Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Post-Infectious Autoimmunity Syndrome (PIFAS): Part 1 Unlocking the Power of PIFAS-Related Pathogenic and Clinical Values Through the Implications in Clinical Cardiology Practice
*Corresponding author:Sergey Suchkov, Russian Academy of Natural Sciences (RANS), Moscow, Russia.
Received: February 27, 2024; Published: March 05, 2024
DOI: 10.34297/AJBSR.2024.21.002887
Abstract
Development and progression of Postinfectious Autoimmunity (PIAI) are stimulated by the immunogenic resources of the host tissue and tissue-associated antigens, while the very initial (pre-early) phases of PIAI were postulated and are proved today to be mediated by mimicking epitopes. Molecular mimicry is one of the key triggering mechanisms in PIAI and autoimmune disorders, and autoimmune process following molecular mimicry can be considered as failed side effect of immune reactions. Molecular mimicry concept provides approaches to etiological agents associated with the PIAI progression identification as well as specific prevention and treatment modalities to control the development of the autoimmune disorders and Autoimmune Myocarditis (AIM), in particular. Hypothesis on the possible involvement of molecular mimicry in the development of PIAI and Post-infectious Autoimmune Syndrome (PIFAS) becomes very intriguing. It provides new approaches to identify etiological agents associated with PIAI, paired microbial and tissue-linked epitopes targeted for autoimmune reactions determination, PIAI pathogenesis recognition and specific prevention and therapy for autoimmune disorders and AIM development.
Soon, genotyping and phenotyping results combined and consolidated under the aegis of IT-assisted algorithms will be used for the creation of unified databanks necessary for per-sonal health biomonitoring of PIFAS and PIFAS-driven conditions. The novel diagnostic ideology is expected to be based on a combination of two categories of investigations:
i. pathogenically oriented (OMICS-based) diagnosis of PIFAS
ii. screening of microbial pathogens (microbiome-related profiling) as the main causal factors of PIFAS.
Introduction
Autoimmunity is still a mystery of clinical immunology and medicine paired with daily clinical practice. Etiology and pathogenesis of autoimmune disorders remain to be unclear, and thus be assessed as a kind of balance between hereditary (genetically based) predisposition, triggering (including environmental) factors and the appearance of autoantibodies (auto Abs) and/or self-reactive T cells (Figures 1A,1B). Autoimmune diseases are conditions in which an individual’s own immune system mistakes its own tissue as an infection or foreign substance (as the NON-SELF). The immune system then creates auto Abs and autoreactive cytotoxic T cells (CTLs) to attack the tissue(s) it identifies as problematic, causing destruction to the tissue and organ structure. There are more than 200 types of autoimmune conditions, and since any body part can be involved in, due to the wide variety of possible Abs and CTLs that can be produced, spectrum and symptoms of autoimmune diseases vary greatly (Figure 2). Despite numerous studies presented in the field, etiology and pathogenesis of autoimmune disorders remain to be unclear and thus need to be studied further. The correlation and balance between a certain autoimmune dis-ease, and the appearance of auto Abs and/or self-reactive T cells are not always appearing to be evident and sufficient to stress the occurrence of proofs for the state of autoimmunity. In this context, to induce autoimmunity that is provoked by the well-known defects in the autoantigen (autoing) recognition by either T or B cells, at least, four conditions are required:

Figure 1A: The balance of immunity.
Note*: The immunity’s ability to distinguish self from nonself is negatively impacted by genetic factors and environmental triggers. A combination of host genetic factors and exposure to environmental triggers promote the development of immune imbalance and thus the autoimmune disease. A balance must be maintained between the regulatory T cells and the pathogenic T effector cells Th1, T helper cell 1; Th17, T helper cell 17; TFH, T follicular helper cells; Treg, regulatory T cell; Tr1, T regulatory type 1 (Tr1) cells rep-resenting a distinct population of T cells, which are induced in the periphery upon antigen exposure under tolerogenic conditions; MHC, Major Histocompatibility Complex; IL-23R, interleukin 23R; IL-7R, interleukin 7R; NOD2, Nucleotide-binding oligomerization domain 2 (an intracellular sensor for small peptides derived from the bacterial cell wall component, peptidoglycan) From: Vojdani, Aristo. (2014). A Potential Link between Environmen-tal Triggers and Autoimmunity. Autoimmune diseases. 2014. 437231. 10.1155/2014/437231.s

Figure 1B: Balancing the Immune System and Dysregulation of Immune Balance.
Note*: Immune balance can be disturbed on two levels: immune tolerance (on the left) or immune activation (on the right). Onset of a disease can be simplified by the shift in the balance on one side or the other. From: Source: Neo Stem, Inc.

Figure 2: A spectrum of autoimmune diseases.
Note*: An autoimmune disorder occurs when the body’s immune system attacks and destroys healthy body tissue by mistake. An autoimmune disease is the result of the immune system accidentally attacking your body instead of protecting it. The exact cause of autoimmune disorders is unknown. One theory is that some microorganisms (such as bacteria or viruses) or drugs may trigger changes that confuse the immune system. This may happen more often in people who have genes that make them more prone to autoimmune disorders. There are more than 200 autoimmune disorders. Common ones include lupus, rheumatoid arthritis, Crohn’s disease, and ulcerative colitis. Autoimmune diseases can affect many types of tissues and nearly any organ in your body. They may cause a variety of symptoms including pain, tiredness (fatigue), rashes, nausea, headaches, dizziness and more. Specific symptoms depend on the exact disease. From: https://www.knowbalance.com/autoimmunity---inflammation
i. the presence of pathogenic self-reactive T and/or B cells in persons with the appropriate HLA genotype at high-risk.
ii. the availability of the major autoantigen and/or mimicking antigens at levels sufficient to the T cell presentation and subsequent T cells differentiation, maturation, and activation.
iii. the generation of additional (costimulatory) signals required to activate T and B cells in a proper way.
iv. the loss of the ability of the regulatory T cells to control mechanisms of the auto-immune inflammation.
Meanwhile, immune responses triggered by foreign antigens can be ignored by the proper immunological surveillance mechanisms because self-reactive T and B cells could survive for some reasons. Among the immunological armamentarium, molecular mimicry (Figures 3A,3B) [1] based on self-reactive T- and B-cell activation by cross-reactive epitopes of infectious (in most of the cases) agents, is of special value is of special value in many fields of clinical medicine [1,2]. The relationship between microbes and autoimmunity could be manifested by the presence of auto Abs, autoimmune complexes, or autoreactive CTCs. The presence of autoimmune phenomena in chronic infections could be related to polyclonal B-cell activation, molecular mimicry be-tween microbial and host antigens, altered self, abnormal expression of immunoregulatory molecules, and the anti-idiotypic network (Figures 4A,4B).

Figure 3A: Postinfectious autoimmunity provoked by microbial infection.
Note*: Autoimmune diseases remain one of the mysteries that perplex immunologists. And autoimmunity is a by-product of the immune response to microbial infection. For decades there have been tantalizing associations between infectious agents and autoimmunity: CMV, HSV and B3 coxsackieviruses and myocarditis; Trypanosoma crazy and Chagas’ disease; diverse viruses and multiple sclerosis; B4 Coxsackievirus, cytomegalovirus or rubella and type 1 diabetes, to name the most frequently cited examples. In addition, animal models have provided direct evidence that infection with a particular microbe can incite a particular autoimmune disease. Nonetheless, many of the associations appear less than convincing and, even for those that seem to be on solid footing, there is no real understanding of the underlying mechanism(s). CMV, ……….; HSV, …………….,.

Figure 3B: Microbes initiate autoimmune responses.
Note*: Molecular mimicry of microbial components with self-peptides results in activation of autoreactive lymphocytes and subsequent cytokine and antibody production against self-antigens. Intramolecular or intermolecular epitope spreading amplifies this response. Viral or bacterial superantigens non-specifically activate T cells, further promoting inflammation. From: Caza, Tiffany & Oaks, Zachary & Perl, Andras. (2014). Interplay of Infections, Autoimmunity, and Immunosuppression in Systemic Lupus Erythematosus. International reviews of immunology. 33. 10.3109/08830185.2013.863305

Figure 4A: Idiotypic/anti-idiotypic interactions.
Note*: Due to the ability of internal-image Abs compete with an antigen for binding to Ab and to alter the biologic activity of an antigen, anti-IDs have become a target in the search for new treatments for autoimmune illnesses, cancer, and some other diseases. The mechanism of the formation of anti-idiotypic antibodies (Jerne’s network) is the following: an antigen stimulates the production of Abs (Ab1). The active centers of Ab1 are recognized by the second class of Abs - anti-IDs (Ab2). In their turn, Ab2 serves as an antigen for the third class of Abs (Ab3) - anti-anti-IDs, and so on. The network finally appeared to be not endless, because Ab3 may be identical or close in their recognizing properties to Ab1. Ab tends to make the system respond by increasing the production of anti-X- or anti-Ab specificities. In this interpretation, the immune system does not care about the self- or non-self-character of recognizable antigens, and rather keeps the idiotype-anti-idiotypic balance. Moreover, according to Jerne’s theory it is nothing but autoimmunity, which serves as the key point of the physiologic autoregulation of the immune system. Source: Aliya K Stanova, et al. Anti-Idiotypic Agonistic Antibodies: Candidates for the Role of Universal Remedy. Antibodies 2020, 9, 19.

Figure 4B: The immune network hypothesis.
Note*: MAntibody-mediated immune responses involve complex interactions between two large classes of lymphocytes - the B and T lymphocytes. In many situations, B and T cells reacting against some antigens have been shown to share similar idiotypic specificity. Cell–cell interaction can, therefore, occur through antigen uptake by one cell and recognition of that antigen by a regulatory cell, or through recognition between idiotypic and anti-idiotypic cell-surface receptors. The anti-idiotypic network amplifies antigenic signals. (A) An anti-body Ab1 is produced in response to a specific antigen. (B) With a defined idiotype, Ab1 induces the production of an anti-idiotypic antibody Ab2. This Ab2 may resemble the original antigen as an internal image. (C) Ab2 can stimulate the synthesis of an anti(anti-idiotypic) antibody Ab3 which principally is of the same specificity as Ab1. The immunization with an antigen (i.e., CA125) generates an anti-antigen antibody called Ab1, which induces the formation of an anti-idiotype antibody (Ab2). Its antigenic determinant mimics the first antigen. So it could be used to generate a third antibody Ab3 also called Ab1’ which recognizes the original antigen identified by Ab1. Source: Giuseppe Bronte, Giuseppe Cicero, Giovanni Sortino. Immunotherapy for recurrent ovarian cancer: A further piece of the puzzle or a striking strategy? January 2014Expert Opinion on Biological Therapy 14(1):103-14.
In most cases, the appearance of self-reactivity in the sera of patients with chronic infections is not associated with clinical manifestations. These findings suggest that autoimmune dis-ease is the result of a combination of factors including immunologic, genetic, hormonal, and environmental. Infectious agents have a role in the breakdown of tolerance and the appearance of autoreactivity. However, only patients with the proper immunogenetic and hormonal background may develop clinical manifestations of autoimmune disease. Despite the extensive knowledge that has accumulated, the specific relationship between infections and autoimmunity is still obscure. Hypotheses regarding the possible involvement of molecular mimicry in the development of Post-infectious Autoimmunity (PIAI) are currently very intriguing. They provide new approaches for identifying etiological agents that are associated with PIAI, paired microbial and tissue linked epitopes targeted for autoimmune reaction determination, PIAI pathogenesis recognition and specific prevention, and therapy for autoimmune disorder development. Hypothesis on possible involvement of molecular mimicry in the development of PIAI emphasizing the significance of adaptive immune resources for the recognition of the cross-reactive (mimicking) determinants and induction of auto aggression becomes very intriguing.
Conceptual Model of PIAI and Post-Infectious Autoimmune Syndrome (PIFAS)
To understand the role of molecular mimicry in PIAI induction, one should refer to the mechanism of interaction between infectious agent and the host immune system. Most of the infections represent a stepwise development of the canonical immune response associated with genetic pre-disposition and featured microbiota or, more individually and specifically, endomicrobiota. Apart from common pathogens known, commensal microbes are also able to interact with the immune machinery to break the control of the host’s defence over endomicrobiota whilst causing a disorder. Interactions of endomicrobiota with the host’s immune machinery in due the course of the immune response would give rise to different types of dysbiosis whilst illustrating an ability of the commensal microbes and pathogens to suppress the immune response at the first (initial) steps of the microbe-driven response (Figure 5).
The commensal microbiota affects many aspects of mammalian health including control of the immune system to such an extent that a “commensalocentric” view of the maintenance of overall health could be suggested. Autoimmunity (PIAI) is a case of mistaken identity: the immune system reacts to self-tissues and cells as if they were pathogens. Autoimmune reactions can be both advanced or blocked by the commensal microbiota, which can affect innate and adaptive arms of immune responses as well as the mechanisms of “innate-adaptive connection.” Whether specific microbial lineages affect immunity and autoimmunity (the “specific lineage hypothesis”) or multiple lineages can tip the homeostatic balance that regulates host/microbiota homeostasis toward reduced or enhanced host reactivity (the “balanced signal hypothesis”) is yet unknown. The complexity of host/microbiota interactions needs to be fully appreciated to find the means for prophylaxis and treatment of autoimmune disorders (including PIAI). The key role of both microbial and immune-related factor to trigger the immune pathogenesis is evidently proved today. However, a comparison of types of the host’s immune responses and the features of microbiota with the immune deviations and imbalances, on one hand, and clinical patterns of the disease, on the other one, is appearing to be a more complicated aim and objective to be cleared further. Meanwhile, the key role in the chronification of the primary (microbe-driven) immune disorder is played by:
i. an invading pathogen, or/and attributed to dysbiosis.
ii. a genetically predisposed host’s immune response to result in a form of post-infectious clinical immunological syndrome (PICIS) (Figure 6).

Figure 6: The interaction of microbial, genomic, and immune-related factors to provoke the immune balance and to trigger the immune pathogenesis of post-infectious clinical immunological syndrome (PICIS)
Note*: (A) In healthy hosts, an efficient immune barrier contains the microbiota in the gut lumen and feedback mechanisms avoid excessive activation of host immune responses. ‘Peacekeeping’ bacteria that release anti-inflammatory products participate in the tuning of host responses towards tolerance and help to prevent the pro-inflammatory effects of any pathobionts that are present in the microbiota, thus maintaining intestinal homeostasis (B & C). In dysbiosis: immunodeficient patients, who lack an important component of the gut barrier (for example, secretory Igs in patients with CVID or a key regulatory pathway (for example, loss-of-function mutations that affect the IL-10R spontaneously develop intestinal inflammation when exposed to the microbiota. Treg, ……….; Th17, ……………………; IL, ……………….,.
One of the issues regarding the latter is mutualism between the host’s immune machinery and endomicrobiota regarding shaping the immune response to mediate the balance between health and disease. And the commensal endomicrobiota affects control over the immune system. Moreover, given the intimate interplay between endomicrobiota and the host immune system, it is not surprising that some members of the microbiota have been linked to PIFAS. So, keeping a delicate balance in the immune system by eliminating invading pathogens or treating dysbiosis, whilst still maintaining self-tolerance to avoid autoimmunity, is critical for the body’s health. And unlocking how beneficial microbes affect the development of the immune system may lead to novel and natural therapies based on harnessing the immunomodulatory properties of the endomicrobiota. So, along with the immune-mediated factor to drive the immunopathology, a microbial factor may also promote development of immune imbalances whilst forming several types of PICIS, owing the endomicrobiota to get armed with intrinsic tools enabling to escape from or to ignore at-tacks of the host’s immune machinery. The severity and duration of CDIO depends on the interaction between the endomicrobiota spoiled and/or invading pathogen, on the one hand, and the antimicrobial immunity machinery, on the other one.
The severity and duration of microbe-driven PICIS depends on the interaction be-tween the endomicrobiota spoiled and/or invading pathogen, on the one hand, and the antimicrobial immunity machinery, on the other hand. At this point, PICIS would determine apart from a type of immune-mediated pathology, an extent of progression of the underlying disease, and assessing the risks of complications is of great predictive and prognostic significance to be valuable and informative for the purposes being personalized, to manage the progression of the process. Due to our long-term experience and practice, PICIS along with versions of canonical Post-Infectious Secondary Immunodeficiency Syndrome (PIFSIS), would include post-Infectious Autoimmune Syndrome (PIFAS) as one of the alternatives of microbe-induced and driven immune imbalances (Figure 7).
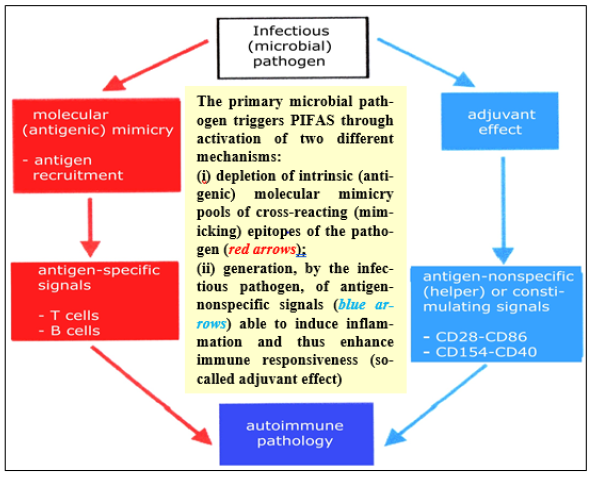
Figure 7: A schematic representation of the Postinfectious Aute Syome (PIFAS)
Note*: A crucial role in formation of PIFAS and progression to develop CDIO is played by inborn (in the first ace, HLA-associated) predisposition coupled with impaired immune responsiveness of the human body invaded and/or having had signs of dysbiosis since recent data has stressed the importance of the end microbiota in immune homeostasis CDIO, chronic diseases of infectious origin; PIFAS, postinfectious auto-immune syndrome.
The situation is drastically different at the initial stages of the aggressive courses and/or at stages of advanced progression of the infectious process, and at its transformation from a subclinical into the clinical stage. In those cases, the incidence of PIFAS increases substantially. PICIS would determine an extent of progressing and chronification of the underlying disease whilst assessing the risks of complications is of great predictive and prognostic significance to be valuable and informative for the preventive and prophylactic purposes. The latter is important so much to illustrate a principally new (systems, in terms of Personalized & Precision Healthcare standards) approach to chronic disease that would focus on the integrated predictive, prognostic, subclinical diagnostic standards to secure the effective prevention of the disorder in individual clients (patients or at-risk persons).
Let us to remind that simple biomarkers that are used to assess CDIO as a multifactorial disorder are effective once the disease is well established, but none, thus far, are reliable for the detection of the pre-early (subclinical) manifestations. Current methods and simple biomarkers that are used to assess CRID are effective once the disease is well established, but none, thus far, are reliable for the detection and monitoring of the pre-early (subclinical) manifestations. And there is thus urgent need to identify combinatorial biomarkers for subclinical PIFAS, to allow preventive/treatment strategies to be instituted early in the disease process. So, a host-mimicking pathogen tandem whilst being a combinatorial biomarker could regulate the responses of the cells to the signalling pathway and thus calibrate the host defence whilst limiting tissue damage and preventing PIAI. In this sense, this approach would be considered as a fundamentally new strategy based on a recognition of multifunctional and combinatorial biomarkers (Figure 8) of the next step generations long before the clinical illness. And there is thus urgent clinical need to identify predictive combinatorial biomarkers for sub-clinical PIFAS to get a panel of upgraded diagnostic tests performed and used the latter (Figure 9) and allow preventive treatment strategies to be instituted at the pre-early stages in the disease pro-cess. The need for biomarkers of the next step generations, newer algorithms, software applications to support integrative data analysis and to secure the databanks becomes critical to the discovery of linkages and concordance between different data types such as OMICS-related, IT-assisted, and clinically rooted ones! For instance, in cardiology-related practice, AIM usually develops in genetically predisposed individuals infected with the CVB3 or related viruses to represent typical manifestations of molecular mimicry. The presence of CM-AR CTLs and anti-CM auto Abs is prerequisite to AIM development to initiate myocardial lesions (Figures 10A,10B).
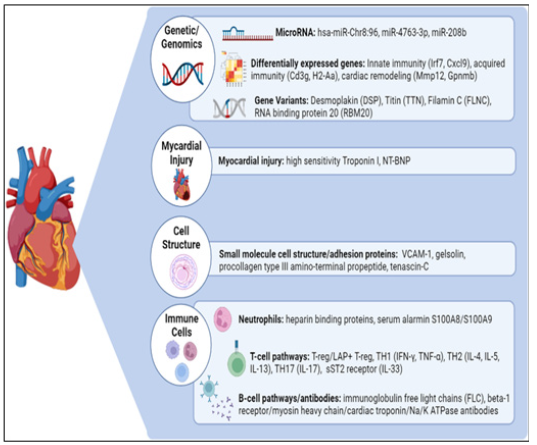
Figure 8: Multifunctional and combinatorial biomarkers for autoimmune myocarditis.
Note*: Myocarditis is a disease caused by cardiac inflammation that can progress to dilated cardiomyopathy, heart failure, and eventually death. The pre-early and accurate diagnosis, and effective treatment remain challenging due to the high heterogeneity. Therefore, the identification of accurate, predictive, and prognostically informative biomarkers is critical for screening and treatment. The biomarkers are categorized into several families based on their site; cardiomyocyte biomarkers, cell-related and microenvironmental biomarkers, immune biomarkers, and macro-environmental biomarkers The functionally valuable and combinatorial biomarkers presented here, have shown great promise in clinical translational research studies and provide clinicians with essential and precision tools for early diagnosis and improved outcomes.
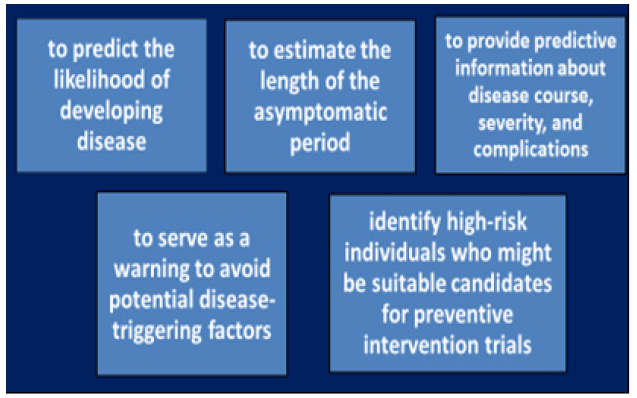
Figure 9: Applications of diagnostic, predictive and prognostic biomarkers in monitoring patients with AIM and persons-at-risk.

Figure 10A: Staging of post-infectious myocarditis.
Note*: Myocarditis is defined as the inflammatory process affecting the myocardium. Regardless of its etiology, acute inflammation may progress to subacute and chronic stages and finally to tissue remodeling, fibrosis, and loss of myocardium architecture and contractile function. The latter chronic damage corresponds to the development of Dilated Cardiomyopathy (DCM). Myocarditis is often caused by viral infections and PIFAS. The most common causes of infectious myocarditis include coxsackievirus, parvovirus, adenovirus, EBV, CMV, HSV, etc. The development of AIM is dependent on the genetic factors of the host, and the MHC may be an important determinant of the type and severity of the disease. CTL, ………………………; IL, ………………………..; TNF-alpha, ……………………..; IFN-gamma,
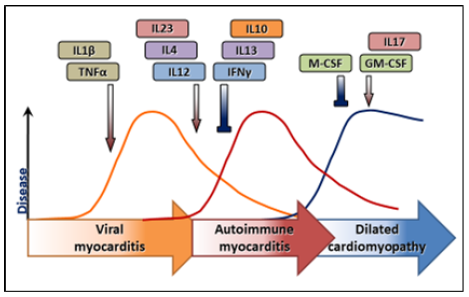
Figure 10B: A schematic representation of the Postinfectious Aute Syome (PIFAS)
Note*: IL, ……………………….; TNF-alpha, …………………….; IFN-gamma, ………………………………….
Clinical manifestations of AIM, with distinct onset, vary from asymptomatic to fatal. But the precise biomarkers to predict the course at initial presentation have not yet been established. Meanwhile, an improved knowledge of the mechanisms of infections to precede the illness should help to get type of PIFAS defined and then be used as combinatorial and/or multifunctional biomarkers, to:
i. Predict the likelihood of developing disease.
ii. Estimate the length of the asymptomatic period.
iii. Provide predictive information about disease course, severity, and complications.
iv. Serve as a warning to avoid potential disease-triggering factors.
v. Identify high-risk individuals who might be suitable candidates for preventive intervention trials.
vi.Develop preventive strategies for quenching PIFAS at the subclinical stages.
So, the discovery of novel biomarkers specifically tailored to PIFAS-related disease type and stage are expected to enable PPM-driven approach by facilitating predictive diagnostics and personalized therapies. Of particular interest would be, for instance, the use of protein microarrays for immune response profiling, through which a PIFAS-specific Ab repertoire may be defined to monitor and thus control subclinical and clinical stages of the disease. And the rapid development of proteomic, microbiomics and immunological methods has designated the latter as an emerging platform to interrogate the targeted immunome and microbiome for the discovery of next generation (presumably, combinatorial) biomarkers exploitable for risk assessment, subclinical detection, prognosis of PIFAS. New strategies, such as immunome-based PIFAS biomarker-driven tests, which would allow to systematically characterize and quantify query sample sets of interest' in bio samples, may dramatically improve next-generation PIFAS-related biomarkers, especially considering the plethora of candidates coming, for instance, from the 'bioreactor' microbiota affecting PIFAS onset and progression. Therefore, future efforts should be focused on the validation of the combinatorial biomarkers, i.e. demonstration of their close correlation to the pathological process.
To stress the above-mentioned comments, let me mention that PIFAS-related auto aggression provoked by insufficient coordination between two immune arms and hyperactivity of the adaptive one is a dominant feature of PIFAS to serve as a combinatorial biomarker. Its unique feature is a broad repertoire of auto Abs responsible for multi-seropositivity and thus to specific autoimmune inflammation biomarkers (e.g., anti-B7-HI auto Abs).
Antigen-Specific Immunological Reactivity and its Role in PIAI and PIFAS
To develop any form of immune response (including PIAI followed by self-reactive cells activation), two types of signals are required, i.e., antigen-nonspecific, and antigen-specific. The absence of antigen-nonspecific signals usually leads to anergy or apoptosis of immunocompetent cells [3-6]. Antigen-specific signals that are substantially realized by means of molecular mimicry are necessary to activate self-reactive immunocompetent cells [7-9]. To date, there are numerous indirect evidence to support the theory saying that development and progression of PIAI are stimulated by high immunogenicity of the host tissue and tissue-associated antigens, while the very initial phases of PIAI are mediated by the mimicking epitopes. In most cases, meanwhile, it is very difficult to define specific immunodominant self-epitope to be responsible for the induction of the autoimmune disorder. Moreover, the presence of massive and heterogeneous pools of pathogenically relevant autoantigens proved to be of crucial importance in many autoimmune diseases. As a result, the hypothesis on single “key and unique” autoantigen that triggers and maintains autoimmune responses was questioned [10-13].
Correlation between Infection and PIAI: Primary and Secondary Autoimmunity
It is of fundamental importance to distinguish between primary and secondary autoimmunity conditions, more precisely, genuine autoimmune diseases and clinical syndromes associated with autoimmunity. Secondary autoimmunity-associated syndromes developing in response to primary injury of organs and tissues represent evolutionary descent physiological (protective) host response. On the contrary, primary autoimmune lesions of tissues and organs caused by infectious agents are less understandable. Those lesions are key pathogenic factors for inducing or significantly worsening the course of autoimmune diseases. Thereupon, it should be mentioned that PIAI mechanisms (with pattern of antigenic peptides or microbial superantigens) differ from that occurred in canonical infectious inflammation. To generate PIAI, the substantial activation of self-reactive T and B cells is needed; the latter is regarded as a major step to bridge canonical post-infectious inflammation and PIAI. To date, there are three possible explanations for this phenomenon:
1. microbial superantigens activation: those superantigens can stimulate T lymphocytes, some of which are specific for autoantigens (including super autoantigens). Canonical infectious inflammation leads to the induction of APC to intensify processing and presentation of the autoantigens in the multiple lesion sites.
2. T cells bystander activation involving epitope spreading with intramolecular and intermolecular diversification. Bystander activation is stimulated by inflammation and promotes proliferation of pre-activated T lymphocytes.
3. Molecular mimicry: those mechanisms are not mutually exclusive. They can play a part in PIAI pathogenesis while being of crucial importance in particular stages of the disease [7,14].
Release of cryptic (mostly, intramolecular) autoepitopes because of the massive tissue destruction caused by persisting infection also contributes to the proliferation and differentiation of self-reactive cell populations (Figure 11) [7,15]; Moudgil KD, Sercarz EE. Crypticity of self-antigenic determinants is the cornerstone of a theory of autoimmunity. Discov Med. 2005;5(28):378-82]. This evidently makes a diversity of primary (microbial) and tissue-associated antigens valuable so much for the implementation of the resources of molecular mimicry into the induction of PIAI.
Definition of the Molecular Mimicry Concept
Modern interpretation of molecular mimicry concept says that germ inducing host immune response possesses toxic potential and offers cross-reactivity between self-epitopes and host epitopes. So let us suggest several criteria to support the involvement of molecular mimicry into the pathogenesis of PIAI [1].
1. The co-existence of the antigenic tandem consisting of the microbial antigen that is structurally homologous to the tissue-associated autoantigen and able to initiate primary T and B cell responses, on one hand, and the autoantigen as itself that maintains and potentiates microbial antigen induced immune response, on the other hand.
2. Established and proof-confirmed significance of the autoimmune response against cross-reactive microbial antigen(s) and self-antigen(s) that are, in turn, of crucial importance as for the canonical (primary) infectious inflammation as well as for PIAI.
3. Direct involvement of autoreactive T lymphocytes pre-activated by the infectious agent, into a cascade of triggering and further development of PIAI. Those T cells can respond to both microbial and cross-reactive host antigens.
The above-mentioned criteria were developed and proposed to define the contribution of molecular mimicry into the direct induction of PIAI. But those criteria do not include other pathogenic factors promoting transformation of the primary infectious process into PIAI [7].
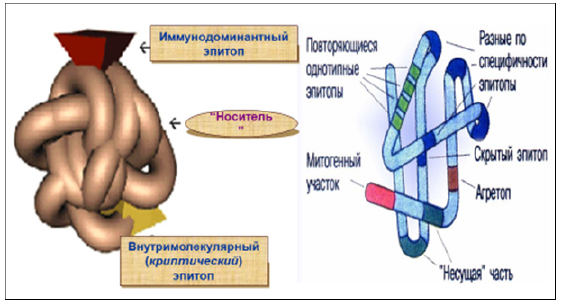
Figure 11: Cryptic autoepitopes involved in PIAI progression.
Note*: The epitopes involved in autoimmune pathogenesis are “cryptic” self-epitopes (i.e. epitopes corresponding to peptides which do not induce a T cell response after immunization with intact antigens, but which do trigger normal responses after direct immunization with the peptide). T cells specific for these epitopes are therefore not tolerized and may become pathogenic if the cryptic epitopes are presented in the periphery. Autoimmunity then results from the pathogenic presentation in peripheral tissues of normally hidden, cryptic epitopes. This, of course, does not exclude the possibility that autoimmunity can also result from breakdown of tolerance against dominant self-epitopes.
Molecular Mimicry and its Practical Value
During clinical autoimmunity induction (in particular, PIAI), initial expansion of the naïve self-reactive T cells usually requires activation of the proper TCR by MHC (HLA)-associated peptides (Figures 12A,12B). Meanwhile, according to the molecular mimicry concept, primary activation of the T cells is mediated by the microbial peptides structurally homologous to some of the host peptides. Molecular mimicry and its implications itself would depend on structural features of the tri-molecular HLA-peptide-TCR complex (Figure 13).
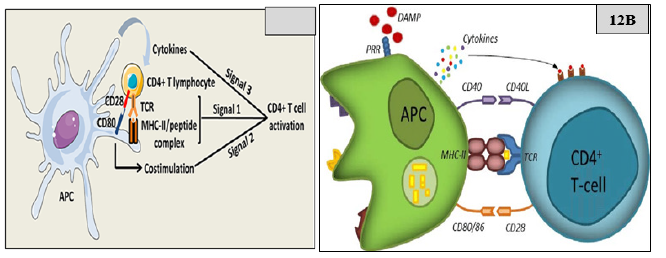
Figure 12: CD4+ T cell activation by APC.
Note*: TCD4+ T cells orchestrate a broad range of immune responses. CD4+ T cells differentiate into various T helper subsets characterized by distinct cytokine secreting profiles that confer effector functions adapted to a variety of infectious or endogenous threats. Regula-tory CD4+ T cells are a specialized subset that plays a fundamental role in the maintenance of immune tolerance to self-antigens. Manipulating effector or regulatory CD4+ T cells responses is a promising immunotherapy strategy for, respectively, chronic viral infections, or PIFAS and severe autoimmune diseases. Initially, the TCR recognizes the antigenic peptide in association with the MHC class II. Then the presence of a danger signal (danger associated molecular pattern, DAMP) leads to the expression of co-stimulation markers on the APC (CD80:86 and CD40). These markers engage the CD28 and CD40L expressed on T-cells. This second signal leads to the secretion of cytokines by the APC which finalizes the activation of the T cell. Three signals are necessary to fully activate CD4+ T cells. The first signal is mediated by the interaction between TCRs and peptide/MHC-II complexes at the APC surface. The second signal is mediated by costimulatory molecules, such as CD80 on APCs, which interacts with CD28 on CD4+ T cells. The third signal is delivered by soluble factors including cytokines, which are notably secreted by the APCs. APC, ………………………….,.

Figure 13: Production of HLA–peptide–TCR complex.
Note*: Depict of the interaction between TCR and HLA-peptide complexes. Polymorphic residues on HLA molecules are crucial for the interaction with the TCR. Specific pockets in the peptide binding groove on HLA molecules interact with anchor side chains on antigenic. APC, ………………………….;.
Those structural features limit specificity of peptide recognition by TCR thus making such specificity as being as degenerative [16-21]. That would allow for the microbial pathogen to avoid immunological surveillance and to persist in a host for a long time. However, such identity is not absolutely secured, and for that reason, immune system can recognize the infectious pathogen as foreign. So, the immune response against this infect is accompanied by destruction of the targeted tissue cells that bear cross-reacting mimicking determinants. Those immune responses (known as autoimmune lysis phenomenon) are mediated by self-reactive T and/or B cells [22-28].
Intensive studies of structural and functional TCR-HLA-peptide interactions have markedly renewed the latest version of the concept of molecular mimicry, presenting the latter as follows:
1. Modifications of amino acid sequences in HLA-presented peptides are far from determining the patterns of TCR-HLA-peptide interaction and T cell activation due to conformational instability and structural polymorphism of TCR domains (known as induced fit phenomenon). There-fore, TCR-mediated recognition of the appropriate and specific target is featured with the degeneracy, so those T cells could be activated by a panel of the structurally diverse peptides. The latter would support the idea that T cells could be activated by a panel of antigenic peptides structurally different from the targeted self-peptide.
2. Structural homology as itself does not induce either autoimmunity conditions or provoke autoimmune disorders. Furthermore, some additional factors are required for T cells activation, i.e., effective processing of an antigen containing mimicking peptide, the appropriate presentation of the processed peptide (known as peptide HLA selection) as well as a pattern of TCR-specificities.
3. The presence of mimicking peptide that is effectively recognized and presented by T lymphocyte is insufficient to induce and develop PIAI, because in T cells activation mode mimicking peptides act as weak agonists or antagonists.
A phenomenon of degeneracy of HLA-peptide immune recognition by TCR has changed molecular mimicry concept rather than just the other phenomenon demonstrating the availability of related and/or similar sequences shared by mimicking peptides within the panel. The instability within TCR-HLA-peptide interactions provide not only T cells activation but leads to generation of the structurally homologous or closely related peptides. Recently, molecular mimicry in the absence of structural sequence homology has been demonstrated [29-38]. Moreover, a pool of T cells recognizing two homologous peptides (microbial antigen and autoantigen in conjunction with HLA of various phenotypes) was identified [39]. TCR recognition of two peptides in the context of different HLA molecules (class I and class II) is also possible when TCR cross-reacts with several bimolecular complexes of diverse HLA and numerous recombinant peptides at once [40-42].
During the last years, structural and functional studies of trimolecular TCR-HLA-peptide complexes have further modified the concept of molecular mimicry and let that be understandable as follows:
1. Modifications in the primary structure of HLA-presenting peptides are far from determining specificity of TCR recognition and its binding as well as T cells activation. These transformations are often designed in TCR plasticity limits. Therefore, TCR recognition of peptide can be referred as degenerative while being comparable with T cells stimulation by a whole set of ligands including those lacking proper specificity (i.e., unrelated ligands).
2. Most mimicking epitopes promote development but not initiation of autoimmune disorders due to HLA limited selection capacity, intracellular primary antigen processing type and T cells repertoire.
3. Intracellular processing, recognition, and presentation of mimicking microbial epitope to the appropriate autoreactive T cells clone do not necessarily mean autoimmunity progression but indicate that mimicking epitope intended for regulatory T cells activation can alternatively act as either weak agonist or antagonist.
To date, four variants of molecular mimicry can be screened for having assessed:
i. molecular mimicry with structural homology (some peptide amino acids are responsible for HLA binding specificity whereas others determine that of TCR).
ii. molecular mimicry without structural homology.
iii. molecular pseudo-mimicry due to autoantigens accumulation on pathogenic bacteria [1].
iv. multi-receptor molecular mimicry due to HLA receptors diversity. In this case, TCR complexing with HLA-peptide (related as well as unrelated peptides) can recognize a variety of HLA receptors. Considering that all people are HLA-DR heterozygous, this variant of molecular mimicry can occur naturally [19,26].
Practical Value of Molecular Mimicry Working Model
Peptide side chain’s structure is of crucial importance for peptide epitope binding with HLA receptors and bimolecular HLA-peptide complex generation. At the same time, these anchor amino acid residues can be replaced by the others thus resulting in peptide structure degeneracy [26,43]. Therefore, replacement of peptide side chains localized in deep cavities does not alter the specificity of MHC-peptide complex TCR recognition [26,44] while specificity of HLA-peptide complex TCR recognition is determined by just a few peptide side chains. As to Myelin Basic Protein (MBP), three side chains required for TCR recognition were described thereby revealing a wide range of bimolecular HLA-peptide complex cross-reactivity [39,45-47]. Hence, TCR cross-reactivity may include (a) TCR recognition of different peptides coupled with the same HLA molecule and (b) TCR recognition of different peptides coupled with various HLA molecules.
Considering that, TCR specificity and TCR cross-reactivity should also be precisely distinguished since binding capacity of the same TCR is more than 106. Total number of natural and synthetic peptides recognized by a proper TCR is relatively large, but the number of microbial and cross-reacting human autologous peptides is much less. Nevertheless, there is a set of diverse peptides for a proper TCR acting as agonists for this receptor. Even greater set of peptides can induce TCR-directed weak signal providing point of initiation of the positive selection of TCR-bearing cells in thymus and prompting peripheral survival of naïve T lymphocytes [48,49].
In a variety of autoimmune disorders, a whole of trigger autoantigens with different specificity and functionality playing pathogenically relevant role in PIFAS induction can be observed. While evaluating clinical manifestations of PIAI, key pathogenic aspects should be considered regardless of the diversity of tissue antigens involved in its induction and development: (a) infectious antigens serve as PIFAS triggering molecules [50-52] and (b) most of the infectious agents possess tropism to certain cells, organs and tissues exerting direct cytopathogenic in-fluence on them. The consequences of such tropism are determined by the hereditary predisposition of a patient and HLA genes expression pattern, in particular [53]. Considering that, PIAI can be subdivided into organ-specific and organ-nonspecific types thus allowing for constructing the appropriate immunodiagnostic protocol in each specific case. The development of organ-specific autoimmune disorders caused by infectious agents is mediated by inflammatory reactions considering as antigen-nonspecific signal that triggers immune response. Mimicking microbial pathogens act as adjuvants thus promoting PIAI due to their cross-reactivity with tissue-associated autoantigens of the diseased patients [54,55]. As to AIM, two thirds’ cases are associated with the prior infection while transformation of primary (infectious) disorder into PIAI is proved to be determined by infectious agents (e.g., CVB3, HSV or CMV) mimicry [56, 57]. Viral particles are not necessarily detected in heart tissue suggesting that in some patients, the virus is eliminated by the time point of PIAI-driven clinical manifestations (PIFAS) as the process gains autoimmune symptoms. To confirm this, autoantibodies and self-reactive T cells clones against cardiac autoantigens can be identified. PIAI progression in response to primary infection is accompanied by cryptic (masked) self-epitopes release thus amplifying autoimmune aggression [58]; Moudgil KD, Sercarz EE. Crypticity of self-antigenic determinants is the cornerstone of a theory of autoimmunity. Discov Med. 2005;5(28):378-82].
Autoimmune Myocarditis (AIM) as A Paradigm of Autoimmune Disease Through the View of PIAI and PIFAS
An association of organ- and tissue-specific autoimmune diseases with viral infection, is strongly supported by clinical observation and epidemiologic study. Several plausible mechanisms have been proposed, including molecular mimicry, changes induced in endogenous anti-gens, and disturbance in the host’s immune response. For instance, Coxsackie Virus (CV) infections frequently initiate autoimmune response in humans and provide a tool for understanding post-infection AIM (Rose, N.R., Neu, N., Neumann, D.A., Herskowitz, A. (1988). Myocarditis: A Postinfectious Autoimmune Disease. In: Schultheiß, HP. (eds) New Concepts in Viral Heart Dis-ease. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3- 642-73610-0_13).
Chronic and multiple infections with viruses, such as EBV, HSV and CMV, and some bacteria may, in susceptible individuals, play a role in the evolvement of PIFAS-driven myocarditis resulting in AIM. As most infections pertain to our resident microbiota and healthy carriage of infections is the rule, we propose to focus on understanding what changes its configurations to PICIS, to the restoration of health, or to the sustaining of illness into a chronic state of CRID to be triggered by PIFAS. Myocarditis can be defined as the inflammatory process affecting the muscular tissues of the heart (myocardium). Regardless of its etiology, the acute inflammation may progress to subacute and chronic stages and finally to tissue remodelling, fibrosis, and loss of myocardium architecture and contractile function. The latter chronic damage corresponds to development of Dilated Cardiomyopathy (DCM). And AIM through the view of PIFAS is an important cause of heart failure among adolescents and young adults (Figure 14).
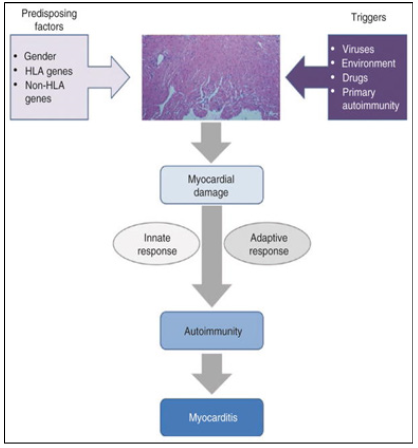
Figure 14: Model for autoimmune myocarditis development.
Note*: Autoimmune myocarditis is considered a multifactorial entity, in which several immunologic mechanisms are involved in its development and progression. Regarding the trigger and initiation of its pathogenesis, so far, no unique sufficient factor has been identified but rather multiple endogenous and environmental confluent factors, in such a way that the myocardium-specific autoimmune process is triggered and sustained. The balance and relative influence of those factors is still unclear but seems to be variable and host dependent. Coexistence of predisposing factors along with specific triggers of myocardium damage leads to exposure of cryptic self-antigens and a consequent inflammatory process. At this point, both the innate and the myocardium-specific adaptive response generate a self-sustained autoimmune phenomenon independent of the original trigger factor. The autoimmune process is responsible for the development of autoimmune myocarditis and progression to DCM. From: Bracamonte-Baran W, Čiháková D. Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol. 2017; 1003:187-221. doi: 10.1007/978-3-319-57613-8_10. PMID: 28667560; PMCID: PMC5706653.
Both virus infection and myosin immunization produce myocardial inflammation and elicit heart-reactive Abs which bind to the myocardium in vivo and which recognize the cardiac myosin heavy chain (N R Rose 1, S L Hill. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996 Sep;80(3 Pt 2):S92-9. doi: 10.1006/clin.1996.0146; Patel H, Madanieh R, Kosmas CE, Vatti SK, Vittorio TJ. Clin Med Insights Cardiol. 2015 May 21;9(Suppl 2):7-14. doi: 10.4137/CMC.S19703. eCollection 2015; Toll-Like Receptors: Are They Taking a Toll on the Heart in Viral Myocarditis? Favere K, Bosman M, Klingel K, Heymans S, Van Linthout S, Del-putte PL, De Sutter J, Heidbuchel H, Guns PJ. Viruses. 2021 May 27;13(6):1003. doi: 10.3390/v13061003).
Usually, at the initial steps, viral myocarditis is considered sometimes an independent entity. Nevertheless, it seems that once the trigger infectious noxa exerts its effect, the final effector mechanisms are like the ones leading to AIM progression and chronic complications. Myocardium-tropic viral infection acts as a trigger and “co-adjuvant” generating a sustained myocardium-specific autoimmune response (PIFAS-driven AIM). The main viruses associated with AIM and endowing with the cross-reactive epitopes are the CVB3, adenovirus, influenza (A,B), parvovirus B19, cytomegalovirus, and Epstein-Barr Virus (EBV). All these viruses cause myocarditis with similar inflammatory features, and all could lead to DCM. Following the above-mentioned considerations, AIM usually develops in genetically predisposed individuals infected with the CVB3 to represent typical manifestations of molecular mimicry. The presence, in circulating blood, of CM-AR CTLs and anti-CM auto Abs is prerequisite to AIM development to initiate myocardial lesions. Clinical manifestations of AIM, with distinct onset, vary from asymptomatic to fatal. But the biomarkers to predict the course at initial presentation have not yet been established. Meanwhile, an improved knowledge of the mechanisms of infections to precede the illness should help to get type of PIFAS defined and then be used as a combinatorial biomarker, to develop preventive and/or prophylactic strategies for quenching PIFAS at the pre-early (subclinical) stages.
The correlation between the stage of immune mediated chronic inflammation and the form of PICIS is also characterized by the involvement of an additional (third) component, viz., clinical form or variant of Chronic Diseases of Infectious Origin (CDIO). Here are several analytical examples related to:
1. Clinical form of PIFSI: in patients with primary infectious myocarditis (PIM), PIFSI is detected in 75% of cases, whereas in patients with AIM the contribution of PIFSI is notably de-creased (to 25%) giving way to auto aggression (the contribution of PIFAS increases to 85%).
2. Stage of clinical reversible immunodeficiency (CRID). At early stages (< 3 months for CPN and < 1 month for myocarditis (М)), PIFSI is detected in 40% of cases; however, at later stages of CRID its share decreases appreciably, while that of autoimmune syndromes increases in contrast.
3. Rate of progression and chronification of CRID. In patients with relapsing or rapidly progressing CRID (e.g., ICIIP or AIM), the contribution of PIFSI does not exceed 32-36%, while the share of autoimmune syndromes reaches 80-100%. In such patients, persistent forms of me-ningoencephalitis (e.g., ICIIP) or AIM associated with myocardial dystrophies are predominant.
These findings suggest that PIFSI is not only the outcome of the infectious process, but also represents a factor responsible for its lingering and chronically relapsing course. Further progression and chronization of CRID are controlled by postinfectious auto-aggression factors, such as PIFA and PIFASID [59-96].
Conclusion
AIM is an extremely complex and multistep immune mediated chronic process. Several risk factors and biologic issues predisposing its trigger and progression have been identified. Those include HLA and non-HLA genetic characteristics, exposure of cryptic antigens, mimicry, interfering autoimmune diseases, and viral infections. Also, the current concept is that once the initial myocardial inflammation is established, then a T cell-dependent autoimmune process takes place despite differences in the specific etiologic factor. That autoimmune process is responsible for the self-sustained inflammation and progression to tissue damage leading to DCM. Molecular mimicry is one of the key triggering mechanisms in PIAI and AIM since any microbe has antigenic determinants (epitopes) immunologically like the host myocardium-related epitopes, therefore, immune response induced by pathogen epitopes can break host epitopes tolerance. In genetically predisposed patients with suitable HLA haplotypes, immune response results in autoimmunity owing to T and B cells cross-reactivity. Hence, autoimmune process following molecular mimicry can be considered as failed side effect of immune reactions.
Despite the growing body of knowledge about the immunopathogenesis of AIM, the specific triggers and factors leading to progression in patients are still a conundrum. Also, since no risk or etiologic factor seems to be sufficient for the initiation and progression processes, post-myocarditis progression to DCM is still unpredictable in clinical practice and does need and require discovery of combinatorial biomarkers of the next step generation. Meanwhile, advances exist in therapeutics, but still relying in global immunosuppression and unspecific immunomodulation, with is still suboptimal results. The expectation is that future basic and translational studies might provide even deeper insights in the pathogenesis of AIM to provide the design-driven and biomarker-based targeted drugs to prevent AIM! That would simultaneously lead to development of better diagnostic tools (including theragnostic) allowing characterization and stratification of stages of AIM progression in each patient or in persons-at-risk. Finally, that translational knowledge would make possible the development of individualized network-targeted combinatorial treatments, using resources of systems polypharmacy.
Acknowledgement
None.
Conflict of Interest
None.
References
- Shoenfeld Y, Rose NR, Nancy Agmon (2004) Levin Infection and autoimmunity, Elsevier BV Amsterdam Holland 768.
- Perl A (2004) Pathogenesis and spectrum of autoimmunity. Methods Mol Med 102: 1-8.
- Cook AD, Davies JM, Myers MA, Mackay IR, Rowley MJ et al. (1998) Mimotopes identified by phage display for the monoclonal antibody CII-C1 to type II collagen. J Autoimmun 11(3): 205-211.
- Kagnoff MF, Paterson YJ, Kumar PJ, Kasarda DD, Carbone FR et al. (1997) Evidence for the role of a human intestinal ad-enovirus in the pathogenesis of coeliac disease. Gut 28(8): 995-1001.
- Ufret Vincenty RL, Quigley L, Tresser N, Pak SH, Gado A et al. (1998) In vivo survival of viral antigen-specific T cells that induce experimental autoimmune encephalomyelitis. J Exp Med 188(9): 1725-1738.
- Olson JK, Croxford LJ, Miller SD (2004) Innate and adaptive immune requirements for induc-tion of autoimmune demyelinating disease by molecular mimicry. Mol Immunol 40(14-15): 1103-1108.
- Benoist C, Mathis D (2001) Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2(9): 797-801.
- Klehmet J, Shive C, Guardia Wolff R, Ines Petersen, Edward G Spack, et al. (2004) T cell epitope spreading to myelin oligoden-drocyte glycoprotein in HLA-DR4 transgenic mice during experimental autoimmune encephalo-myelitis. Clin Immunol 111(1): 53-60.
- Liang B, Mamula MJ (2000) Molecular mimicry and the role of B lymphocytes in the processing of autoantigens. Cell Mol Life Sci 57(4): 561-568.
- Bach JF (1995) Organ-specific autoimmunity. Immunol Today 16(7): 353-355.
- Bach JF, Koutouzov S, Van Endert PM (1998) Are there unique autoantigens triggering autoimmune diseases? Immunol Rev 164: 139-155.
- Graham KL, Utz PJ (2005) Sources of autoantigens in systemic lupus erythematosus. Curr Opin Rheumatol 17(5): 513-517.
- Salaman MR (2003) A two-step hypothesis for the appearance of autoimmune disease. Autoim-munity 36(2): 57-61.
- Rose NR, Mackay IR (2000) Molecular mimicry: a critical look at exemplary instances in human diseases. Cell Mol Life Sci 57(4): 542-551.
- *Molecular mimicry is a reasonable explanation for the often-described association of infec-tion with autoimmune disease. However, there are yet no firm examples of a human disease based on such a mechanism. So, this mechanism is reevaluated in broader terms.
- Pleister A, Eckels DD (2003) Cryptic infection and autoimmunity. Autoimmunity Rev 2(3): 126-132.
- Panoutsakopoulou V, Sanchirico ME, Huster KM, M Jansson, F Granucci, et al. (2001) Analysis of the relationship be-tween viral infection and autoimmune disease. Immunity 15(1): 137-147.
- Wucherpfennig KW (2001) Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 108(8): 1097-1104.
- Wucherpfennig KW (2001) Insights into autoimmunity gained from structural analysis of MHC-peptide complexes. Curr Opin Immunol 13(6): 650-656.
- Wucherpfennig KW (2001) Structural basis of molecular mimicry. J Autoimmun 16(3): 293-302.
- Wucherpfennig KW (2004) T cell receptor crossreactivity as a general property of T cell recogni-tion. Mol Immunol 40(14-15): 1009-1017.
- Wucherpfennig KW (2005) The structural interactions between T cell receptors and MHC-peptide complexes place physical limits on self-nonself discrimination. Curr Top Microbiol Immunol 296: 19-37.
- Davidson A, Diamond B (2001) Autoimmune diseases. N Engl J Med 345(5): 340-350.
- Davies JM (1997) Molecular mimicry: can epitope mimicry induce autoimmune disease? Immu-nol. Cell Biol 75(2): 113-126.
- Farris AD, Keech CL, Gordon TP, J McCluskey (2000) Epitope mimics and determinant spreading: pathways to autoimmunity. Cell Mol Life Sci 57(4): 569-578.
- Karlsen AE, Dyrberg T (1998) Molecular mimicry between non-self, modified self and self in autoimmunity. Semin Immunol 10(1): 25-34.
- Kohm AP, Fuller KG, Miller SD (2003) Mimicking the way to autoimmunity: an evolving theory of sequence and structural homology. Trends Microbiol 11(3): 101-105.
- Pedersen GN, Nyborg J, Clark BF (1999) Macromolecular mimicry of nucleic acid and protein. IUBMB Life 48(1): 13-18.
- Rose NR (1998) The role of infection in the pathogenesis of autoimmune disease. Semin Immunol 10(1): 5-13.
- Bankovich AJ, Girvin AT, Moesta AK, K Christopher Garcia (2004) Peptide register shifting within the MHC groove: theory becomes reality. Mol Immunol 40(14-15): 1033-1039.
- Carl PL, Temple BRS, Cohen PL (2005) Most nuclear systemic autoantigens are extremely disordered proteins: implications for the etiology of systemic autoimmunity. Arthritis Res Ther 7: 1360-1374.
- Cohn M (2005) Degeneracy, mimicry and cross reactivity in immune recognition. Mol Immunol 42(5): 651-655.
- Dunker AK, Brown CJ, Lawson JD, Lilia M Iakoucheva, Zoran Obradović, et al. (2002) Intrinsic disorder and protein function. Biochemistry 41(21): 6573-6582.
- Reiser JB, Darnault C, Gregoire C, Thomas Mosser, Gilbert Mazza, et al. (2003) CDR3 loop flexibility contributes to the degenera-cy of TCR recognition. Nat Immunol 4(3): 241-247.
- Carrizosa AM, Nicholson LB, Farzan M, S Southwood, A Sette, et al. (1998) Expansion by self-antigen is necessary for the induction of experimental autoimmune encephalomyelitis by T cells primed with a cross-reactive environmental antigen. J Immunol 161(7): 3307-3314.
- Hemmer B, Fleckenstein BT, Vergelli M, G Jung, H McFarland, et al. (0997) Identification of high potency microbial and self-ligands for a human autoreactive class II-restricted T cell clone. J Exp Med 185(9): 1651-1659.
- Hiemstra HS, Schloot NC, van Veelen PA, S J Willemen, K L Franken, et al. (2001) Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci USA 98(7): 3988-3991.
- Hudrisier D, Riond J, Burlet-Schiltz O, M G von Herrath, H Lewicki, et al. (2001) Structural and functional identification of ma-jor histocompatibility complex class I-restricted self-peptides as naturally occurring molecular mimics of viral antigens. Possible role in CD8+ T cell-mediated, virus-induced autoimmune dis-ease. J Biol Chem 276(22): 19396-19403.
- Zhao R, Loftus DJ, Appella E, E J Collins (1999) Structural evidence of T cell xeno-reactivity in the ab-sence of molecular mimicry. J Exp Med 189(2): 359-370.
- Lang HL, Jacobsen H, Ikemizu S, Christina Andersson, Karl Harlos, et al. (2002) A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol 3(10): 940-943.
- Fourneau JM, Bach JM, van Endert PM, Jean-François Bach (2004) The elusive case for a role of mimicry in au-toimmune diseases. Mol Immunol 40(14-15): 1095-1102.
- Gronski MA, Boulter JM, Moskophidis D, Linh T Nguyen, Kaisa Holmberg, et al. (2004) TCR affinity and negative regulation limit autoimmunity. Nat Med 10(11): 1234-1239.
- Kamradt T, Volkmer Engert R (2004) Cross-reactivity of T lymphocytes in infection and auto-immunity. Mol Divers 8(3): 271-280.
- Hammer J, Valsasnini P, Tolba K, D Bolin, J Higelin, et al. (1993) Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell 74(1): 197-203.
- Stern LJ, Wiley DC (1994) Antigenic peptide binding by class I and class II histocompatibility proteins. Structure 2(4): 245-251.
- Burrows SR, Khanna R, Burrows JM, Moss DJ (1994) An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: impli-cations for graft-versus-host disease. J Exp Med 179(4): 1155-1161.
- Wucherpfennig KW, Sette A, Southwood S, M Matsui, J L Strominger, et al. (1994) Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by hu-man T cell clones. J Exp Med 179(1): 279-290.
- Wucherpfennig KW, Strominger JL (1995) Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80(5): 695-705.
- Hiemstra HS, Drijfhout JW, Roep BO (2000) Antigen arrays in T cell immunology. Curr Opin Immunol 12(1): 80-84.
- Marusic Galesic S, Pavelic K (1990) Dynamics of positive and negative selection in the thymus: review and hypothesis. Immunol Lett 24(3): 149-154.
- Plotz PH (2003) The autoantibody repertoire: searching for order. Nature Rev 3(1): 73-78.
- Dahlquist G, Frisk G, Ivarsson SA, L Svanberg, M Forsgren, et al. (1995) Indications that maternal coxsackie B virus infec-tion during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia 38(11): 1371-1373.
- Steck AJ, Tschannen R, Schaefer R (1981) Induction of antimyelin and antioligodendrocyte an-tibodies by vaccinia virus. An experimental study in the mouse. J Neuroimmunol 1(1): 117-124.
- Nepom GT, Kwok WW (1998) Molecular basis for HLA-DQ associations with IDDM. Diabetes 47(8): 1177-1184.
- Conrad B (2003) Potential mechanisms of interferon-alpha induced autoimmunity. Autoimmunity 36(8): 519-523.
- Kidd P (2003) Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 8(3): 223-246.
- Kandolf R (2004) Virus etiology of inflammatory cardiomyopathy. Dtsch Med Wochenschr 129(41): 2187-2192.
- Muir P, Nicholson F, Illavia SJ, T S McNeil, J F Ajetunmobi, et al. (1996) Serological and molecular evidence of enterovirus in-fection in patients with end-stage dilated cardiomyopathy. Heart 76(3): 243-249.
- Warnock MG, Goodacre JA (1997) Cryptic T-cell epitopes and their role in the pathogenesis of autoimmune diseases. Br. J. Rheumatol 36(11): 1144-1150.
- Cunningham MW, Quinn A (1997) Immunological crossreactivity between the class I epitope of streptococcal M protein and myosin. Adv Exp Med Biol 418: 887-892.
- Tomer Y, Davies TF (1993) Infection, thyroid disease and autoimmunity. Endocrine Rev 14(1): 107-120.
- Deshpande SP, Lee S, Zheng M, B Song, D Knipe, et al. (2001) Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol 75(7): 3077-3088.
- Whittle RM, Wallace GR, Whiston RA, Dumonde DC, Stanford MR, et al. (1998) Human antiretinal antibodies in toxoplasma retinochoroiditis. Br J Ophthalmol 82(9):1017-1021.
- Arscott P, Rosen ED, Koenig RJ, Kaplan MM, Ellis T, et al. (1992) Immunoreactivity to Yersinia enterocolitica anti-gens in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 75(1): 295-300.
- Burch HB, Nagy EV, Lukes YG, W Y Cai, L Wartofsky, et al. (1991) Nucleotide and amino acid homology between the human thyrotropin receptor and HIV-l nef protein: identification and functional analysis. Biochem Biophys Res Commun 181(1): 498-505.
- Amedei A, Bergman MP, Appelmerk BJ, Annalisa Azzurri, Marisa Benagiano, et al. (2003) Molecular mimicry between Helicobacter pylori antigens and H+K+-adenosine triphosphatase in human gastric autoimmunity. J Exp Med 198(8): 1147-1156.
- D Elios MM, Appelmerk BJ, Amedei A, Mathijs P Bergman, Gianfranco Del Prete, et al. (2004) Gastric autoimmunity: the role of Helicobac-ter pylori and molecular mimicry. Trends Mol Med 10(7): 316-323.
- Fotheringham J, Jacobson S (2005) Human herpesvirus 6 and multiple sclerosis: potential mecha-nisms for virus-induced disease. Herpes 12(1): 4-9.
- Molina V, Shoenfeld Y (2005) Infection, vaccines and other environmental triggers of autoim-munity. Autoimmunity 38(3): 235-245.
- Many environmental factors are known to affect the immune system and may play a role as triggers of the autoimmune mosaic. Infections are known to induce and exacerbate autoimmune diseases, mainly by the mechanism of molecular mimicry. Vaccines were found to be temporally followed by a new onset of autoimmune diseases.
- Zachou K, Rigopoulou E, Dalekos GN (2004) Autoantibodies and autoantigens in autoimmune hepatitis: important tools in clinical practice and to study pathogenesis of the disease. J Autoimmune Dis 1(1): 2.
- Vergani D, Mieli-Vergani G (2003) Autoimmune hepatitis. Autoimmunity Rev 2(5): 241-247.
- Vergani D, Mieli-Vergani G (2004) Mechanisms of autoimmune hepatitis. Pediatr Transplant 8(6): 589-593.
- Wicher K, Wicher V (1990) Autoimmunity in syphilis. Immunol Ser 52: 101-124.
- Balandraud N, Roudier J, Roudier C (2004) Epstein-Barr virus and rheumatoid arthritis. Autoimmun Rev 3(5): 362-367.
- Alvarez Lafuente R, Fernandez Gutierrez B, De Miguel S, Jover JA, Rollin R, et al. (2005) Potential relationship be-tween herpes viruses and rheumatoid arthritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 64(9): 1357-1359.
- Soulas P, Woods A, Jaulhac B, Anne Marie Knapp, Jean Louis Pasquali, et al. (2005) Autoantigen. innate immunity and T cells cooperate to break B cell tolerance during bacterial infection. J Clin Invest 115(8): 2257-2267.
- Riemekasten G, Hahn BH (2005) Key autoantigens in SLE. Rheumatology 44(8): 975-982.
- Moon UY, Park SJ, Oh ST, Wan Uk Kim, Sung Hwan Park, et al. (2004) Patients with systemic lupus erythematosus have abnor-mally elevated Epstein-Barr virus load in blood. Arthritis Res Ther 6(4): 295-302.
- McClain MT, Heinlen LD, Dennis GJ, Jon Roebuck, John B Harley, et al. (2005) Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med 11(1): 85-89.
- Roitt IM, Delves PJ (2001) Essential immunology. Blackwell Publishing Ltd Oxford UK 481.
- Kono DH, Theofilopoulos AN (2021) Autoimmunity and tolerance. In: Firestein GS, Budd RC, Gabriel SE, Koretzky GA, McInnes IB, O'Dell JR, eds. Firestein & Kelley's Textbook of Rheumatology. 11th ed. Philadelphia, PA: Elsevier chap 22.
- Kumar V, Abbas AK, Aster JC (2021) Diseases of the immune system. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed Philadelphia PA Elsevier 6.
- Peakman M, Buckland MS (2021) The immunity. In: Feather A, Randall D, Waterhouse M, eds. Kumar and Clarke's Clinical Medicine. 10th ed Philadelphia PA Elsevier 3.
- Bracamonte Baran W, Čiháková D (2017) Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol 1003: 187-221.
- Root-Bernstein R, Fairweather D (2015) Unresolved issues in theories of autoimmune disease using my-ocarditis as a framework. J Theor Biol 375: 101-23.
- Rose NR. (2016) Viral myocarditis Curr Opin Rheumatol 28(4): 383-9.
- Fairweather D, Kaya Z, Shellam GR, Lawson CM, Roseet NR al. (2001) From infection to autoimmunity. J Autoimmun 16(3): 175-86.
- Caforio AL, Mahon NJ, McKenna WJ (2001) Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity 34(3): 199-204
- Massilamany C, Arunakumar Gangaplara, David Steffen, Jay Reddy (2011) Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cell Immunol. 271(2): 438-49
- Li Y, Janet S Heuser, Luke C Cunningham, Stanley D Kosanke, Madeleine W Cunningham, et al. (2006) Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol 177(11): 8234-8240.
- Abramson J, Husebye ES (2016) Autoimmune regulator and self-tolerance – molecular and clinical as-pects. Immunol Rev 271(1): 127-40
- Metzger TC, Anderson MS (2011) Myocarditis: a defect in central immune tolerance? J Clin Invest 121(4): 1251-1253.
- Noel R Rose (2014) Learning from myocarditis: mimicry, chaos and black holes. F1000Prime Rep 6:25.
- Lifang Zhao Zhaoying Fu (2018) Roles of Host Immunity in Viral Myocarditis and Dilated Cardi-omyopathy. Journal of Immunology Res 2018: 5301548.
- Antonio Cannata, Jessica Artico, Piero Gentile, Marco Merlo, Gianfranco Sinagra, et all. (2019) Myocarditis evolving in cardiomyopathy: when genetics and offending causes work together. Eur Heart J Suppl 21(Suppl B): B90-B95.
**There are several mechanisms for activation of autoreactive T and B cells by infectious agents, i.e., molecular, mimicry, bacterial and viral superantigens, bystander activation etc. Criteria for establishing the role of infectious agents in autoimmune diseases are being given as well.
**A progress was made in the identification of such microbial peptides based on the analysis of structural features that are important for TCR recognition of MHC-bound peptides.
**The progression from benign autoimmunity to pathogenic autoimmune disease depends on the balance of cytokines produced during the inflammatory process accompanying infection. In many autoimmune diseases, the cytokine profile favors the proinflammatory cytokines, IFN-gamma and IL-1, which support the production of disease.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.