibrin Glue on Chronic Wound Healing in Rats. Biomed Res Int 2015: 846062.
- Mathen C, Ghag Sawant M, Gupta R, Dsouza W, Krishna SG (2021) Evaluation of Potential Application of Wharton's Jelly-Derived Human Mesenchymal Stromal Cells and its Conditioned Media for Dermal Regeneration using Rat Wound Healing Model. Cells Tissues Organs 210(1): 31-44.
- Jin MH, Yu NN, Jin YH, Mao YY, Feng L, et al (2021) Peroxiredoxin II with dermal mesenchymal stem cells accelerates wound healing. Aging (Albany NY) 13(10): 13926-13940.
- Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, et al (2010) Mesenchymal stem cells' interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen 18(6): 655-661.
- Jung H, Kim HS, Lee JH, Lee JJ, Park HS (2020) Wound Healing Promoting Activity of Tonsil-Derived Stem Cells on 5-Fluorouracil-Induced Oral Mucositis Model. Tissue Eng Regen Med 17(1): 105-119.
- Yamanaka S (2020) Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 27(4): 523-531.
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10(1): 68.
- Liang X, Lin F, Ding Y, Zhang Y, Li M, et al (2021) Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res Ther 12(1): 295.
- Bojanic C, To K, Hatoum A, Shea J, Seah KTM, et al (2021) Mesenchymal stem cell therapy in hypertrophic and keloid scars. Cell Tissue Res 383(3): 915-930.
- Nagata M, Iwasaki K, Akazawa K, Komaki M, Yokoyama N, et al (2017) Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A 23(9-10): 367-377.
- Wang H, Liu S, Zhang W, Liu M, Deng C (2022) Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosome Repairs Endometrial Epithelial Cells Injury Induced by Hypoxia via Regulating miR-663a/CDKN2A Axis. Oxid Med Cell Longev 2022: 3082969.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.