Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Stem Cell Technologies to Integrate Bio Design- Related Tissue Engineering within the Frame of Cell- Based Regenerative Medicine: Towards the Preventive, Therapeutic and Rehabilitative Resources and Benefits
*Corresponding author:Sergey Suchkov, University of World Politics & Law, Moscow, Russia, Russian Academy of Natural Sciences (RANS), Moscow, Russia, European Association of Predictive Preventive and Personalized Medicine (EPMA), International Society for Personalized Medicine (ISPM), Tokyo, Japan, Personalized Medicine Coalition (PMC), Washington, DC, USA, AMEE (Association for Medical Education in Europe), Centre for Medical Education, University of Dundee, Dundee, Scotland, ACS (American Chemical Society), Washington, DC, USA, AHA (American Heart Association), Dallas, TX, USA, ARVO (The Association in Research in Vision & Ophthalmology), Rockville, MD, USA, ISER (International Society for Eye Research), Anchorage, AK, USA.
Received: December 21, 2023; Published: January 12, 2024
DOI: 10.34297/AJBSR.2024.21.002811
Abstract
Cardiovascular disease is the leading cause of death, with 80% of cases occurring in developing countries. Innovative therapies are required to reduce mortality and limit or abolish the necessity for cardiac transplantation. Over the last decade, stem cells have been a promise for the cure of several diseases not only due to their plasticity but also to their capacity to act in a paracrine manner and influence the affected tissue. Human SC-based therapy derivatives are extremely attractive for therapeutic development because they have direct pharmacologic utility in clinical applications, unlike any other adult cells. Moreover, stem cell-derived paracrine factors have been shown to suppress inflammation and apoptosis, stimulate angiogenesis, and amplify the proliferation and differentiation of resident Cardiac Stem Cells (CSCs). And SC therapies are thus viable alternatives to conventional treatments with substantial therapeutic potential; market opportunities are huge, as multiple product candidates are expected to be approved over the coming decade.
Introduction
The goal for regenerative medicine is to channel multipotent human cells with high proliferative capacity into pre-defined scenarios and specified differentiation programs within the human body. Meanwhile, end-stage heart failure is a global scourge, and cardiovascular disease (CVD) is a major health problem and the leading cause of morbidity and mortality in the Globe. And thus, the treatment and prevention of CVD are the public health priorities. The latter means that CVD is a life course disease that begins with the evolution of risk factors that in turn contribute to the development of subclinical (pre-early and symptom-free) atherosclerosis.
The idea of extending the lifetime of the human heart has been fuelled by a series of major advances in transplantation and drug therapies. Nevertheless, atherosclerotic complications and myocardial infarction is characterized by the irreversible loss of cardiac myocytes because of the ischemic necrosis. Therefore, the need to re-establish the structural and functional features of native heart tissue represents a major challenge for the field of cardiac regeneration and engineering as the latest avenue to move ahead.
Stem Cell (SC)-based therapy has been considered as a promising alternative in the treatment of ischemic heart disease. CSCs are directly involved in cardiac cellular homeostasis during aging and adaptation to physiological and pathological stress and being transplanted into damaged hearts, CSCs have the capacity to generate de novo myocardial tissue. The niche for CSCs can be activated by several active biomolecules (including cytokines and growth factors), or through the injection of systemic drugs, such as statins, to obtain beneficial results like those of CSC transplantation [1].
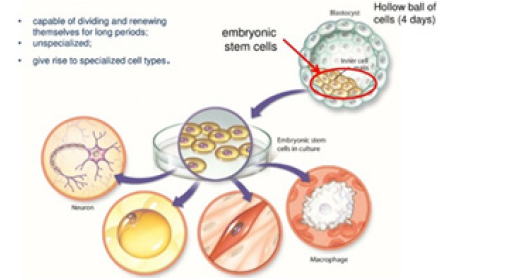
Meanwhile, current understanding defines the heart as an organ comprised of heterogeneous population of myocytes, which continue to die and self-renew, thereby maintaining cardiac integrity throughout the life. However, the cell source for heart self-renewal, purportedly by the re-placement of senescent Cardiac Myocytes (CMs) with juvenile cells, as well as the underlying mechanisms remain obscure (Figure 1).
So far, current therapies merely delay its inexorable progression, and the lack of a clinically-suitable and highly productive human Cardiac Myocyte (CMC) source with proper myocardium regenerative potential has been the major setback in regenerating the damaged human heart, either by endogenous cells or by cell-based transplantation or cardiac tissue remodelling and engineering via procedures of bio- and/or drug design being based on drug discoveries [1,2]. The latter does strongly need much more advanced and promising techniques to improve morphological and electromechanical properties of the diseased heart (Figure 2).
Multiple techniques to improve morphological and electromechanical properties of the diseased heart:
1. In vitro cardiac differentiation of different stem cell types.
2. Tissue engineering approaches combining cells with biomaterials to design in vitro cardiac patches or injectable scaffolds for transplantation into the infarcted heart area.
3. Cell-and gene-based strategies secreting cytokines, growth factors and microRNAs to promote cardiac regeneration.
4. Stem-cell derived exosomes as an innovative cell-free therapy in heart regenerative medicine.
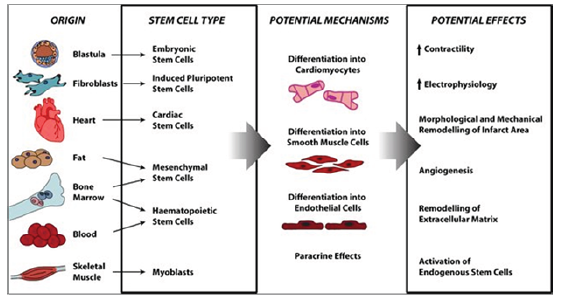
Right now, different cell types are under evaluation regarding their regenerative potential. First-generation cell types including Skeletal Myoblasts (SMs), Bone Marrow Mononuclear Cells (BMMNCs), Hematopoietic Stem Cells (HSCs), Endothelial Progenitor Cells (EPCs), and Mesen-Chymal Stem Cells (MSCs) were initially introduced. Despite promising preclinical studies, first-generation approaches displayed heterogeneous clinical outcomes [3].
The CSC's Main Role is to Replace Myocardial Dying CMCS and Reconstruct Damaged Areas in the Heart
Among families of SCs are the heart muscle cells (cardiac myocytes/CMCs) being specific for congenital and chronic inflammatory heart cases, ischemic disorders or for heart attack victims. For instance, Human Embryonic Stem Cells (HESCs) and Induced Pluripotent Stem Cells (IPSCS) can self-renew indefinitely, while maintaining the capacity to differentiate into adult and useful CMCs [4].
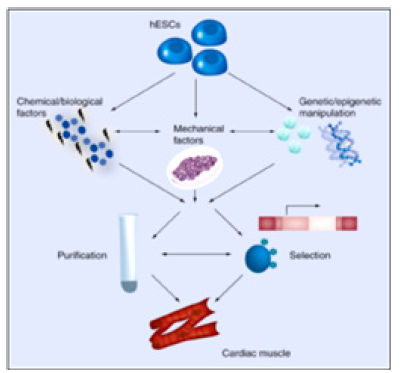
Human Embryonic Stem Cell (HESC)-Based Drugs: Currently, the hESC CMC therapy derivatives are the only available human cell sources with ad-equate capacity to regenerate contractile heart muscles, vital for heart repair in the clinical setting (Figure 3).
Directed differentiation of HESCs has generated much interest in the field of regenerative medicine. Because of their ability to differentiate into any cell type in the body, hESCs offer a novel therapeutic paradigm for myocardial repair by furnishing a supply of CMCs that would ultimately restore normal myocardial function when delivered to the damaged heart (Figure 4). Moreover, immunosuppressive regimens for hESC-based therapeutics may not need to be as rigorous as conventional organ transplantation [5]. Nevertheless, transplanted hESC-derived CMs will be susceptible to immune rejection to some degree (Figure 4).
Researchers are focusing on chemical (e.g., 5-azacytidine and p38 MAPK inhibitors) and biological (e.g., activin A, bone morpho-genetic protein, basic FGF, VEGF and Dickkopf homolog 1) factors, genetic (e.g., miRNAs) and epigenetic (e.g., miRNAs and chromatin remodeling) manipulation, and mechanical factors (e.g., hydrodynamics and surface tension) to direct cardiomyocyte differentiation from hESCs. These approaches are complemented by purification methods that take advantage of the biochemical properties of human cardiomyocytes (e.g., Percoll density centrifugation and mitochondrial content), and selection strategies that rely on the expression of cardiac-specific genes (e.g., reporter lines and molecular beacons) and surface markers. hESC: Human embryonic stem cell.
On the other hand, studies report that spontaneous CMC differentiation of hESCs is an inefficient process that yields very low numbers of CMCs, the need for new methods of directed differentiation of hESCs into functional CMCs and cardiac progenitors has led to an explosion of research utilizing translational strategies to direct cardiac differentiation and enrich populations of cardiac cells for therapeutic use. Further improving policy making and funding situation for hESC research and translational applications would open up a new dimension of cell therapy-based future medicine to provide new treatments for life-threatening cardiovascular diseases and failures. Transforming pluripotent hESCs into fate-restricted therapy derivatives would dramatically increase the clinical efficacy and safety of hESC-derived cellular products, bringing cell-based regenerative medicine to a turning point [6].
Induced Pluripotent Stem Cells (IPSCs): A novel approach in cardiac regeneration has been proposed with the discovery of Induced Pluripotent Stem Cells (iPSCs) (Figure 5).
iPSCs nearly identically resemble ESCs and can give rise to all cell types (including CMCs) in the body, and thus have opened new opportunities for PPM and new ways of modelling human dis-eases. iPSCs can efficiently differentiate into CMCs and thus hold a real regenerative potential for future clinical applications [7]. Better understanding and control of the reprogramming process should enable enhanced efficiency and higher fidelity in reprogramming. Toward those trend and path, the possibility to directly induce conversion of fibroblasts into CMCs has recently emerged as a promising area for in situ cardiac regeneration. And better understanding and control of the reprogramming process should enable enhanced efficiency and higher fidelity in reprogramming.
Over the past few years, various combinations of biomolecules (including transcription factors/TFs), have successfully been developed to create iPSCs. Inspired by the iPSC approach using multiple TFs, many studies have shown that, with the proper conditions, somatic cells can also be transdifferentiated into another cell fate both within and outside of their germ layer, which is also called lineage-specific reprogramming [8].
Pluripotent Stem Cells (PPSCs): The First Steps Towards the Bioengineering Strategies for Cardiac Regeneration: Embryonic Stem Cells (ESCs) and Induced Pluripotent Stem Cells (iPSCs), which are collectively called PPSCs, have emerged as a promising source for regenerative medicine and cardiology [9]. Particularly, Human Pluripotent Stem Cell-Derived Cardiomyocytes (hPSC-CMCs) have shown robust potential for regenerating injured heart, opening the door for clinical application to secure the cardiac repair [10]. Cell therapy with hPSC-CMCs has shown great potential for individualized therapy of injured heart, is expected to serve as an integral component of PPM and is potentially viewed as a treatment that would revolutionize the management of patients with severe heart failure (Figure 6).
The panel (A) shows a schematic representation of the different hPSC-based methodologies used for regenerative purposes in the cardiac field. The panel (B) provides a timeline that summarizes the key milestones reached in the field, starting from the simple injection of hPSC-CMs into the heart to the development of tissue-like structures with enhanced hPSC-CM maturation, more com-plex perfusable and personalized constructs and injectable hydrogels. However, more studies are needed to ensure the precise therapeutic effects, underlying mecha-nisms, and safety, before this technology can be applied clinically.
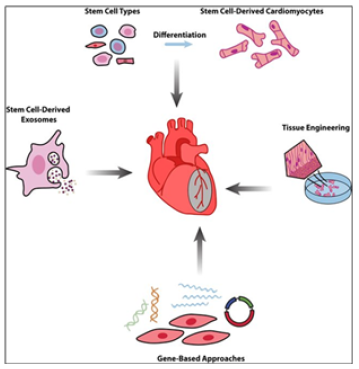
SC-Derived Paracrine Factors the Newest Effective Tools to Repair the Myocardial Damages and to Manage Cardiac Healthy Entity
Some focus of basic research in the field and translational applications have since shifted to SC-derived paracrine factors, including cytokines, growth factors, mRNA, and miRNA (notably, the latter can enter the extracellular space either in soluble form or packed into membrane vesicles) (Figure 7).
Schematic representation of the main experimental cardiac medicine approaches suggested to address myocardial injury and aiming at stimulating endogenous mechanisms of repair and myocardial restoration by means of stem cell-derived paracrine effectors and biomaterials. And then the paracrine factors have been shown to suppress inflammation and apoptosis, to stimulate angiogenesis, and to induce and amplify the proliferation and differentiation of resident Cardiac SCs (CSCs). Such features have led to exosomes being considered as potential drug candidates affording myocardial regeneration. The search for chemical signals capable of stimulating cardio myogenesis is still ongoing despite continuous debates regarding the ability of mature cardiac myocytes to divide or to dedifferentiate, and the ability of CSCs to differentiate in-to cardiac myocytes [11]. In this sense, one of the most effective tools to manage cardiac entity and to control the balance of CMCs within the myocardium whilst preventing subclinical stages of the real-time disorder and the disease, to treat the latter and to regenerate the post-treatment complications and damages could be SCs and biomolecules secreting by those cells and cells in the SC microenvironment.
Mesenchymal Stem Cell-Based Drugs and their Applications in Cardiology: Along with the introductory SC-related reference, the stroma-related cells, or Mesenchymal Stem Cells (MSCs) (Figure 8), being multipotent progenitor cells constitute a minute proportion of (Figure 8).
Soluble factors released by MSC play an essential role in the post-ischemic reparative process improving angiogenesis, cytoprotecting, and endogenous cardiac regeneration and reducing fibrosis. Ang-1 angiopoietin 1, HGF hepatocyte growth factor, MSC mesenchymal stem/stromal cells, VEGF vascular endothelial growth factor the bone marrow, represented as a rare population of cells that makes up 0.001 to 0.01% of the total nucleated cells [12]. MSCs can differentiate into both mesenchymal, as well as, non-mesenchymal cell lineages, such as myocytes, both in vitro and in vivo. In this sense, several stud-ies have documented the substantial clinical improvements observed in animal models, when MSC were systemically introduced as a therapy in mouse models of myocardial ischemia and infarct. As described previously, MSCs are characterized by their hypo immunogenicity which would secure allo-MSC engraftment in cardiac tissues in the absence of immunosuppression, whilst favourably altering ventricular function [13]. The allo-MSC engraftment occurred without evidence of immunologic rejection and in the absence of assisted immunosuppressive therapy emphasizing some of the apparent advantages of those cells over other cell populations for cellular cardiomyopathy (Figure 9).
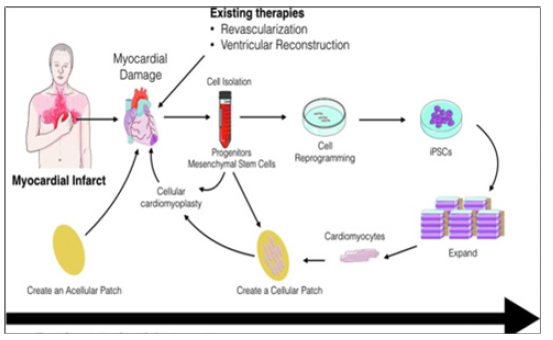
Figure 9: Cellular cardiomyopathy as a new translational and therapeutic approach involving engineering culture systems, the use of novel biomaterials for mechanical support of the cells and for controlled release of therapeutics, and tissue engineering.
Diagrammatic representation of the different approaches that can be used to repair infarcted myocardial tissue whilst securing the cellular cardiomyopathy. An acellular patch can be used as an off-the-shelf product that can be implanted soon after myocardial infarction. Alternatively, cells can be harvested (i.e., progenitor cells) and injected back into the patient. Another approach can be the isolation of somatic cells (i.e., blood cells), reprogrammed, expanded, differentiated, and assembled into a bioengineered cardiac tissue that can then be implanted back into the patient as an autologous patch. These approaches have different timing and expenses associated with them that can have potential impact on their clinical use.
The concept of adult SC plasticity implies that SCs and CSCs can transdifferentiate into mature cell types outside their original lineage in response to microenvironmental cues. For example, HSCs may transdifferentiate into CMCs, thereby improving heart function and survival [14]. The data accumulated from preclinical and clinical data indicate that bone marrow derived MSCs have, in addition to their therapeutic purposes in regenerative medicine, effects that can result from their anti-inflammatory properties. In addition, the therapeutic effectiveness of MSCs relies heavily on their ability to modify microenvironments. Those modifications occur through the release of cytokines, and anti-apoptotic and trophic molecules that promote the repair and protection of damaged (including cardiac) tissues [15].
Understanding SC-Based Therapy of the Second-Step Generation: The Novel Cell Type Candidates to Secure Regenerative Cardio Myogenesis
SCs and CSCs, as subjects to be utilized in treating cardiovascular diseases, are envisioned as a replacement for lost functional cells (e.g., CMCs), a means of trophic support, and, more recently, a cell-based therapeutic tool for in vitro modelling to understand disease and to screen and personalize treatments. The need for new and improved pharmacotherapies in modern cardiology to treat and, moreover, to prevent the disease while minimizing preclinical manifestations is driving interest by the specialists in personalized and precision medicine (PPM).
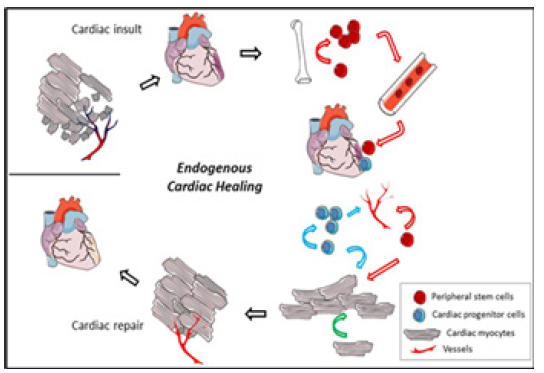
Consistent with the traditional view that the heart is a “postmitotic” organ that possesses minimal capacity for self-repair, much of the preclinical and clinical work has focused exclusively on introducing SCs into the heart, with the hope of differentiation of these cells into functioning CMCs. This approach is ongoing and retains promise but to date has yielded inconsistent successes. More recently, it has become widely appreciated that the heart possesses endogenous repair mechanisms (Figure 10) that, if adequately stimulated, might regenerate damaged cardiac tissue from in situ cardiac stem cells [16] (Figure 10).
After a cardiac insult, a significant number of CMCs die, and vessel density gets reduced. In a very limited way, the heart has the ability to regenerate but it is insufficient to compensate for the total damage. The cellular participants in the endogenous regeneration process may include CMCs (pink cells, black lines), local cardiac progenitor cells (blue cells), and recruited peripheral stem cells (red cells). These cells have the potential to proliferate and participate in the regeneration of CMCs, angiogenesis, and the release of trophic factors that may reduce cardiac cell death. Modes of the cell’s participation are identified by coloured arrows (peripheral stem cell, red; cardiac progenitor cell, blue; cardiac myocyte, green).
Accordingly, much recent work has focused on engaging and enhancing endogenous cardiac re-pair mechanisms. So, understanding the biological activity of SCs, CSCs and progenitor cells, and their ability to contribute to the repair, regeneration and remodelling of the human heart is just crucially important. Among the latest SC-based products is Baxter Healthcare’s Phase III trial of intramyocardially administered autologous HSCs (harvested from peripheral blood), intended to be used in patients with refractory chronic myocardial ischemia. Findings to date indicate that the product can repair heart tissue, increase blood flow, and allow the patient to exercise.
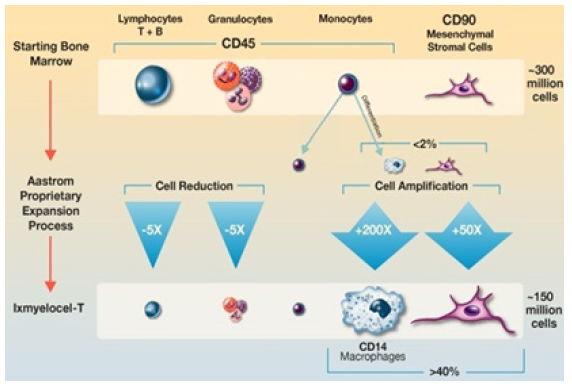
One direct competitor to Phase III trials Baxter’s treatment is AMR-001 (NeoStem), which uses autologous enriched HSCs from the bone marrow to treat ST elevation myocardial infarction. Injected into the infarct-related artery, AMR-001 targets the site of ischemic injury, where chemokine CXC Receptor 4 (CXCR4) on HSCs binds to Stromal-Derived Factor 1 (SDF1), which is induced by Hypoxia-Inducible Factor (HIF) produced by ischemic tissue. Other late-stage candidates include Aastrom’s Ixmyelocel‑T (Figure 11),
Ixmyelocel T is composed of a mixture of cell types that include those expected to be found in the BM-MNC population. These include myeloid cells (granulocytes, monocytes, and mixed myeloid progenitors) and lymphoid cells (T cells, B cells, and mixed lymphoid progenitors) that express CD45 on the cell surface, CD90+ MSCs, and CD45+CD14+ auto fluorescent+ (CD14+Auto+) macrophages. The numbers of CD90+ and CD14+Auto+ cells are significantly greater in ixmyelocel T because of expansion during the Aastrom (now Vericel) proprietary expansion process. Which consists of autologous cells derived from BM and proved to promote immunomodulation, angiogenesis and tissue remodelling [17]. The product is in Phase III trials for critical limb ischemia and dilated cardiomyopathy.
Bio heart and Cytori also have Phase III C‑Cure (Cardio3 Bioscience) which is based on autologous MSCs differentiated via its cardiopoiesis platform into CMCs, which are re-injected into the heart. Cytori’s adipose-derived MSCs for acute myocardial infarction underwent Phase II/III trials. Evidence has been presented that a fraction of CMCs may be able to renter the cell-cycle and that limited regeneration can occur through recruitment of resident and circulating SCs. Some clinicians may regard these new ideas as being mere curiosities, because of their everyday experience that endogenous repair mechanisms are overwhelmed in patients with Acute Myocardial Infarction (AMI), advanced coronary artery disease, and chronic heart failure. However, the existence of endogenous repair mechanisms suggests that cardiac repair may be achieved therapeutically in these clinical settings.
The occurrence of viable cells internalized within different kinds of host cells has been recognized for more than 100 years and usually to illustrate:
1. cannibalism,
2. emperipolesis and
3. and entosis, which differ in both effector and host cell identity, mechanism of penetration, and function. Both emperipolesis and entosis appear to share similar features with the development of intracellularly localized CSCs which are being encapsulated can replicate followed by the partial cardiomyogenic differentiation (Figure 12 A-C).

Figure 12A: The CSCs inside CMCs and the formation of CSC-containing CICSs in the cultures obtained from newborn and 20- and 40-dayold rats.

Figure 12C: Cell-In-Cell Structures (CICSs) identified in the suspension of freshly isolated myocardial cells (ex vivo) of 20- and 40-day-old rats.
a. Experimental design. The cells were plated and cultured for up to 30 days, followed by immunostaining or time-lapse microscopy. (B–G) Immunocytochemistry. The nuclei of the cells have been stained with Hoechst. Transmitted light and fluorescent images are merged.
b. c-kitC CSC inside a CM obtained from a newborn rat (day in vitro 6).
c. Isl1C CSC inside a CM obtained from a newborn rat (day in vitro 4). As documented by the expression of Ki67, both the CSC and the host cell exhibit proliferative ability.
d. ScaC CSC encapsulated between the nuclei of the host cell (20-day-old rat, day in vitro 11).
e. A mature c-kitC CSC-containing CICS with a prominent coating (“capsule”) with 3 pores (white arrows, 40-day-old rat, day in vitro 6). Optical sectioning shows the host cell nucleus (blue) just above the CICS (see sections 13–15 in Video S1). (F-G) The CICS capsule in detail.
f. Erosion of the Isl1C CSC-containing CICS capsule (black arrow) obtained from a 40-day-old rat, day in vitro The pores are also visualized (white arrows). The capsule interior is positive for sarcomeric aactinin, also observed in (G).
g. Erosion of the c-kitC CSC-containing CICS capsule (black arrow) obtained from a newborn rat, day in vitro The pores are seen (white arrows) (Figure 12 B).
The optical sections were spaced 1.01 mm (A, B) and 2.01 mm along the z-axis (C). Images are placed in consecutive order from the bottom to the top of structures.
a. C-kitC CSC-containing CICS in the culture obtained from a 40 day old rat (FITC, green, day in vitro 6) After counterstaining for a-sarcomeric actin (Alexa 543 nm, red), the nuclei (Hoechst, blue) of both the CSC and the host cardiomyocytes are visualized; the vertical dimension of the CICS is 20 mm (slices 3 to 20). Transmitted light and fluorescent images are merged.
b. The Isl1C CSC containing CICS (FITC, green, left column) inside a given host CM obtained from a newborn rat (day in vitro 11), with a vertical dimension of 7 mm (slices 2 to 6). Cytoskeletal actin (rhodamine phalloidine, red, central column) can be seen. Green and red fluorescent images are merged and presented in the right column.
c. The Isl1C CSC-containing CICS in the culture of newborn rat (day in vitro 4). Isl1C CSCs (FITC, green), cytoskeletal actin (rhodamine phalloidine, red), the nuclei (Hoechst, blue) are merged with transmitted light images (Figure 12 C).
Transmitted light and fluorescent images are merged.
a. Isl1C CSCs inside cardiomyocytes of 20-day-old rats (Isl1, green), asarcomeric actin, red).
b. c-kitC CICS. (40-day-old rat, c-kit, green; Ki67, red).
c. c-kitC CICS. (40-day-old rat, c-kit, green; asarcomeric actin, red).
Our results suggest that self-renewal of cells in myocardium is driven primarily by proliferation and differentiation of CSCs inside the colonies and by division and partial differentiation inside the bodies of small myocardial cells (TACs and young CMs) [18]. The presence of CSC-derived colonies, CICSs and TACs in myocardium proved our viewpoint about two pathways that generate new CMCs in adult heart. Moreover, TACs may play a central role in self-renewal of myocardium throughout the lifetime. By studying the behaviour of CSCs, we proved that the formation of new CMCs from resident CSCs occurs through colony formation and consequent to their intracellular development inside the CMCs, forming Cell-In-Cell Structures (CICSs). Our data indicate that cardiomyogenic stimuli should be focused on TACs, rather than on CSCs or mature CMCs. And the proliferative activity of TACs is enhanced following ischemia and hypoxia and that their cardiomyogenic differentiation ability renders them as top candidates for therapeutic cardio myogenesis in the diseased heart [19].
In studies of the last decade, it was shown that Apoptotic Bodies of Cardiomyocytes (APBC) stimulate the development of colonies of resident stem cells of cardiomyocyte precursors in the heart muscle of animals. This is accompanied by an increase in myocardial contractility. The introduction of Apoptotic Bodies of Fibroblasts (APBF) to experimental animals caused the development of colonies with markers of endothelial cells in combination with a decrease in myocardial contractility (Figure 13).

Figure 13: Detection of CSC colonies and “Cell-In-Cell Structures” (CICSs) in the myocardium of “old” rats after exposure to Apoptotic Cells of Cardiomyocytes (ApBc) and Fibroblasts (ApBf).
Earlier on syngeneic animals using the label - GFP was shown that a local laser apoptotic effect on tissues causes an intensive transition of mesenchymal bone marrow stem cells (HSCs) from the bloodstream to the zone of programmed cell death (Figure 14).
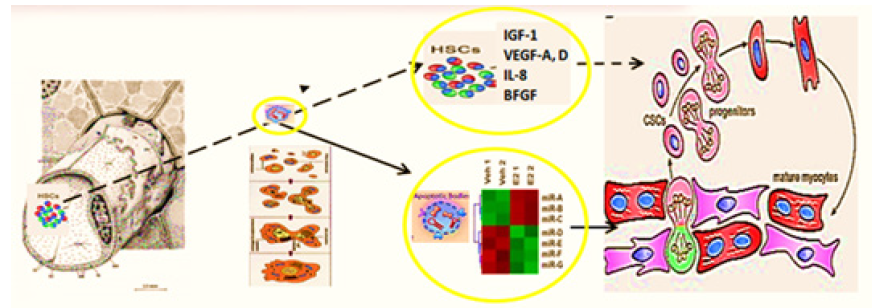
Figure 14: Hypothesis of pathogenesis of restoration of myocardial integrity after injury involving Apoptotic Bodies of Cardiomyocytes (ApBc), resident myocardial Stem Cells (CSCs), and mesenchymal bone marrow stem cells (HSCs).
Apoptotic Bodies (APB) of cardiomyocytes combine the functions of CSC and HSCs in the areas of myocardial regeneration. Signalling molecules are located on the surface of ApB, which mediate the homing and chemotaxis of HSCs to the area of the damaged myocardium. HSCs provides targeted delivery of growth factors and cytokines required to maintain CSC proliferation. ApB contains a complex of molecules, carriers of "epigenomic memory" about the tissue belonging of a dead cell. It is likely that simultaneously with the triggering of the effector link of apoptosis, which ends with the formation of ApB, RNA is expressed, the new profile of which is the “code” of the tissue belonging to the dead cell. When ApB enters the CSC via endocytosis, a specific set of long and short non-coding RNAs express genes that determine the direction of differentiation of resident myocardial stem cells. It can be assumed that this hypothesis is true not only for the heart, but also for other organs and tissues [20].
An important aspect of cardiac assessment is the diagnosis of the degree and specificity of cardi-ac muscle damage due to ischemia-reperfusion syndrome, which is an inherent event in myocardium during stenting and aortocoronary bypass of cardiac vessels. The fact is that not only cardiomyocytes, but also other cells forming the heart (fibroblasts, endotheliocytes, vascular smooth muscle cells) are subjected to ischemic and reperfusion damage. Each of these cell types is capable of producing tissue-specific microRNAs into the intercellular medium while remaining viable. The first studies towards evaluation of tissue-specific damage profiles in cardiac ischemia showed that microRNAs are promising biomarkers of myocardial cell state. Thus, the assessment of microRNA-208a levels is the most promising for determining organ-specificity of myocardial damage. Determination of tissue-specific microRNA profiles opens the prospect of personalized assessment of myocardial damage taking into account the whole cell pool [21]. Exosomes of Cardiosphere Derived Cells (CDCs) inhibit the apoptosis and promote the proliferation of CMs, along with enhancing angiogenesis. Inhibition of exosome production blocks these regenerative processes. It has been shown that CDC exosomes contain a certain miRNA repertoire, with particular enrichment of miR-146a. Moreover, isolated administration of a miR-146a mimic reproduces some but not all the benefits of CDC exosomes [19].
Realizing the translational and therapeutic potential of CICSs has been hindered by some obstacles which would be surmounted and brought SC-based therapy of the future towards clinical applications, including establishing defined culture systems for de novo derivation and maintenance of clinical-grade progenitor cells and lineage-specific differentiation of the latter by siRNA-driven modulation. Such milestone advances and medical innovations in CICSs research al-low generation of a large supply of clinical-grade cardiac progenitor-based therapy derivatives targeting for cardiac problems, bringing cell-based regenerative medicine to a turning point. We expect that advanced modalities that integrate cellular, bioengineering, and Information (IT) technologies via clinical studies and translational applications as new consolidated entities will enhance the efficacy of cardiac cell therapy and further contribute to cardiac regenerative medicine.
SC Therapy and World Market: The Future of SC Drugs and Upgrading Niches
The global cell therapy market is continuously evolving in order to meet the needs of the patients who have been affected by any particular diseases, and ischemic failure, on particular! The in-creasing prevalence of the latter is shifting the market trends from traditional treatments to novel cell therapies. Meanwhile, SC therapies and regenerative medicine as a whole have also been an area of interest for major pharma companies, many of which have set up their own R&D units or have acquired stakes/invested in regenerative medicine companies. In this space, cell therapy is the fastest growing segment of regenerative medicine and also the largest.
The global SC-based therapy market is driven by factors such as increasing awareness related to the therapy in effective disease management and growing demand for regenerative medicines [22]. However, high cost related with SC-based therapy is likely to obstruct the growth of the market during the forecast period. The growing research and development activities in some are-as, including Asia Pacific and Oriental regions is expected to offer huge growth opportunity for SC therapy market.
Based on Type, the SC-based therapy market is segmented into adult SC therapy, iPPSC therapy, ESC therapy, and other SC therapies. In 2019 adult SC-based therapy held the largest share of the market [23]. However, iPPSC therapy are expected to register the highest CAGR in the market during the forecast period (Figure 15).
Based on Treatment, the SC-based therapy market is segmented into allogeneic and autologous. Allogeneic held the largest share of CVD segment in the global market. However, autologous segment is expected to grow at the faster rate during the forecast period. Factors contributing in the growth of autologous market are low risk associated, affordable treatment and no risk of graft versus host diseases. As autologous SC therapy becomes a reliable treatment in ischemic diseases, and biopharma companies will evaluate business models to determine the commercial opportunity associated with investment. As a business model, allogeneic cell sources are more aligned with the pharma business practice of centralized product production and distribution to health care providers. However, for pharma to aggressively adopt allogeneic adult cell therapy, multiple issues will need to be addressed, including cell expansion and manufacturing, product consistency, product de-livery to the patient, and successful well-designed, well-controlled clinical trials showing significant benefits over standard of care.
Based on a kind of Implementation, the market would pretend for a set of translational applications and resources to be utilized to secure the impact of the initiatives and the quality of the final product.
Based on End User, the SC-based therapy market is segmented into academic and research institutes, hospitals, and specialty clinics. The academic and research institutes held the largest share of the end user segment in the global market and are expected to grow at the fastest rate during the forecast period.
Regenerative medicine and SC-based therapy initiatives are now attracting new public and private funding. Although SC therapy will continue to be the largest market segment of regenerative medicine, cross segment therapies that combine the use of immunology, genetic, SC therapy and IT armamentarium are rapidly advancing.
Meanwhile, pharma and biotech companies have taken an increased interest in SC biology as the fundamental core of the business to be translated into the practice. The specific use of SC-based tools in conventional drug discovery programs are varied but based on the reproducibility of de-riving clinically relevant cell types as cardiac myocytes, in particular. And major accent has been put onto discovery programs that stimulate the endogenous activation of cardiac progenitors for Congestive Heart Failure (CHF) or Myocardial Infarction (MI) [24]. The opportunity to generate novel molecules that modify endogenous SCs is very much in scope and will likely lead to new therapeutic approaches using small molecules and biologics to enhance the body’s natural repair mechanisms. So, the move to true cell-based therapeutics by pharma is still modest, and some companies have preferred to take equity stake in active biotech companies while others are adopting a ‘‘watchful waiting’’ approach until the myriad of clinical trials currently underway read out definitively one way or another before actively investing in the space.
Strategic Partnerships and Alliances on the Way to Get the Ideal SC-Based Drug Developed
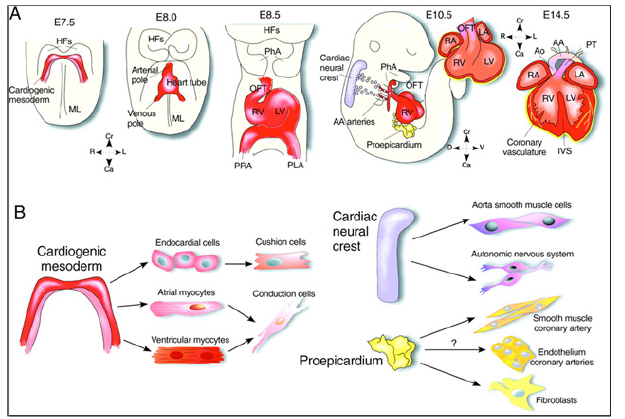
The market players operating in the SC therapy market adopt the strategy of collaborations to enlarge customer base across the world, which also permits the players to maintain their brand name globally. Among the high-profile collaborations are Pfizer’s deal with Athersys, involving milestone payments of up to $105 million. Novartis has a drug discovery alliance with Epistem, whereas Astellas Pharma has invested in Cytori’s SC programs. Increasing vindication of R&D in the sector in the form of new approvals will increase its visibility and spur further investment: in the near term this is likely to come from novel hematopoietic SC transplantation options as well as cardiovascular therapies. In the long term, SCs have great promise for wider applications in regenerative medicine, including organ regeneration and replacement. It is expected that partnerships with biopharmaceutical companies will develop following the demonstration of clinically safe and efficacious approaches. And the next decade will usher in further advances in our understanding of the biology of PPSCs that will bring SC therapeutics closer to the clinic. These will likely include the establishment of a comprehensive tree for the human cardiac lineage (Figure 16).
A) Contribution of the three populations of embryonic heart progenitors, cardiogenic mesoderm (red), cardiac neural crest (purple) and proepicardial organ (yellow) to different heart compartments during cardiac morphogenesis in the mouse. Progenitors of the cardiogenic mesoderm are first recognizable under the Head Folds (HFs) of the embryo at E7.5, then move ventrally to the Midline (ML) and form initially the linear heart tube and ultimately the four chambers of the heart. After the looping of the heart tube (E8.5), cardiac neural crest progenitors migrate from the dorsal neural tube to engulf the aortic arch arteries and contribute to vascular smooth muscle cells of the Outflow Tract (OFT) around E10.5. At the same time in mouse development, the proepicardial organ pre-cursors contact the surface of the developing heart, give rise to the epicardial mantle (yellow) and contribute later to the coronary vasculature. In the fetal heart (∼E14), the chambers separate due to septation and are connected to the Pulmonary Trunk (PT) and Aorta (Ao). Cranial (Cr)-Caudal (Ca), Right (R)-Left (L), and Dorsal (D)-Ventral (V) axes are indicated.
(B) Cardiac cell types that arise through the lineage diversification of the three embryonic precursor pools in the mouse heart. Whereas the contribution of the proepicardium to the smooth muscle cells of the coronary system and to the mesenchymal cells of the heart is well accepted, the origin of the endothelial lineage in the coronary vasculature is still controversial. AA, aortic arch; IVS, interventricular septum; LA, left atrium; LV, left ventricle; PhA, pharyngeal arches; PLA, primitive left atrium; PRA, primitive right atrium; RA, right atrium; RV, right ventricle.
profiles of cell surface marker expression that define specific cardiac progenitor pools; methods to derive and isolate cardiac progenitors and specialized CMC subtypes to high purity and in sufficient quantities; strategies to circumvent immune rejection; and preclinical large animal models of heart failure for assessing cell engraftment, host immune response and myocardial function in both the short and long term [25]. Indeed, human SC-based therapy derivatives are extremely attractive for therapeutic development because they have direct pharmacologic utility in clinical applications, unlike any other adult cells. The human SC as a special entity is emerging as a new type of potential therapeutic agent of cellular entity in cell-based regenerative medicine, because human SC-based therapy derivatives have the potential for human tissue and function restoration that the conventional drug of molecular entity lacks. There are currently many challenges facing the cell-based therapy industry. And the requirements for high levels of process, translational pipelines and product characterization will result in significant direct costs in all process stages, from establishment of a master cell bank to final product testing and expertise.
As an industry, cell-based therapies are still in the early stages of translational applications and clinical development. And developers considering the range of available manufacturing technologies need to balance the competing pressures discussed. So, moving forward, we must better characterize cell-based therapy clinical trials with accessible information for a host of variables, including cell dose, patient numbers and cell providence. This will allow for efficient and accurate data collection on cell-based therapy clinical trials, facilitating decision making across the cell-based therapy sector. As understanding of the cell-based products increases, we will likely experience step change improvements in manufacturing capability [26].
Anyway, we would have to focus on bringing cell-based strategies into the therapeutic pipeline through generating and differentiating novel cell types using the latest drug design and bioengineering approaches. Pharma’s primary strengths are the process by which lead compounds are turned into a marketable drug. Although the pharmaceutical industry has embraced SCs as tools in drug discovery, few companies have taken the risk to deliver SC-based medicines. In reality, we still do not know whether regenerative medicine will provide niche benefit or will revolutionize PPM and healthcare as a whole. Should significant benefit be demonstrated by SC-based medicine, one must anticipate a flurry of acquisitions and partnering deals to make way for the future. If cell therapies are to achieve their full clinical and commercial potential, significant challenges must be overcome with regards to current abilities to produce clinical grade cells at commercially relevant scales [27].
Given the complementary strengths of academic institutions and their skills in identification and validation of novel therapeutic targets, a collaborative approach between pharma and academia is essential to bring the exciting potential of regenerative therapeutics into a reality. We do hope that this viewpoint will illuminate key issues that currently limit synergistic relationships between pharma, bio designers, clinicians and basic researchers and may even stimulate initiation of the multidisciplinary collaborative projects.
Acknowledgement
None.
Conflict of Interest
None.
References
- Kolios G, Moodley Y (2013) Introduction to stem cells and regenerative medicine. Respiration 85(1): 3-10.
- Choi B, Lee SH (2018) Nano/Micro-Assisted Regenerative Medicine. Int J Mol Sci 19(8): 2187.
- Mazo M, Pelacho B, Prósper F (2010) Stem cell therapy for chronic myocardial infarction. J Cardiovasc Transl Res 3(2)79-88.
- Zhang Y, Mignone J, MacLellan WR (2015) Cardiac Regeneration and Stem Cells. Physiol Rev 95(4): 1189-1204.
- Dupont G, Yilmaz E, Loukas M, Macchi V, De Caro R, et al. (2019) Human embryonic stem cells: Distinct molecular personalities and applications in regenerative medicine. Clin Anat 32(3): 354-360.
- Müller P, Lemcke H, David R (2018) Stem Cell Therapy in Heart Diseases - Cell Types, Mechanisms and Improvement Strategies. Cell Physiol Biochem 48(6): 2607-2655.
- Yamanaka S (2020) Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 27(4): 523-531.
- Shi Y, Inoue H, Wu JC, Yamanaka S (2017) Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16(2): 115-130.
- Parsons XH (2013) The Designation of Human Cardiac Stem Cell Therapy Products for Human Trials. J Clin Trial Cardiol 1(1): 02.
- Notably, Menasché (2015) reported the first clinical application of cardiac patches composed of human ESC-derived cardiac progenitor cells in a patient suffering from severe ischemic left ventricle dysfunction, demonstrating the possibility to use an engineered based approaches for cardiac repair.
- Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regen Med 5(1): 121-143.
- Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9(1): 63.
- Zhang B, Zhang J, Zhu D, Kong Y (2019) Mesenchymal stem cells rejuvenate cardiac muscle after ischemic injury. Aging (Albany NY) 11(1): 63-72.
- Wagers AJ, Weissman IL (2004) Plasticity of adult stem cells. Cell 116(5): 639-648.
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, et al. (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410(6829): 701-705.
- Bar A, Cohen S (2020) Inducing Endogenous Cardiac Regeneration: Can Biomaterials Connect the Dots? Front Bioeng Biotechnol 8: 126.
- Bartel RL, Cramer C, Ledford K, Longcore A, Parrish C, et al. (2012) The Aastrom experience. Stem Cell Res Ther 3(4): 26.
- Belostotskaya GB, Nerubatskaya IV, Galagudza MM (2018) Two mechanisms of cardiac stem cell-mediated cardiomyogenesis in the adult mammalian heart include formation of colonies and cell-in-cell structures. Oncotarget 9(75): 34159-34175.
- Belostotskaya G, Sonin D, Galagudza M (2021) Intracellular Development of Resident Cardiac Stem Cells: An Overlooked Phenomenon in Myocardial Self-Renewal and Regeneration. Life (Basel) 11(8): 723.
- Tyukavin A, Belostotskaya G Zakharov E, Suchkov S (2019) 4th World Congress & Expo on Pharmaceutics and Drug Delivery Systems.
- Ivkin DY, Lisitsky DS, Zakharov EA, Lyubishin MM, Karpov AA, et al. (2015) microRNAs as promising diagnostic and pharmacological agents. Astrakhan Medical Journal.
- Aggarwal S, Sardana C, Ozturk M, Sarwat M (2020) Plant stem cells and their applications: special emphasis on their marketed products. 3 Biotech 10(7): 291.
- (2020) The Stem Cell Therapy Market to 2027 - Global Analysis and Forecasts by Type; Treatment; Application; End User, and Geography. Research And Markets.com's, Dublin.
- Wu et al., 2004
- Meilhac SM, Lescroart F, Blanpain C, Buckingham ME (2014) Cardiac cell lineages that form the heart. Cold Spring Harb Perspect Med 4(9): a013888.
- Arora P, Sindhu A, Dilbaghi N, Chaudhury A, Rajakumar G, et al. (2012) Nano-regenerative medicine towards clinical outcome of stem cell and tissue engineering in humans. J Cell Mol Med 16(9): 1991-2000.
- Li RA (2014) Cardiovascular regeneration. Stem Cell Res Ther 5(6): 141.








 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.