Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Study on Extraction Method of Flavonoids in Star Anise
*Corresponding author:Chen Sui and Du Juan, Zhuhai College of Science and Technology, Zhuhai, China.
Received: February 26, 2024; Published: February 29, 2024
DOI: 10.34297/AJBSR.2024.21.002878
Abstract
In this experiment, star anise was used as raw material to study the effects of three different extraction solvents, water, anhydrous ethanol and 4% NaOH, on the extraction of flavonoids from star anise. The results showed that the yield of flavonoids was 8.18% with water as the extraction solvent. With ethanol as the extraction solvent, the yield of flavonoids was 13.12%. With sodium hydroxide as the extraction solvent, the yield of flavonoids was 11.12%. Therefore, the use of ethanol as the extraction solvent has a better effect on the extraction of flavonoids from star anise.
Keywords: Star anise, Flavonoids, Process optimization
Introduction
Star anise, also known as fennel, is rich in flavonoids. It is a common medicinal plant with strong fragrance and rich nutrients. Because of its unique taste, it has become an important condiment [1]. Star anise is mainly produced in Guangxi, Fujian, Guangdong, Taiwan, and other provinces south of 25° north latitude [2]. Flavonoids have certain antioxidant and antibacterial effects and are widely used in food preservatives such as meat and food processing [3]. In China, studies have shown that flavonoids have strong antioxidant capacity, which can neutralize free radicals and reduce the oxidation reaction in food [4]. It showed selective and mild inhibitory activity against Pseudomonas aeruginosa, L.anatipestifer, Streptococcus agalactiae and Bacillus subtilis, respectively [5]. In addition, the volatile oil of star anise had strong scavenging ability to hydroxyl radical, superoxide anion radical and DPPH radical [6,7].
Materials and Methods
Materials and Reagents
Octagonal, bought from the farmers' market Rutin standard (purity 98%, Tianjin Xiensi Biochemical Technology Co., Ltd.); anhydrous ethanol (analytical purity, Sinopharm Chemical Reagent Co., Ltd), 4% sodium hydroxide (analytical purity, Guangzhou Chemical Reagent Factory), 5 % sodium nitrite (analytical purity, Fuchen (Tianjin) Chemical Reagent Co., Ltd), 10 % aluminum nitrate (analytical purity, Shanghai Aladdin Biochemical Technology Co., Ltd).
Instruments and Equipment
AR224CN electronic balance Ohaus Instruments (Shanghai) Co., Ltd.; dongguan Fangtai Electric Appliance Co., Ltd.; 800A Multifunctional Crusher; sHZ-B water bath constant temperature oscillation box Shanghai Boxun Industrial Co., Ltd. Medical Equipment Factory; fC5706 Centrifuge Aohaus Instruments (Shanghai) Co., Ltd.; l5 spectrophotometer.
Experimental Methods
Sample Processing: The dried star anise was crushed by a high-speed grinder and placed in a sealed bottle through a 40-mesh sieve for later use. The 7.000g star anise sample was accurately weighed, placed in a conical flask, added with an extractant with a certain solid-liquid ratio, and placed in a constant temperature oscillation box for 120r/min oscillation and extraction, and then centrifuged with a centrifuge at 5000r/min for 15min. The supernatant was taken, that is, the crude flavonoid extract.
Determination of the Maximum Absorption Wavelength: 0mL of rutin control solution and 1.0mL of star anise sample solution were put into 10mL cuvette, and then added anhydrous ethanol to constant volume to 5.0ml, added 5 % sodium nitrite to dissolve 0.3ml, shaken and stood for 6min. Add 10% aluminum nitrate solution 0.3ml, shake well, and then stand for 6min ; continue to add 4% sodium hydroxide solution 4ml, and finally add 75% ethanol to the scale, shake well, stand for 15min, and measure the absorbance between 450-600nm [8]. The absorption peak of rutin standard solution is basically the same as that of octagonal column, and the maximum absorption peak is near 505 nm. Therefore, 505 nm is selected as the detection wavelength in this experiment [9].
Establishment of Rutin Standard Curve: A total of 5.0mg of rutin reference substance was accurately weighed, placed in a 25mL volumetric flask, added with an appropriate amount of anhydrous ethanol, heated, and dissolved in a water bath at about 45°C, cooled, diluted to the scale, and shaken well to obtain the rutin reference solution. Precisely absorb rutin control solution 0.0,0.5,1.0,2.0,3.0,4.0,5.0ml, respectively, placed in 10mL cuvette, and then added anhydrous ethanol to a constant volume of 5.0ml, and then added 5% sodium nitrite solution 0.3ml shaken well, standing for 6min after adding 10% aluminum nitrate solution 0.3ml, shaken well, and then standing for 6min ; add 4% sodium hydroxide solution 4ml, and then add 75% ethanol to constant volume to the scale, shake well, stand for 15min, with anhydrous ethanol as blank, determine its absorbance in 200-700nm. The standard curve was drawn with absorbance as the ordinate and concentration as the abscissa [10].
Determination of Flavonoids: 100uL of crude flavonoid extract was accurately extracted and placed in 10mL cuvette, then added absolute ethanol to constant volume to 5.0ml, added 5% sodium nitrite to dissolve 0.3ml, shaken and stood for 6min ; add 0.3ml 10% aluminum nitrate solution, shake well, and then stand for 6min ; add 4% sodium hydroxide solution 4ml, and then add 75% ethanol to constant volume to the scale, shake well, stand for 15min, with the extractant as a blank, and measure the absorbance at 505nm. The mass concentration of flavonoids in the test sample was obtained by drawing the standard curve, and the extraction rate of flavonoids was calculated according to the following formula.
Extraction rate of flavonoids (%) = (mass concentration of total flavonoids (mg/mL) * dilution multiple)/(m*1000)
Effect of Water Bath Temperature on the Extraction of Flavonoids from Star Anise: Accurately weighed 7.000g star anise powder, under the conditions of solid-liquid ratio of 1: 7, extraction time of 90min, water bath temperature of 30°C, 40°C, 50°C, 60°C, 70°C, 80°C, 90°C, the content of flavonoids in star anise powder was determined according to the method of 4.3.4.
Effect of Water Bath Oscillation Time on the Extraction of Flavonoids from Star Anise: Accurately weighed 7.000g star anise powder, under the conditions of solid-liquid ratio of 1: 7, temperature of 70°C, water bath oscillation time of 30min, 60min, 90min, 120min, 150min and 180min, the content of flavonoids in star anise powder was extracted and determined according to the method of 4.3.4.
Effect of Solid-Liquid Ratio on the Extraction of Flavonoids from Star Anise: Accurately weighed 7.000 g star anise powder, under the conditions of water bath temperature of 70°C, extraction time of 90 min, solid-liquid ratio of 1:3,1:5,1:7,1:9,1:11,1:13, the content of flavonoids in star anise powder was extracted and determined according to the method of 4.3.4 [11].
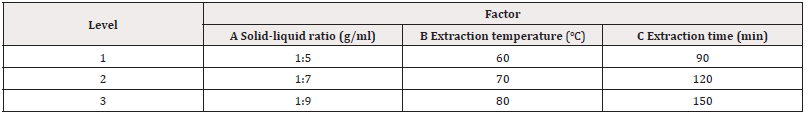
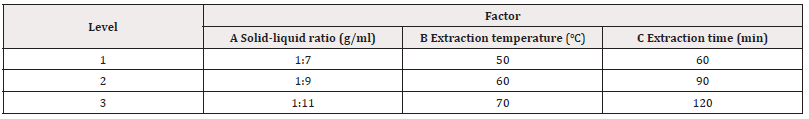
Orthogonal Test of the Optimum Process Conditions for the Extraction of Flavonoids from Star Anise by Water, Anhydrous Ethanol and 4% NaOH Water Bath Oscillation Method: Based on the single factor experiment of water bath oscillation process, L9(33) orthogonal test design (see Tables 1-3) was used to further study the effects of solid-liquid ratio, temperature, extraction time and other factors on the extraction rate of flavonoids when water, ethanol and NaOH were used as extractant solutions, and the optimal process conditions for extracting flavonoids from star anise with different solvents were determined (Tables 1-3).
Result and Discussion
Rutin Standard Curve
According to the linear fitting of rutin standard quality and absorbance, the regression equation y=0.2831x+0.02, R2=0.9996 was obtained. The results showed that rutin standard had a good linear relationship in a certain linear range (Figure 1).
Analysis of Single Factor Test Results of Water Bath Oscillation Process Conditions
Effect of Water Bath Temperature on the Extraction of Flavonoids from Star Anise: When three different solvents were used to extract flavonoids from star anise, the extraction rate of flavonoids increased with the increase of temperature, and then decreased slowly. At different extraction temperatures, the yield of flavonoids extracted by water was the lowest, and the yield of flavonoids extracted by ethanol was the highest (see Figure 2). With water as the extractant, when the temperature is between 30°C and 70°C, the extraction rate of octagonal flavonoids increases with the increase of temperature, and then decreases. Anhydrous ethanol was used as the extraction agent, when the temperature was between 30°C and 60°C, the extraction rate of octagonal flavonoids increased with the increase of temperature, and then decreased. With 4% NaOH as extractant, when the temperature was between 30°C and 50°C, the extraction rate of star anise flavonoids increased with the increase of temperature, and then decreased.
The extraction temperature varies with the boiling point of different solvents. When the temperature rises, the movement rate between molecules will be faster and faster, and the penetration, diffusion and dissolution will also be faster and faster. At the same time, high temperature can also change the structure of the cell membrane, prompting flavonoids from the outermost cells into the solvent. But high temperature will destroy some unstable flavonoids. Therefore, when the extraction was carried out at different temperatures, the extraction temperature was positively correlated with the extraction rate of flavonoids. However, when the temperature continues to rise and reaches a certain temperature value, it will decline. Therefore, when extracting flavonoids with water, the extraction temperature should be between 30-70°C ; when extracting flavonoids with anhydrous ethanol, the extraction temperature should be between 30°C and 60°C ; when extracting flavonoids with 4% NaOH, the extraction temperature should be between 30°C and 70°C. Because the octagonal flavonoids contain different components of molecular structure, when different extraction solvents are in contact with different flavonoids, the solubility of flavonoids in different solvents is quite different, because the extraction temperature of different solvents is different (Figure 2).
Effect of Water Bath Shaking Extraction Time on the Extraction of Flavonoids from Star Anise: When three different solvents were used to extract flavonoids from star anise, the extraction rate of flavonoids increased first and then decreased with the increase of extraction time. Different extraction time, the yield of flavonoids extracted by water was the lowest, and the yield of flavonoids extracted by ethanol was the highest. When anhydrous ethanol and 4% sodium hydroxide were used as extractants, when the extraction time was in the range of 30min-90min, the extraction rate of star anise flavonoids increased with time, and then decreased. When water was used as the extractant, when the extraction time was in the range of 30min~120min, the extraction rate of star anise flavonoids increased with time, and then decreased.
When the water bath oscillation extraction time is too small, the flavonoids in the star anise cannot be fully dissolved into the extractant. Therefore, within a certain range, the extraction rate of flavonoids was positively correlated with the extraction time; when the water bath oscillation extraction time is too long, flavonoids may be oxidized and decomposed, resulting in a decrease in the extraction rate of flavonoids [12]. Therefore, when anhydrous ethanol and 4% NaOH were used as extractant, the optimum extraction time was 60min~120min; when water was used as the extractant, the optimal extraction time was 90min~150min (Figure 3).
Effect of Solid-Liquid Ratio on the Extraction of Flavonoids from Star Anise: When three different solvents were used to extract flavonoids from star anise, with the increase of solid-liquid ratio, the extraction rate of flavonoids from star anise increased first and then decreased. When water and 4% NaOH were used as extractants, when the solid-liquid ratio was between 1:3-1:7, the extraction rate of star anise flavonoids increased with the increase of solid-liquid ratio, and then showed a slight downward trend, but the overall change was gentler. When anhydrous ethanol was used as the extractant, the extraction rate of flavonoids from star anise with the increase of the ratio of material to liquid, it always presents an upward trend. When the solid-liquid ratio reached 1: 9, the extraction rate of flavonoids from star anise increased significantly (Figure 4).
When the ratio of material to liquid is too much, on the one hand, it will increase the difficulty of subsequent evaporation and concentration, on the other hand, it is also very likely to dissolve some other substances, affecting the extraction rate of flavonoids [13,14]. Therefore, when the extraction agent is water and 4 % NaOH, the ratio of material to liquid should be selected in the range of 1:5-1:9, and when the extraction agent is ethanol, the ratio of material to liquid should be selected in the range of 1:7-1:11.
Analysis of Orthogonal Test Results of the Optimum Process Conditions for the Extraction of Flavonoids from Star Anise by Different Extraction Solvents
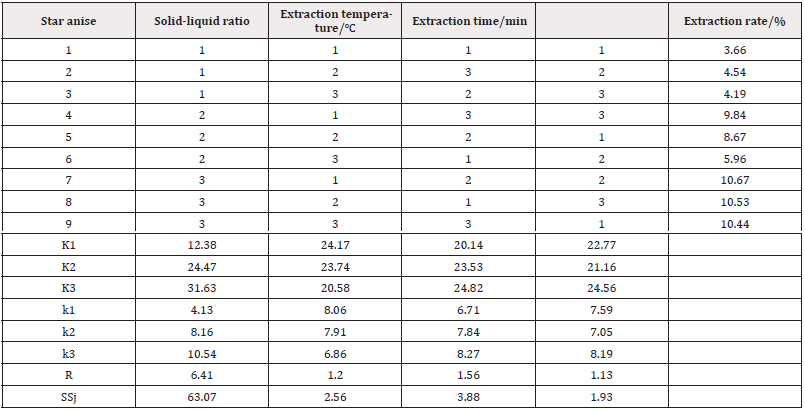
Optimum Conditions for Extraction of Flavonoids from Star Anise by Aqueous Solution: (Tables 4,5) It can be seen from Table 5 that F0.05 (2,2) <F solid-liquid ratio < F0.01 (2,2), F extraction time < F0.05 (2,2), F extraction temperature < F0.05 (2,2). Therefore, the liquid-to-material ratio had a significant effect on the extraction of flavonoids, and the extraction time and extraction temperature had no significant effect on the extraction rate of flavonoids. According to the orthogonal experiment know, the highest extraction rate of flavonoids from star anise was up to 8.18% under the optimum conditions of solid-liquid ratio of 1:9(g/mL), extraction temperature of 70°C and extraction time of 90min.
The Optimum Conditions for the Extraction of Flavonoids from Star Anise by Anhydrous Ethanol: (Tables 6,7) From Table 7, it can be seen that F0.05 (2,2) < F solid-liquid ratio < F0.01 (2,2), F0.05 (2,2) < F extraction temperature < F0.01 (2,2), F extraction temperature < F0.05 (2,2). The liquid-temperatures have a significant effect on the extraction of flavonoids, and the extraction time and extraction temperature have all no significant effect on the extraction rate of flavonoids. According to the orthogonal experiment konw, under the optimum conditions of solid-liquid ratio 1:9(g/mL), extraction temperature 60°C and extraction time 90min, the highest extraction rate of flavonoids from Star anise was up to 13.12%.
The Optimum Conditions of Extracting Flavonoids from Star Anise with 4% NAOH: (Tables 8,9) From Table 9, we can see that F0.05 (2,2) < F solid-liquid ratio < F0.01 (2,2), F extraction temperature < F0.05 (2,2), F extraction temperature < F0.05 (2,2), liquid-solid ratio has a significant effect on the extraction of flavonoids, extraction time and extraction temperature have no significant effect on the extraction of flavonoids. According to the orthogonal experiment know, the optimum conditions for the extraction of 4% flavonoids from star anise were as follows: the ratio of liquid to material was 1:9, the extraction temperature was 40°C, and the extraction time was 90min. Under these conditions, the yield of flavonoids was 11.12%.
Conclusions and Discussions
The optimum process conditions for extracting flavonoids from water, absolute ethanol and 4% sodium hydroxide were obtained by orthogonal test. Under the optimum conditions, the yield of water extraction of flavonoids was 8.18%. The yield of flavonoids extracted by ethanol was 13.12%. The yield of flavonoids extracted by 4% sodium hydroxide was 11.12%. The results showed that the extraction rate of anhydrous ethanol > 4 % NaOH extraction rate > water extraction rate. The extraction rate of flavonoids from star anise was the highest when ethanol was used as the extraction solvent, and the average extraction rate was as high as 13.12%.
Because the flavonoids are more complex, the structure of flavonoids extracted from different solvents to identified to select the best reagents and parameters for the extraction of flavonoids, which provides a certain theoretical basis for the extraction of star anise flavonoids.
Acknowledgment
Fund Project
1. Guangdong College Students' Innovation and Entrepreneurship Training Program in 2022(S202213684025).
2. Guangdong University Characteristic Innovation Program (Natural Science) Fund (2021KTSCX173).
Conflict of Interest
None.
References
- Lu Xiaohong (2014) Plant MSG star anise. Guangxi Forestry (03): 26-28.
- Li Lin, Dai Ming (2023) Literature research on star anise. Chinese medicinal materials (03): 773-779.
- Lou Nan, Han Xiaoyu, Hu Rongliu (2023) Extraction method of flavonoids and its application in food preservation. Food Safety Guide (15): 169-171.
- Yan Xuyu, Li Juan, Li Ling (2021) Response surface optimization of ultrasonic-assisted extraction of flavonoids from okra fruit and its ability to scavenge hydroxyl radicals. Chinese condiment 46(10): 62-69.
- (2017) Study on flavonoids and antibacterial activity in the leaves of Illicium gongshanense. Shanghai Jiaotong University.
- Xie Zhixin, Chen Linlin, Zhang Wenzhou (2018) Ultrasonic-assisted extraction and antioxidant activity of volatile oil from star anise. Chinese condiment 43(04): 124-128.
- Zhao Erlao, Xu Weifang, Liu Le (2019) Research progress on antioxidant activity of star anise. Chinese condiment 44(05): 194-196.
- Qu Falin, Liu Guirong, Zhao Hanqing (2004) Study on the Content Determination of Total Brass in Hypericum perforatum. Shizhen Traditional Chinese Medicine (07): 395-396.
- Fang Qingxia, Jin Ge (2004) Determination of total brass in okra. Pharmaceutical introduction (09): 675-676.
- Li Guizhen, Qin Rongxiu, Li Yonghong (2011) Study on the extraction process of total brass from cinnamon. Guangxi Forestry Science 40(03).
- Lin Kuan, Xu Congyue, Liang Zheng (2019) Extraction and purification of flavonoids from cinnamon and its antioxidant activity in vitro. Food Science and Technology 44(07): 267-272.
- Mao Judai Yalmamaiti, Zhu Jiangmei, Yuan Hui (2011) Study on Extraction Technology of Flavonoids from Foeniculum vulgare Mill. Journal of Central China Normal University (Natural Science Edition) 45(02): 295-298.
- Zhang Min, Li Liangdao, Zhong Xiaohong (2013) Study on the reflux extraction process of total brass from the peel of zhoupi citrus. Hunan Forestry Science and Technology 40(04): 14-17.
- Xu Hongyu, Kuai Yiyun, Zhao Jie (2019) Optimization of extraction process of total brass from Aralia elata by response surface methodology. Food Research and Development 40(03): 106-111.













 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.