Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Treatment of Transplant Renal Artery Stenosis by Angioplasty and Stent Placement: Case Report
*Corresponding author:Andres Tlacaelel Balderas Chairez, Radiology and Imaging Department, High Specialty Medical Unit, Mexico City.
Received: February 20, 2024; Published: February 27, 2024
DOI: 10.34297/AJBSR.2024.21.002870
Abstract
TRAS is the most commonly recognized vascular complication. It is more common at the site of the anastomosis following renal transplantation defined as the angiographic evidence of transplant renal artery narrowing> 50%. Endovascular intervention is the preferred first-line treatment option for patients with TRAS (with or without stents). This report presents a 62-year-old man with kidney transplant since 2022, with risk factors and history of Covid-19 infection. Where studies revealed renal artery stenosis (reason of elevated creatinine and refractory hypertension). Endovascular treatment was performed using stent and after that the patient studies and laboratories returned to normal parameters.
Abbreviations: AT: Acceleration Time; CIT: Cold Ischemia Time; CT: Computed Tomography; CVM: Cytomegalovirus; DFG: Delayed Graft Function; DM: Diabetes Mellitus; DSA: Digital Subtraction Angiography; TRAS: Transplant Renal Artery Stenosis; PSV: Peak Systolic Velocity; PTA: Percutaneous Transluminal Angioplasty; PTAS: Percutaneous Transluminal Angioplasty with Endovascular Stenting.
Introduction
The recipient complications after kidney transplant can be classified as vascular, urological or related to graft rejection. Early recognition of these complications leads to reduced morbidity, mortality, and the preservation of the allograft. Vascular complications, including thrombosis, arteriovenous fistulae, and Transplant Renal Artery Stenosis (TRAS) [1]. TRAS is the most commonly recognized vascular complication, with an incidence ranging from 1% to 25%. Associated with risk factors such as the presence of atherosclerosis or previous trauma in the donor artery, or any technical intraoperative aspects. Must be suspected when treatment-resistant hypertension is present and its more common at the site of the anastomosis; defined as the angiographic evidence of transplant renal artery narrowing > 50%, with a reported incidence of 1-23% [1,2].
The main predisposing factors for TRAS include Cytomegalovirus (CVM) infection and Delayed Graft Function (DFG) [3], but other main risk factors include Diabetes Mellitus (DM), acute rejection, and increased Cold Ischemia Time (CIT) [1]. The clinical presentation is due to renal hypoperfusion, which results in activation of the renin–angiotensin–aldosterone system; with worsening or refractory hypertension, fluid retention, and often DFG [4]. However, in patients with COVID 19 infection, apart from pulmonary symptoms, in some of them it has been reported that they may present atypical clinical manifestations [5,6].
Case Report
A 62-year-old man with end-stage renal disease due to DM nephropathy, recipient of a deceased-donor kidney transplant in 2022 with serum creatinine (Cr) of 4.8 mg/dL. Previous medical history included history of hypertension, DM, and COVID-19 infection in 2022. Course with DFG requiring 3 sessions of hemodialysis secondary to acute tubular necrosis. Previous Doppler ultrasounds with Maximum Systolic Velocity (PSV) of 370 cm/s, which warranted follow-up, gradually raised the PSV to 461cm/s, as well as their blood pressures, reason for which Angio tomography was requested in 2023, which reports endoluminal narrowing of the arterial anastomosis of up to 70%. It was decided to hospitalize him to start the surgical protocol. At the first evaluation of the patient, he was in good general condition, with no evidence of other alterations. The abdominal and the rest of the examination had normal parameters. Physical examination revealed a body temperature of 37.2°C, blood pressure of 175/80 mm Hg, pulse of 70 beats per minute, respiratory rate of 20 breaths per minute, and blood oxygen saturation of 95% on room air. The blood workup on peripheral blood show glucose 168 mg/dl, creatinine 6.29 mg/dl, and serum electrolytes such as Na 142, K 5.28, and CL 109. Hemoglobin 13.3 gr/dL, hematocrit 39.5. WBC count was 5.42×109/L and with a platelet count of 176×109/L. Liver transaminases and coagulation were within normal reference values. Thereafter, a consultation with the interventional radiology service is carried out, where they decide to perform angioplasty with stent placement. Pre-operative assessments are carried out with any reported contraindication for surgical performance. On the fifth day of the hospital stay, the patient was transferred to the hemodynamics service with antihypertensive drugs (oral nifedipine 30 mg BID and prazosin 1mg TID). The procedure was started with angiography, which corroborated stenosis already reported in previous studies. Measurements were taken for stent planning; angioplasty was performed in the lesion area and a stent was placed. The procedure lasted approximately 1 hour and 30 minutes, with an instillation of approximately 180 cc of contrast medium, and no complications inherent to the procedure were reported. After 7 days from hospital admission and 48 hours after endovascular treatment, the patient does not present any eventualities or complications, within good general clinical conditions, laboratory analysis without alterations, and blood pressures with improvement to the initial ones (150/75 mmHg)., it was decided to discharge him from the hospital with the aforementioned anti-hypertensive treatment and anticoagulants, to continue follow-up in the outpatient clinic. The patient comes to the clinic in three weeks, with the results of the Doppler ultrasound study, which reports a renal artery graft with adequate patency with PSV 165 cm/s.
Discussion
TRAS is a frequent, potentially curable cause of refractory hypertension and allograft dysfunction that usually presents between 3 months and 2 years after, but it can present at any time [7]. Noninvasive imaging commonly used to assess the transplanted kidney includes ultrasound, Computed Tomography (CT) angiography, or Digital Subtraction Angiography (DSA) [8], depending on institutional resources and preferences.
Doppler ultrasound accepted flow measurement most commonly accepted is a PSV of 200 cm/s in the transplant renal artery, although the possibility and sensitivity increases when the speed is ≥ 300 cm/s with an Acceleration Time (AT) ≥ 0.1 s. [9,10]. CT provides three-dimensional images of the vascular tract that may be superior to DSA, with the advantage that it does not need artery puncture and requires less contrast medium [11], but in both modalities, the diagnostic criteria employed for TRAS was a 50% or greater narrowing in the renal artery intraluminal diameter [12,13]. The first image modality used in our case was the Doppler ultrasound, where an increase in the PSV of 461.0 cm/s was identified and an at> 1 on the renal artery (Figure 1A). Subsequently, Angio tomography was performed and the presumptive diagnostic is corroborated, observing an abrupt decrease in the ostium of the anastomosis of the arterial graft with a minimum diameter of 1.24 mm, a length of 13 mm, corresponding to a stenosis of 70% (Figure 1B-C).
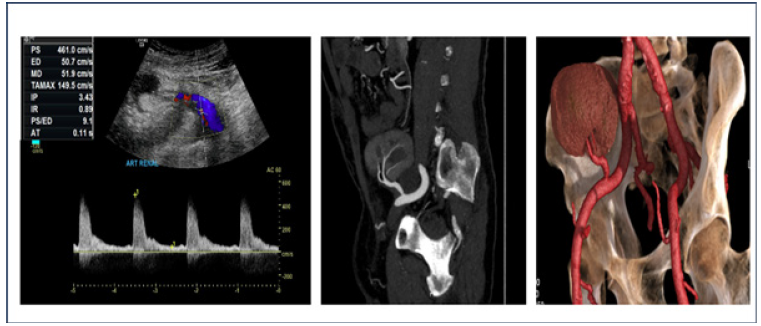
Figure 1: A; Color Doppler ultrasound of the kidney graft with spectral analysis of the renal artery, with peak systolic velocities of 461cm/s and AT of 0.11 s. B; Angio tomography in which a reduction in the renal artery is observed at the level of the ostium. C; Angio tomography in 3D reconstruction.
Endovascular intervention is the preferred first-line treatment option for patients with TRAS, Percutaneous Transluminal Angioplasty (PTA) with or without endovascular stenting (PTAS) has been demonstrated to be efficacious. A technical success rate of >90% has been reported [7]. Balloon angioplasty has a restenosis rate of 5% to 30% at 6 to 8 months. Stenting reduces the incidence of restenosis. However, PTAS is more expensive than PTA, and patients with PTAS need long-term oral anticoagulant [14,15]. In the case the patient was protocolized for endovascular treatment, confirming clinical and radiological diagnosis. The approach was carried out through the right common femoral artery, vascular access sheath 6 FR was inserted by which hydrophilic guide and endovascular catheter were raised. The anastomosis of arterial graft was selectively catheterized and visualizing an approximate 80% stenosis. Subsequently, the performance of angioplasty was decided, ascending catheter with radio-opaque polyethylene balloon, positioned at the level of the injury and insufflated for 20-30 s with maximum pressures of 12 atm. Finally, a 6 Fr x18 mm stent system was introduced, releasing it with fluoroscopic control at the level of the renal graft ostium. Post-dilatation with stent placement angiograms were taken to corroborate the position and permeability correction of the stent (Figure 2).
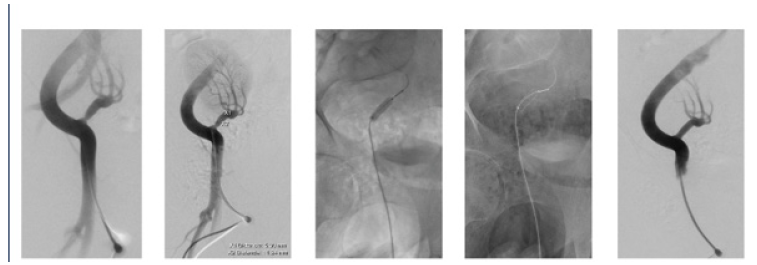
Figure 2: A; DSA with hydrosol is non-ionic iodinated contrast medium, identifying a decrease in caliber at the level of the anastomosis of the transplanted kidney artery. B; Measurement and planning for stent placement. C; Performing angioplasty prior to stent placement. D; Be Smooth Stent System 6 Fr x 18 mm with balloon. E; Release of the stent at the level of the ostium of the artery. F; Angiographic control with adequate opacification of the renal artery.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Acknowledgments
The workers at the Imaging and Urologic Department collaborated on the execution of the procedures.
References
- Gabriel Kanhouche, Gustavo Rocha Feitosa Santos, Henry Campos Orellana, Attilio Galhardo, Ana Carolina Buso F, et al. (2022) Risk factors of transplant renal artery stenosis in kidney transplant recipients. Clinics (Sao Paulo) 77: 100087.
- Ngo AT, Markar SR, De Lijster MS, Duncan N, Taube D, et al. (2015) A Systematic Review of Outcomes Following Percutaneous Transluminal Angioplasty and Stenting in the Treatment of Transplant Renal Artery Stenosis. Cardiovasc Intervent Radiol 38(6): 1573-1588.
- V Audard, M Matignon, F Hemery, R Snanoudj, P Desgranges, et al. (2006) Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant 6(1): 95-99.
- Chen W, Kayler LK, Zand MS, Muttana R, Chernyak V, et al. (2015) Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J 8(1): 71-78.
- Elena Guillen, Gaston J Pineiro, Ignacio Revuelta, Diana Rodriguez, Marta Bodro, et al. (2020) Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant 20(7): 1875-1878.
- Paredes Manjarrez C, Avelar Garnica FJ, Balderas Chairéz AT, Arellano Sotelo J, Córdova Ramírez R, et al. (2023) Lung Ultrasound Elastography by SWE2D and “Fibrosis-like” Computed Tomography Signs after COVID-19 Pneumonia: A Follow-Up Study. Journal of Clinical Medicine 12(24): 7515.
- Bruno S, Remuzzi G, Ruggenenti P (2004) Transplant renal artery stenosis. J Am Soc Nephrol 15(1): 134-141.
- Gaddikeri S, Mitsumori L, Vaidya S, Hippe DS, Bhargava P, et al. (2014) Comparing the diagnostic accuracy of contrast-enhanced computed tomographic angiography and gadolinium-enhanced magnetic resonance angiography for the assessment of hemodynamically significant transplant renal artery stenosis. Curr Probl Diagn Radiol 43(4): 162-168.
- Abate MT, Kaur J, Suh H, Darras F, Mani A, et al. (2011) The use of drug-eluting stents in the management of transplant renal artery stenosis. Am J Transplant 11(10): 2235-2241.
- Ghaneh Fananapazir, John P McGahan, Michael T Corwin, Susan L Stewart, Catherine T Vu, et al. (2017) Screening for Transplant Renal Artery Stenosis: Ultrasound-Based Stenosis Probability Stratification. AJR Am J Roentgenol 209(5): 1064-1073.
- Mell MW, Alfrey EJ, Rubin GD, Scandling JD, Jeffrey RB, et al. (1994) Use of spiral computed tomography in the diagnosis of transplant renal artery stenosis. Transplantation 57(5): 746-748.
- Chow KM, Szeto CC, Lee PS, Ho SS, Leung CB, et al. (2007) Revascularization for post-transplant renal artery stenosis. Nephrology (Carlton) 12(4): 406-412.
- C J Olbricht, K Paul, M Prokop, A Chavan, C M Schaefer Prokop, K Jandeleit, et al. (1995) Minimally invasive diagnosis of renal artery stenosis by spiral computed tomography angiography. Kidney Int 48(4): 1332-1337.
- Gunawardena T, Sharma H (2022) Transplant Renal Artery Stenosis: Current Concepts. Exp Clin Transplant 20(12): 1049-1057.
- Yanqiao Ren, Fu Xiong, Xuefeng Kan, Kun Qian, Yanyan Cao, et al. (2020) Endovascular management of transplant renal artery stenosis: A single-center retrospective study. Catheter Cardiovasc Interv 95(3): 429-436.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.