Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Post-Infectious Autoimmunity Syndrome (PIFAS): Part 2 Features of Management of Post-Infectious Autoimmune Myo-carditis in Clinical Practice - The Integrated Viewpoint of Researchers, Biodesigners and Clinical Practitioners
*Corresponding author:Sergey Suchkov, Russian Academy of Natural Sciences (RANS), Moscow, Russia.
Received: February 27, 2024; Published: March 13, 2024
DOI: 10.34297/AJBSR.2024.21.002896
Abstract
Development and progression of Postinfectious Autoimmunie Diseases (PIAD) are stimulated by the immunogenic resources of the host tissue and tissue-associated antigens, while the very initial (pre-early) phases were postulated and are proved today to be mediated by mimicking epitopes. Molecular mimicry concept provides approaches to etiological agents associated with the PIAD-driven Autoimmune Myocarditis (AIM) progression identification as well as specific prevention and treatment modalities to control the development of AIM. Clinical heterogeneity of myocarditis makes diagnosis challenging. Given the diverse presentation and varying course of myocarditis, personalized treatment approaches would be optimal; however, a standardized management protocol remains elusive. Myocarditis is not only heterogeneous in its clinical presentation but also in pro-cesses governing its pathogenesis depending on etiology. A thorough understanding of those issues is needed to optimize patient care, implement new smart therapeutic strategies as well as to better understand factors that influence clinical presentation, patient outcomes, and response to treatment. Recent translational studies sug-gest that targeted and/or multi-targeted treatments tailored to specific causes of AIM may impact clinical out-comes when added to guideline-directed medical care. Soon, genotyping and phenotyping results combined and consolidated under the aegis of IT-assisted algorithms will be used for the creation of unified data-banks necessary for personal health biomonitoring of PIAD-driven AIM. The novel diagnostic ideology is expected to be based on a combination of two categories of investigations:
i. Pathogenically oriented (OMICS-based) diagnosis of PIAD.
ii. Screening of microbial pathogens (microbiome-related profiling) as the main causal factors of AIM.
And until more definitive studies have been reported, individual patient decisions should be made with shared decision-making that includes the uncertainties in our present knowledge of myocarditis management strategies.
Abbreviations: PIAD: Postinfectious Autoimmune Diseases; AIM: Autoimmune Myocarditis; Abs: Antibodies, Ags: Antigens; CTLs: Cytotoxic T-Cells; APS: Antiphospholipid Antibody Syndrome; EBV: Epstein-Barr Virus; CMV: Cytomegalovirus; SLE: Systemic Lupus Erythematosus; T1D: Type 1 Diabetes; MS: Multiple Sclerosis; MGO: Myelin Glycoprotein Oligodendrocyte; CVB3: Coxsackievirus B3; DCM: Dilated Cardiomyopathy; PIM: Primary Infectious Myocarditis; CRM: Clinical Reversible Myocarditis
Keywords: Autoimmune diseases, Autoimmune myocarditis, Autoimmune inflammation, Personalized treatment, Anti-idiotypic antibodies, Autoantibodies, Dilated cardiomyopathy, Biomarkers, Post-infectious auto-immune disorders, Viruses, Infection, Targeted Drugs
Introduction
Autoimmune diseases represent a significant burden on global health. While genetics play a role, environmental factors, particularly infections, are increasingly recognized as pivotal triggers for autoimmunity, with mechanisms such as molecular mimicry, epitope spreading, and bystander activation contributing to the onset and severity of autoimmune diseases. Infections can instigate pro-inflammatory cell death programs, induce the release of host nuclear autoantigens, and promote their recognition by the immune system, leading to an autoimmune response mediated by autoreactive T and B lymphocytes. The complex interplay between microbial anti-gens and the immune system can result in the production of autoantibodies and autoreactive cytotoxic T cells, exacerbating tissue damage and contributing to the heterogeneous spectrum of autoimmune diseases. Understanding the intricate mechanisms underlying infection-induced autoimmunity is essential for developing targeted therapeutic interventions. By clarifying the role of anti-idiotypic antibodies in regulating autoantibody levels and exploring the potential of antigen-specific immunotherapy, there is promise for tailored treatments that selectively suppress autoimmune responses while preserving normal immune function. Furthermore, advancements in biomarker discovery and omics-based approaches offer the potential for predictive diagnostics and personalized therapies, enabling early intervention and prevention of autoimmune dis-eases. Through a multidisciplinary approach integrating basic and translational research, there is hope for the development of individualized treatments that target specific immune pathways, ultimately improving outcomes for patients with autoimmune diseases.
The Mechanism of Autoimmune Disease Development
There are more than 150 identified autoimmune diseases [1], which are chronic pathologies triggered by the loss of immunological tolerance to self-antigens, which can cause systemic or organ specific damage.
Meanwhile, multiple factors are thought to contribute to the development of immune response to self, including genetics and environment. Among a variety of the environmental (exposomal) factors, the most important triggers of autoimmunity, that can contribute to autoimmune disease onset and severity, are infections to induce the autoimmunity-related conditions. Autoimmunity conditions and/or autoimmune-related risks occur when the immune system recognizes and attacks host tissue. In addition to genetic factors, environmental triggers (in particular, viruses) are thought to play a key provocative role in the development of autoimmune conditions and diseases as well. And thus, a strong immune response to an invading pathogen could disrupt the systemic regulation and control leading to autoimmunity, whilst creating autoantibodies (auto Abs) and Autoreactive Cytotoxic T Cells (auto CTLs) to attack the tissue(s) and causing destruction to the tissue and organ structure. And since any body part can be involved in, due to the wide variety of possible Abs and CTLs that can be produced, spectrum and symptoms of autoimmune diseases vary greatly [1,2].
Infection Agents and Autoimmune Diseases
Infections can instigate pro-inflammatory cell death programs, induce extracellular release of host nuclear autoantigens, and promote their recognition in an immunogenic context by activating the innate and adaptive immune systems. Autoimmune response towards the infection is mediated by autoreactive T and B lymphocytes responsible to produce soluble mediators (e.g., cytokines) and auto Abs [3]. Infectious agents can trigger some autoimmune diseases through different mechanisms. In general, infections are thought to play a role in the development of autoimmune disease, contributing to abnormal immune re-sponses through molecular mimicry, epitope spreading, and bystander activation (Figure 1A,1B,1C) [4-6].
Molecular mimicry involves the activation of cross-reactive immune cells that recognize both microbial and self-antigens, leading to autoimmune responses. Epitope spreading occurs when persistent infection triggers immune cells to attack self-tissues, causing the release of self-peptides and spreading the immune response to multiple self-epitopes. Bystander activation refers to the nonspecific activation of self-reactive immune cells due to inflammation, further perpetuating autoimmune reactions [7]. Many types of infection may influence one or more of those diseases, and a single organism may be able to trigger more than one disease. For instance, high levels of IgM antibodies against Rubella, Toxoplasma gondii, Cytomegalovirus (CMV), and hepatitis C virus have been found in patients with Anti-Phospholipid Syndrome (APS) [8-11]. Epstein-Barr Virus (EBV), Rubella, Toxoplasma gondii, Helicobacter pylori and CMV can trigger the pro-duction of auto Abs with subsequent development of manifestations in Systemic Lupus Erythematosus (SLE) patients [3,12-14]. CMV, adenovirus, EBV a006Ed rotavirus infection may trigger the clinical manifestations associated with -Type 1 Diabetes (T1D) [15-18]. CMV and EBV may favor the development of multiple sclerosis (MS). These infections are associated with the occurrence of auto Abs against myelin basic protein and myelin glycoprotein oligodendrocyte due to their similarity with some bacterial molecules [19,20].
Interactions between Antigens and Immunity
The relationship between microbes and autoimmunity could be manifested by the presence of auto Abs, autoimmune complexes, or autoreactive CTCs. The presence of autoimmune phenomena in chronic infections could be related to polyclonal B-cell activation, molecular mimicry between microbial and host antigens, altered self, abnormal expression of immunoregulatory molecules, and the anti-idiotypic network (Figure 2).
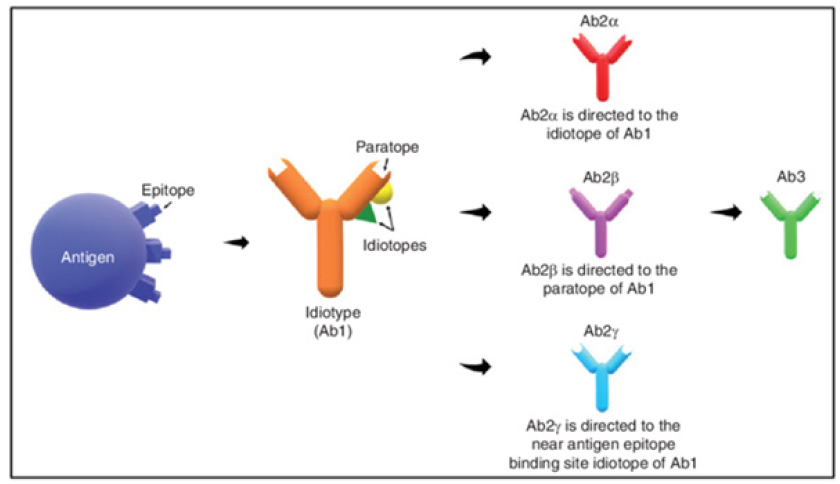
Anti-idiotypic (anti-ID) Abs are important in maintaining a balanced idiotypic regulatory network by neu-tralizing and inhibiting the secretion of auto Abs. In this context, the latter have been advanced as an alternative form of immunotherapy as they can specifically target auto Abs, cause less toxicity and side effects, and could provide long-lasting immunity [21]. The immune response could be modulated through reactions to idiotypes, which represent unique features of immunoglobulins or T-Cell Receptors (TCR). These idiotypes (Ab1) have the capability to recognize antigens and are acknowledged by Ab2 to uphold immune system stability. In a state of equilibrium, the presence of epitopes or antigenic determinants triggers the generation of antigen-specific antibodies (Ab1), subsequently prompting the production of anti-ID antibodies (Ab2) to sustain balance. Ab2 has the capacity to induce the synthesis of anti-anti-ID antibodies (Ab3), which possess similar binding abilities to Ab1 (Figure 3).
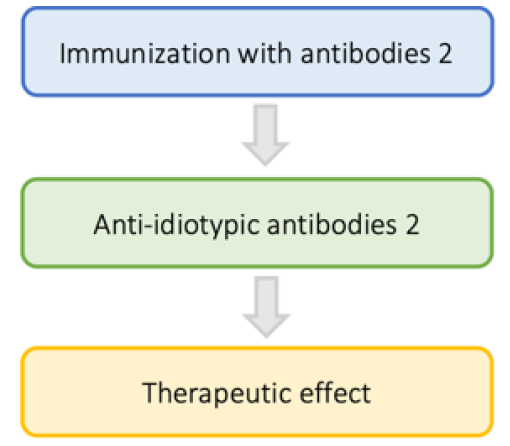
The production of anti-ID antibodies aims to regulate autoantibodies by neutralizing and inhibiting their secretion, ultimately aiding in the prevention of autoimmune diseases. Considering the immunomodulatory potential of anti-ID Abs as antigen-specific immunotherapy, they are likely to play a key role in future attempts to generate an effective treatment for autoimmune diseases (Figure 4) [22].
Anti-ID antibodies not only impede the binding of antigen-antibody complexes and suppress the generation of autoantibodies but also possess a significant advantage in their ability to specifically target and suppress only the corresponding autoantibodies. Unlike conventional medications and immunotherapies, which often exert nonspecific immunosuppression, leading to both protective and harmful immune responses being suppressed, anti-ID antibodies offer a targeted approach. This enables them to effectively dampen a specific autoimmune response while preserving the normal functioning of the immune system. Additionally, anti-ID antibodies induce a memory response by generating T-helper memory cells, ensuring sustained immunity posttreatment, and reducing the likelihood of relapses. Being naturally occurring in the body, anti-ID antibodies elicit immune responses akin to those prompted by nominal antigens, thus ensuring their safety, and minimizing the risk of toxicity. Vaccination with Ab2 offers several advantages, including the presentation of the antigen's internal image. Moreover, since anti-ID antibodies are sourced from the patient, concerns regarding compatibility and rejection by the recipient are practically nonexistent. These antibodies have also demonstrated efficacy in individuals unresponsive to conventional autoimmune disease treatments. However, challenges persist in the development of anti-ID vaccines, particularly in determining the duration of anti-ID immunity [21].
In reality Anti-idiotypic Abs regulate both auto Ab binding and their levels by
i. Neutralizing auto Abs.
ii. Inhibiting the secretion of auto Abs.
The exploration of the role of auto Abs in autoimmune disease has been spurred, in part, by increasing recognition that development of autoimmune diseases is influenced by regulatory Abs (anti-idiotypic Abs) directed against the unique binding (canonical Abs) or catalytic (abzymes) site of auto Abs. Meanwhile, etiology and pathogenesis of post-infectious autoimmune disorders and autoimmunity conditions remain to be unclear, and thus be assessed as a kind of balance between hereditary (genetically based) predisposition, triggering (including environmental) factors and the appearance of autoantibodies (auto-Abs) and/or self-reactive T cells. So, autoimmunity is still a mystery of fundamental and clinical immunology and medicine paired with daily clinical practice as a whole. In this context, to induce autoimmunity that is provoked by the well-known defects in the autoantigen (auto Ag) recognition by either T or B cells, at least, four conditions are required:
i. The presence of pathogenic self-reactive T and/or B cells in persons with the appropriate HLA genotype at high-risk.
ii. The availability of the major autoantigen and/or mimicking antigens at levels sufficient to the T cell presentation and subsequent T cells differentiation, maturation, and activation.
iii. The generation of additional (costimulatory) signals required to activate T and B cells in a proper way.
iv. The loss of the ability of the regulatory T cells to control mechanisms of the autoimmune inflammation [23,24].
Immune reactions triggered by microbial antigens (Ags) can be ignored by tools of immune surveillance (apoptosis or immune suppression), and auto-reactive T- and B-cells can survive for molecular mimicry phenomenon based on the activation of auto-reactive lymphocytes by cross-reactive epitopes of the pathogen. Several plausible mechanisms have been proposed, including molecular mimicry and mimicry-provoked disturbance in the host’s immune response [25-27].
Autoimmune Myocarditis
The outcome of the event would result in manifestations of Post-Infectious Autoimmune Disease (PIAD). Wherein the evaluation of triggering role of infection in the pathogenesis of PIAD is often difficult since the time for provoking the disease to be transformed into PIAD may begin prior specific manifestations of PIAD would form. Thus, for Autoimmune Myocarditis (AIM) to make a bridging link with the infection is established for two-thirds of all the patients, and transformation of primary or infectious phase into PIAD is initiated by mimicking epitopes of, for instance, CVB3 and/or CMV, herewith presence of auto-reactive CTLs and anti-CM auto-Abs, to re-lease sequestered auto Ags and to facilitate the induction and/or development of PIAD is required. Coxsackie Virus (CV) infections frequently initiate autoimmune response in humans and provide a tool for understanding PIAD, including myocarditis [28,29].
Despite a vast armamentarium of approaches to assess PIAD, there are still no obvious clinical and laboratory criteria to get the syndrome validated. The latest design driven innovations in OMICS-approaches will give an opportunity to reveal the sequence of events between induction and progressing of PIAD and allow to control induction and progression of PIAD and thus chronification of the disease to prevent the latter in time. For instance, in cardiology-related practice, AIM usually develops in genetically predisposed individuals infected with the Coxsackie Virus B3 (CVB3) or related viruses to represent typical manifestations of molecular mimicry. The presence of anti-CMV auto Abs is prerequisite to AIM development to initiate myocardial lesions (Figure 5A,5B) [30].
Clinical manifestations of AIM, with distinct onset, vary from asymptomatic to fatal. But the precise biomarkers to predict the course at initial presentation have not yet been established. Meanwhile, an improved knowledge of the mechanisms of infections to precede the illness should help to get type of PIAD-driven AIM de-fined and then be used as combinatorial and/or multifunctional biomarkers, to:
i. Predict the likelihood of developing disease.
ii. Estimate the length of the asymptomatic period.
iii. Provide predictive information about disease course, severity, and complications.
iv. Serve as a warning to avoid potential disease-triggering factors.
vIdentify high-risk individuals who might be suitable candidates for preventive intervention trials.
vi. Develop preventive strategies for quenching PIFAS at the subclinical stages [31, 32].
vii. Biomarkers of PIFAS-related disease.
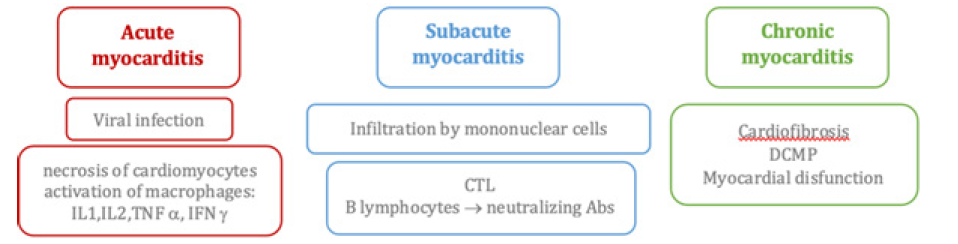
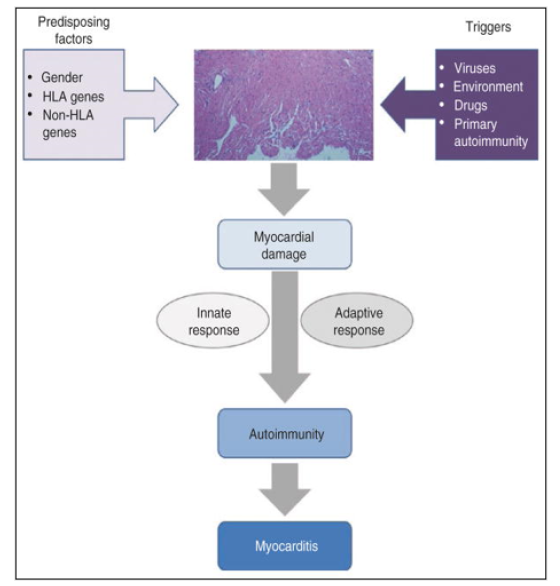
So, the discovery of novel biomarkers specifically tailored to PIFAS-related disease type and stage are expected to enable PPM-driven approach by facilitating predictive diagnostics and personalized therapies. And to stress the above-mentioned comments, let me mention that PIAD-driven AIM provoked by insufficient coordination between two immune arms and hyperactivity of the adaptive one is a dominant feature of PIAD to serve as a combinatorial biomarker. Its unique feature is a broad repertoire of auto Abs responsible for multi-seropositivity and thus to specific autoimmune inflammation biomarkers (e.g., anti-B7-HI auto Abs). An association of AIM with viral infection is strongly supported by clinical observation and epidemiologic study [32]. Myocarditis can be defined as the inflammatory process affecting the muscular tissues of the heart (myocardium). Regardless of its etiology, acute inflammation may progress to subacute and chronic stages and finally to tissue remodeling, fibrosis, and loss of myocardium architecture and contractile function. The latter chronic damage corresponds to the development of Dilated Cardiomyopathy (DCM). Auto-Immune Myocarditis (AIM) is considered a multifactorial entity, in which several immunologic mechanisms are involved in its development and progression. Regarding the trigger and initiation of its pathogenesis, so far, no unique sufficient factor has been identified but rather multiple endogenous and environmental confluent factors, in such a way that the myocardium-specific autoimmune process is triggered and sustained. The balance and relative influence of those factors is still unclear but seems to be variable and host dependent.
Coexistence of predisposing factors along with specific triggers of myocardium damage leads to exposure of cryptic self-antigens and a consequent inflammatory process. At this point, both the innate and degenerates specific adaptive response generate a self-sustained autoimmune phenomenon independent of the original trigger factor. That autoimmune process is responsible for the development of autoimmune myocarditis and progression to Dilated Cardiomyopathy (DCM). Through the view of PIAD it is an important cause of heart failure among adolescents and young adults (Figure 6).
Usually, at the initial steps, viral myocarditis is considered sometimes an independent entity. Nevertheless, it seems that once the trigger infectious noxa exerts its effect, the final effector mechanisms are like the ones leading to AIM progression and chronic complications. Myocardium-tropic viral infection acts as a trigger and “co-adjuvant” generating a sustained myocardium-specific autoimmune response (PIAD-driven AIM). All viruses cause myocarditis with similar inflammatory features, and all could lead to DCM. Following the above-mentioned considerations, AIM usually develops in genetically predisposed individuals infected with the CVB3 to represent typical manifestations of molecular mimicry. The presence, in circulating blood, of CM-AR CTLs and anti-CM auto Abs is prerequisite to AIM development to initiate myocardial lesions. Clinical manifestations of AIM, with distinct onset, vary from asymptomatic to fatal. But the biomarkers to predict the course at initial presentation have not yet been established. Meanwhile, an improved knowledge of the mechanisms of infections to precede the illness should help to get type of PIFAS defined and then be used as a combinatorial biomarker, to develop preventive and/or prophylactic strategies for quenching PIFAS at the pre-early (subclinical) stages.
Forms of Autoimmune Myocarditis
The correlation between the stage of immune-mediated chronic inflammation and the form of AIM is characterized by:
i. The clinical form of primary infectious myocarditis (PIM), which is detected in 75% of cases, whereas in patients with AIM the contribution of PIM is notably decreased (to 25%) giving way to auto aggression. PIM most commonly results from an external inflammatory trigger inducing the host immune response, which may range from minimally transient response to fulminant overwhelming cellular infiltration. If the immune system does not eliminate the infectious pathogen early on, chronic infection develops with or without accompanying inflammation.
ii. Stage of clinical reversible myocarditis (CRM): at early stages (< 3 months for chronic progressive nephropathy and < 1 month for myocarditis, CRM is detected in 40% of cases; however, at later stages of CRM its share decreases appreciably, while that of autoimmune syndromes increases in contrast; CRM is not only the outcome of the infectious process, but also represents a factor responsible for its lingering and chronically relapsing course. Further progression and chronification of CRM are controlled in AIM patients by post-infectious auto-aggression factors.
iii. Rate of progression and chronification of myocarditis: in patients with relapsing or rapidly progressing myocariditis (e.g., AIM), the contribution of CRM does not exceed 32-36%, while the share of autoim-mune syndromes reaches 80-100%. Since the pathological conditions take place at the cellular level, AIM can be suggested but not diagnosed clinically. Accurate diagnosis demands simultaneous histologic, im-munohistochemical and molecular biological workup of the tissue. If the primary infectious or immune-mediated causes of the disease are carefully defined by clinical and biopsy-based tools, specific antiviral treatment options in addition to basic symptomatic therapy are available under certain conditions. These may allow tailored cause-specific treatment that improves symptoms and prognosis of patients with AIM [32-34].
So, if the primary infectious or immune-mediated causes of myocarditis are carefully defined by clinical, OMICS- and biopsy-based tools, specific antiviral, and targeted immunosuppressive treatment options in addition to basic symptomatic therapy are available under certain conditions. Adopting this approach allows for a tailored, cause-specific treatment that improves the prognosis of patients suffering from acute and chronic diseases, while preventing Dilated Cardiomyopathy (DCM). Personalized treatment should also be tailored within the time frame from infection to innate and adaptive response [21, 35-37]. There is still much work to be done.
Prospective Approaches to Treatment
In this context, there is a growing body of therapeutic knowledge which, despite frequently based on case reports and uncontrolled trials, suggests that targeted immune modulation/suppression is a useful approach in AIM, even in viral-triggered cases if it is used together with proper antiviral treatment. In general, the future directions should focus on the proper predictive and prognostic diagnostics, not only on the etiologic aspect but also on the specific stage of evolution of the immune/inflammatory process and specific stage of AIM. That would allow to determine specific treatments depending on the underlying cause and on the predominant immune process taking place in the patient now of the intervention or in persons-at-risk now of generating pre-illness (subclinical) conditions.
The positive side of the story is the exponentially growing knowledge about the 3 stage-based pathogenesis of myocarditis, which certainly will end up in development of effective diagnostic, prognostic, and therapeutic strategies. Advances exist in therapeutics, but still relying on global immunosuppression and unspecific immuno-modulation. The expectation is that future basic and translational studies might provide even deeper insights in the pathogenesis of AIM to provide the design-driven and biomarker-based targeted drugs to prevent AIM! That would simultaneously lead to development of better diagnostic and predictive tools (including theragnostic) allowing characterization and stratification of stages of AIM progression in each patient or in persons-at-risk [38,39]. Finally, that translational knowledge would make possible the development of individualized net-work-targeted combinatorial treatments, using resources of systems polypharmacy.
Conclusion
Autoimmune diseases represent a diverse group of chronic pathologies characterized by the breakdown of immunological tolerance to self-antigens, resulting in systemic or organ-specific damage. While the exact mechanisms underlying autoimmune disease development remain complex and multifaceted, both genetic predisposition and environmental triggers, particularly infections, are known to contribute significantly to the initiation and progression of autoimmune responses. The relationship between microbial antigens and autoimmunity is well-established, with infections capable of triggering abnormal immune responses through mechanisms such as molecular mimicry, epitope spreading, and bystander activation. These processes can lead to the production of autoantibodies and autoreactive T cells, ultimately resulting in tissue and organ damage characteristic of autoimmune diseases.
The association between infections and autoimmune diseases has been observed across various conditions, with specific pathogens implicated in different autoimmune disorders. Understanding the mechanisms underlying infection-induced autoimmunity is crucial for developing targeted interventions and preventive strategies. Furthermore, autoimmune myocarditis serves as a paradigm for studying the relationship between infections and autoimmune diseases. The progression from viral infection to autoimmune heart disease involves complex immune interactions and inflammatory processes, highlighting the need for comprehensive diagnostic and therapeutic approaches. Moving forward, prospective approaches to treatment should focus on personalized and targeted immune modulation, considering the specific stage and underlying cause of the autoimmune process. Advances in basic and translational research hold promise for the development of more effective diagnostic tools, prognostic markers, and individualized treatment strategies, ultimately improving outcomes for patients with autoimmune diseases.
Acknowledgement
None.
Conflict of Interest
None.
References
- Sun L, Su Y, Jiao A, Wang X, Zhang B (2023) T cells in health and disease. Signal Transduct Target Ther 8(1): 235. Committee for the Assessment of NIH Research on Autoimmune Diseases. Board on Population Health and Public Health Practice. Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Enhancing NIH Research on Autoimmune Disease. Washington (DC): National Academies Press (US).
- Carlton LH, McGregor R, Moreland NJ (2023) Human antibody profiling technologies for autoimmune dis-ease. Immunol Res 71(4): 516-527.
- Connie C Qiu, Roberto Caricchio, Stefania Gallucci (2019) Triggers of Autoimmunity: The Role of Bacteri-al Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front Immunol 8(10): 2608.
- Rose NR (2001) Infection, mimics, and autoimmune disease. J Clin Invest 107(8):943-944
- Habibi MA, Nezhad Shamohammadi F, Rajaei T, Namdari H, Pashaei MR, et al. (2023) Immunopathogenesis of viral infections in neurological autoimmune disease. BMC Neurol 23(1): 201.
- Ercolini AM, Miller SD (2009) The role of infections in autoimmune disease. Clin Exp Immunol 155(1): 1-15.
- Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, et al. (2019) Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 11(8): 762.
- Blank M, Asherson RA, Cervera R, Shoenfeld Y (2004) Antiphospholipid syndrome infectious origin. J Clin Immunol 24: 12–23.
- Denham C, Tissier G, Golding A (2019) Antiphospholipid antibody syndrome with thrombotic splenic in-farcts associated with acute cytomegalovirus infection. Access Microbiol 1(7): e000032.
- Shapira Y, Agmon Levin N, Selmi C, Petríková J, Barzilai O, et al. (2012) Prevalence of anti-Toxoplasma antibodies in patients with autoimmune diseases. J Autoimmun 39(1-2): 112-116.
- Ordi Ros J, Villarreal J, Monegal F, Sauleda S, Esteban I, et al. (2000) Anticardiolipin antibodies in patients with chronic hepatitis C virus infection: characterization in relation to antiphospholipid syndrome. Clin Diagn Lab Immunol 7(2):241-244.
- Pérez Mercado AE, Vilá Pérez S (2010) Cytomegalovirus as a trigger for systemic lupus erythematosus. J Clin Rheumatol 16(7): 335-337.
- Frau J, Coghe G, Lorefice L, Fenu G, Cocco E (2023) The Role of Microorganisms in the Etiopathogenesis of Demyelinating Diseases. Life (Basel) 13(6): 1309.
- Qiu CC, Caricchio R, Gallucci S (2019) Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front Immunol 10: 2608.
- Ekman I, Vuorinen T, Knip M, Veijola R, Toppari J, et al. (2019) Early childhood CMV infection may decelerate the progression to clinical type 1 diabetes. Pediatr Diabetes 20(1): 73-77.
- Arafa AA, Abdel Moneim A, Khalil RG, El Senousy WM, Kamel MM, et al. (2022) Association between Paediatric Adenovirus Infection and Type 1 Diabetes. Children (Basel) 9(10): 1494.
- Burke RM, Tate JE, Jiang B, Parashar UD (2020) Rotavirus and Type 1 Diabetes-Is There a Connection? A Synthesis of the Evidence. J Infect Dis 222(7): 1076-1083.
- Klatka M, Rysz I, Hymos A, Polak A, Mertowska P, et al. (2023) Effect of Epstein-Barr Virus Infection on Selected Immunological Parameters in Children with Type 1 Diabetes. Int J Mol Sci 24(3): 2392.
- Langer Gould A, Wu J, Lucas R, Smith J, Gonzales E, et al. (2017) Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 89(13): 1330-1337.
- Barnes K (2022) Infection with Epstein Barr virus increases risk of multiple sclerosis. Commun Med (Lond) 2: 13.
- Pan SY, Chia YC, Yee HR, Fang Cheng AY, Anjum CE, et al. (2020) Immunomodulatory potential of anti-idiotypic antibodies for the treatment of autoimmune diseases. Future Sci OA 7(2): FSO648.
- Stanova AK, Ryabkova VA, Utekhin SV, Shoenfeld VJ, Churilov LP, et al. (2020) Anti-Idiotypic Agonistic Antibodies: Candidates for the Role of Universal Remedy. Antibodies (Basel) 9(2): 19.
- Ziogas A, Bruno M, van der Meel R, Mulder WJM, Netea MG (2023) Trained immunity: Target for prophy-laxis and therapy. Cell Host Microbe 31(11): 1776-1791.
- Yu X, Wax J, Riemekasten G, Petersen F (2023) Functional autoantibodies: Definition, mechanisms, origin and contributions to autoimmune and non-autoimmune disorders. Autoimmun Rev 22(9): 103386.
- Trier NH, Houen G (2023) Antibody Cross-Reactivity in Auto-Immune Diseases. Int J Mol Sci 24(17): 13609.
- Petrova G, Ferrante A, Gorski J (2012) Cross-reactivity of T cells and its role in the immune system. Crit Rev Immunol 32(4): 349-372.
- Bazyleva Ekaterina Vladlenovna, Trevor Marshall, Elena Antonova, Kusum Ahmedilova, Sergey Suchkov (2018) Preventive and Personalized Medicine & Molecular Diagnostics. 8th European Conference on Predictive, Italy.
- Salaman MR (2003) A two-step hypothesis for the appearance of autoimmune disease. Autoimmunity 36(2): 57-61.
- Wucherpfennig KW (2001) Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 108(8): 1097-1094.
- Cherepahina N, Agirov M, Tabaksoeva J, Ahmedilova K, Suchkov S (2011) Postinfectious Autoimmune Syndrome as a Key Factor in Chronization of the Infectious Disease. Autoimmune Disorders - Pathogenet-ic Aspects.
- Mone K, Reddy J (2023) The knowns and unknowns of cardiac autoimmunity in viral myocarditis. Rev Med Virol 33(6): e2478.
- Matsumori A (2023) Myocarditis and autoimmunity. Expert Rev Cardiovasc Ther 21(6): 437-451.
- Bracamonte Baran W, Čiháková D (2017) Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol 1003: 187-222.
- Zhao L, Fu Z (2018) Roles of Host Immunity in Viral Myocarditis and Dilated Cardiomyopathy. J Immunol Res 2018: 5301548.
- Ammirati E, Moslehi JJ (2023) Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 329(13): 1098-1113.
- Gong J, Neilan TG, Zlotoff DA (2023) Mediators and mechanisms of immune checkpoint inhibitor-associated myocarditis: Insights from mouse and human. Immunol Rev 318(1): 70-80.
- Shao J, Liu C, Wang J (2023) Advances in research on molecular markers in immune checkpoint inhibitor-associated myocarditis. Cancer Innov 2(6): 439-447.
- Chary M, Barbuto AF, Izadmehr S, Tarsillo M, Fleischer E, et al. (2023) COVID-19 Therapeutics: Use, Mechanism of Action, and Toxicity (Vaccines, Monoclonal Antibodies, and Immunotherapeutics). J Med Toxicol 19(2): 205-218.
- Weber BN, Garshick M, Abbate A, Youngstein T, Stewart G, et al. (2023) Acute cardiovascular complica-tions of immune-mediated systemic inflammatory diseases. Eur Heart J Acute Cardiovasc Care. 12(11): 792-801.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.