Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Main Determinants Impacting the Survival of Malignant Tumors of the Small Intestine
*Corresponding author: Imane Ouafki, Medical Oncology Department, Hassan II University Hospital, Sidi Mohamed Ben Abdellah University, Fez, Morocco.
Received: January 02, 2024; Published: January 11, 2024
DOI: 10.34297/AJBSR.2024.21.002808
Abstract
Introduction: Malignant neoplasms of the small intestine constitute an extremely rare entity. Their insidious nature as well as their almost asymptomatic evolution are responsible for a late diagnosis, sometimes following a hemorrhagic or occlusive complication. Reason why these tumors are often accompanied by a poor prognosis. This article made it possible to analyze the main determinants impacting the survival of these patients in order to evaluate the effectiveness of the therapeutic means deployed in their treatment.
Materials and Methods: This is a retrospective cohort study, having collected patients with a malignant tumor of the small intestine, treated in the medical oncology department, of the Hassan II University Hospital Center in Fez, from January 2016 to January 2020. A description of patient characteristics was carried out before analyzing the 2-year overall survival (OS) according to the different risk factors reported in the literature.
Results: We included 43 patients. The average age was 57 years, with a female predominance. A history of smoking was common to 34.8% of patients. The ileal localisation was the most found (58.1%). Adenocarcinoma (ADK) was the most common histological type (42%). The disease was diagnosed at an advanced stage in 58.1% of cases. Optimal surgery was performed in more than half of patients (53.4%). Chemotherapy was administered to 88.3% of patients. The median OS at 2 years was 23.8 months (95% CI: 18.6-28.9), representing a survival rate estimated at 25.6%. We observed a significantly short survival in patients over 50 years old (11.6 months; 95% CI: 7.6-15.5; p=0.002), with a performance status (PS) greater than 1(11.5 months; 95% CI: 6.4-16.5; p=0.017), hypertensive patients (6.5 months; 95% CI: 5.26-7.8; p=0.019), in the case of duodenal location of the primary tumor (8.6 months; 95% CI: 1.8-15.4; p=0.041), for neuroendocrine carcinomas (NEC) (8.6 months; 95% CI:2.3-15.0; p= 0.026) and in stages IV (8.6 months; 95% CI: 7.4-9.8; p=0.000). For the other factors studied: male sex, history of smoking, alcoholism, celiac disease and neurofibromatosis, , we found a numerical drop in survival but without statistically significant benefit. While there was no impact on survival in diabetic patients, with a history of Crohn’s disease and breast cancer. However, in multivariate analysis, the factors that were found to be prognostic for survival were advanced age, impaired PS, duodenal location of the primary tumor, the metastatic stage of the disease compared to localized stages and sarcomatous histology unlike ADK which had better survival.
Conclusion: This study confirms, in our context, the results of the literature. Although we have not been able to prove the significant association with survival of all the factors already validated. This is probably justified by the lack of statistical power relating to the retrospective design of the study and the low representativeness of the workforce.
Keywords: Determinants, Survival, Tumors, Malignant, Intestine, Small
Opinion
Malignant tumors of the small intestine are very rare and represent 2 to 6% of all cancers of the gastrointestinal tract [1,2]. The histological types found in the small intestine are adenocarcinomas (ADK) which are the most common, sarcomas, neuroendocrine carcinomas (NEC), gastrointestinal stromal tumors (GIST) and lymphomas [3]. These patients often have a poor prognosis, insofar as median overall survival (OS) is of the order of 19 months, with a 5-year OS around 30% [4]. Indeed, the 5-year survival rates do not exceed 28% for ADK, are 30% for lymphomas and 60% for NEC [5]. The symptoms can be chronic or intermittent, but most tumors of the small intestine are clinically silent for a long time. Nearly half of tumors are found incidentally, during surgery or exploration visualizing the intestine. As a result, patients can remain asymptomatic until the final stages of the disease or during an indicated intervention for acute intestinal obstruction [6]. The heterogeneity of these tumors as well as the limited number of cases have meant that all currently available data are based on small studies or retrospective series [6]. Due to the low incidence of small intestinal neoplasms, it is not easy to recruit a sufficient number of patients for a study. To better understand this question, we collected patients with small intestine cancers treated in our medical oncology department. The main objective of this work is to analyze the determinants impacting the survival of these patients in order to evaluate the effectiveness of the therapeutic means used in their treatment as well as their prognosis.
Materials and Methods
This is a retrospective cohort study, including patients with a malignant tumor of the small intestine, treated in the medical oncology department, of the Hassan II University Hospital Center in Fez, on a period spread from January 2016 to January 2020. The inclusion criteria were: age over 18 years; histologically confirmed cancer of the small intestine; localized or metastatic. The exclusion criteria were lymphoma histology and non-consent of the patient. Eligible patients were searched from the “HOSIX” information system database, by selecting patients treated for a malignant tumor of the small intestine. The non-opposition of patients to the use of data concerning them was verified before the analysis by verbal consent. The agreement of the regional ethics committee was obtained. The data are entered using Excel software, then analyzed using SPSS software version 20. The significance level was set at p<0.05 for all statistical tests with a 95% confidence interval (95% CI). The descriptive analysis focused on the age distribution which was transformed into a binary qualitative variable (<50 years and >50 years), sex, performance status (PS), history, tumor locations, histological types noted, stage of the disease, metastatic sites found. We have also described the medico-surgical therapeutic modalities including: surgery ,monitoring and chemotherapy. We also detailed the different chemotherapy protocols. The 2-year survival which is the main objective of the study, was estimated from the date of diagnosis for a 2-year follow-up. The estimation of survival and 95% CI were determined using the Kaplan-Meier method. Comparison between patient groups was performed using the log-rank test for the factors studied. Cox regression analysis was used to calculate respective hazard ratios and 95% CIs. In multivariate analysis, Cox regression was used to test the independence of significant factors in univariate analysis. The significance level was set at p<0.05 for all statistical tests.
Results
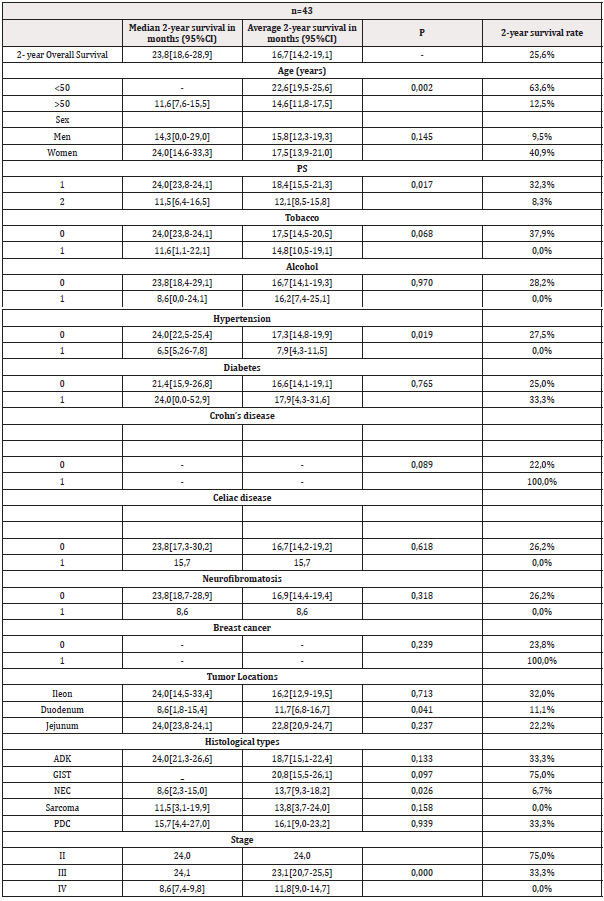
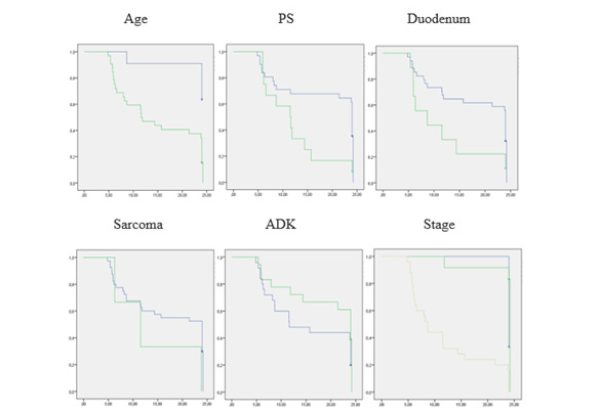
the 43 patients who met the eligibility criteria, the average age was 57 years (35-86) of which 74.4% were over 50 years old. The male/female sex ratio was 0.95. The PS was 1 in 72.1% of patients. A history of smoking was noted in 34.8% of patients. Other pathological histories were hypertension, diabetes, Crohn’s disease, celiac disease, neurofibromatosis and breast cancer. Ileal location was reported in 58.1% of cases. ADK was found in 42% of cases (Figure 1). The disease was diagnosed at an advanced stage in 58.1% of patients (Table 1). Optimal surgery was performed in 53.4% of cases. Monitoring after surgical treatment was decided in 11.6% of patients and 88.3% had received chemotherapy. The CAPOX regimen (Capecitabine + Oxaliplatin) was administered in 44.1% of patients (Table 2). The median 2-year overall survival (OS) was 23.8 months (95% CI 18.6-28.9) with a survival rate estimated at 25.6%. In univariate analysis, among those under 50 years old the 2-year survival rate was 63.6% compared to 12.5% among the oldest (p=0.002). Patients with a PS of 1 had a median survival of 24 months compared to 11.5 months in those with a PS of 2 (p=0.017). The 2-year survival rate was 37.9% for nonsmokers and 0% in those with a history of smoking (p=0.068). Hypertensive patients had a survival rate of 0% compared to 27.5% for the others (p=0.019). Duodenal location was accompanied by a median survival of 8.6 months compared to 24 months for ileal and jejunal tumors (p=0.041). Survival rates in ADK, GIST, NEC, sarcomas and poorly differentiated carcinomas (PDC) were respectively 33.3%; 75.0%; 6.7%; 0.0% and 33.3%. Stages IV had a median survival of 8.6 months compared to 24 months for stages II and III (p=0.000) (Table 3) (Figure 2). In multivariate analysis, the factors significantly associated with survival were age (p=0.000), PS (p=0.030), duodenal location of the primary tumor (p=0.001), stage of the disease (p= 0.000), sarcomas (p=0.004) and ADK (p=0.010) (Table 4).

Figure 1: Distribution of histological types found in the 43 patients followed for malignant tumors of the small intestine, medical oncology department, Hassan II University Hospital, Fez, Morocco, January 2016-January 2020.

Table 1: Characteristics of patients followed for malignant tumors of the small intestine, medical oncology department, Hassan II University Hospital, Fez, Morocco, January 2016-January 2020.
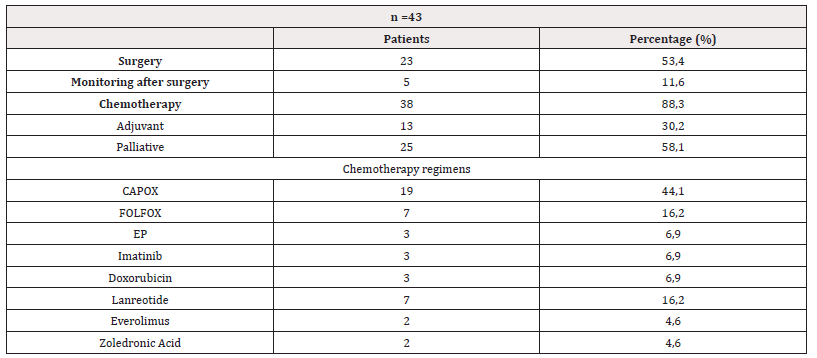
Table 2: Therapeutic modalities of patients followed for malignant tumors of the small intestine, medical oncology department, Hassan II University Hospital, Fez, Morocco, January 2016-January 2020.
Note*: CAPOX capecitabine plus oxaliplatin;FOLFOX fluorouracil/leucovorin plus oxaliplatin ;EP etoposide plus platin (cisplatin or carboplatin).p




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.