Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Vitamin D Deficiency in Children
*Corresponding author: Berfin Özgökçe Özmen, Mersin City and Training and Research Hospital, Pediatric Infectious Diseases Polyclinic, Mersin Turkey
Received: December 19, 2024; Published: January 03, 2024
DOI: 10.34297/AJBSR.2024.21.002795
Abstract
Objective: To determine the frequency of serum 25-OH vitamin D deficiency by gender, age groups and puberty in children in the Van region
Material and Methods: A total of 1242 children consisting of 617 girls and 625 boys were included in the study. The children were divided into three different age groups (infancy, prepubertal and pubertal). Serum calcium, phosphorus, magnesium, alkaline phosphatase, parathormone and 25-OH vitamin D levels were determined in all cases. All parameters were statistically evaluated according to infancy, prepubertal and pubertal groups.
Results: Evaluating the frequency of 25-OH vitamin D deficiency in serum of our patients in all groups, severe deficiency was found in 2.9% (36 children), mild deficiency in 19.6% (244 children) and deficiency in 15.9% (197 children). Overall deficiency was calculated as 38.4% (in 477 children), and serum 25-OH vitamin D level was considered sufficient, i.e. normal, in 61.6% (in 765 children). The frequency of serum 25-OH vitamin D deficiency according to age groups was found to be 15.7% in infants (196 children), 9.5% in prepubertal children (118 children) and 13.1% (163 children) in pubertal children. There was no statistically significant difference between gender and 25-OH vitamin D deficiency.
Conclusion: Vitamin D deficiency was lower in Van children than in previous studies. We attribute this to the free distribution of Vitamin D initiated by the Ministry of Health, easy access to healthcare providers, and increased community awareness. Anyway, from any perspective, vitamin D deficiency is a significant public health problem in terms of its prevalence in different countries. Vitamin D deficiency/insufficiency is still high in our region and is a problem. New approaches adapted to each region and individual are needed to solve or prevent the vitamin D problem.
Keywords: Calcium, Phosphorus, Magnesium, Alkaline phosphatase, Parathormone, Vitamin D
Introduction
The most common cause of nutritional rickets is vitamin D deficiency [1]. The primary source of vitamin D in humans is sunlight, and any factor that prevents ultraviolet rays from reaching the skin (such as indoor air, increased skin pigmentation, veiling for religious or social reasons, use of sunscreens, etc.) reduces the synthesis of vitamin D in the skin [2]. Vitamin D deficiency also varies regionally as vitamin D synthesis is influenced by many factors such as skin color, frequency of sun exposure, angle of incidence of rays on earth, sunscreen, clothing style, air pollution, indoor air, indoor work, exclusive breastfeeding, inadequate dietary habits (e.g.veg etarian diet), fat malabsorption and intake of anticonvulsants [1] The socioeconomic level of the society and the level of health service utilization in the region are also critical. Few foods naturally contain vitamin D, such as fatty fish, mushrooms and eggs [2].
Since vitamin D deficiency has been described as a serious public health problem [3], many topics such as prevention of vitamin D deficiency in children, methods of vitamin D prophylaxis, vitamin D deficiency in mothers, subclinical vitamin D deficiency, and vitamin D deficiency in adolescents in Western countries have been newly discussed in recent years. In addition, new functions of 25-OH vitamin D, such as regulation of immune functions, have been defined. As a result, recently, a decrease in multiple sclerosis, inflammatory bowel disease, allergies, asthma, infectious diseases, kidney disease, diabetes mellitus, cancer incidence, and cardiovascular disease has been reported [4,5].
The Van region, where our study patients lived, is geographically located at 37-39°N latitude and 42-44 °E longitude. Although this region is a sunny place, the solar benefit is low because it is a relatively cold place.
This study aims to discuss the frequency of serum 25-OH vitamin deficiency by gender, age, and puberty in children aged 0-18 years in Van region in the light of current literature.
Materials And Methods
This prospective study included children aged 0-18 years who presented to the General Polyclinics of Child Health and Diseases of Yüzüncü Yıl University Faculty of Medicine between December 1 and August 31. Those who had clinical findings of rickets on history, physical examination, and laboratory findings, those who had chronic disease, those who were taking anticonvulsant medications, and those who were taking vitamin D supplements and calcium tablets were excluded. A total of 1242 children (617 girls and 625 boys) were included in the study. The children were divided into three groups according to chronological age and puberty: Group 1: infants 0-36 months, Group 2: prepubertal children (Tanner stage 1), Group 3: adolescents (Tanner Stage 2 and above). Those who had obtained written and verbal informed consent from their families were included in the study. Their physical examinations were then conducted. The pubertal development of the children was determined according to Tanner’s pubertal developmental stages. All measurements were taken by the same person. The venous blood collected was analyzed in the biochemistry and hormone laboratories of Yüzüncü Yıl University Faculty of Medicine. Routine biochemical analyzes of calcium, phosphorus and magnesium were determined using routine analytical procedures and Roche (Germany) brand commercial kits in the Hitachi PP Modular Autoanalyzer. Parathyroid Hormone measurement was performed with Architect i 4000 SR instrument from Abbott brand, USA, using chemiluminescence. Serum 25-OH Vitamin D level was measured with Agilent Technologies 1200 Series (Germany) instrument and Chromsystems kit using High-Performance Liquid Chromatography (HPLC) method.
A serum 25-OH vitamin D level <5 ng/ml was considered as severe vitamin D deficiency, between 5-15 ng/ml as mild vitamin D deficiency, between 15-20 ng/ml as vitamin D insufficiency, and between 20-100 ng/ml as vitamin D sufficiency [6]. Our study was approved by Ethics Committee of Yüzüncü Yıl University, Faculty of Medicine, Research and Application Hospital and supported by the Department of Scientific Research Projects of Yüzüncü Yıl University.
Statistical Analysis: Descriptive statistics were reported as mean, Standard Deviation (SD), minimum and maximum values, and percentage (%). Pearson correlation coefficient was calculated to determine the relationships between continuous variables. The statistical significance level was taken as P<0.05 in the analyzes. Calculations were performed using SPSS 15 statistical package program for Windows.
Results
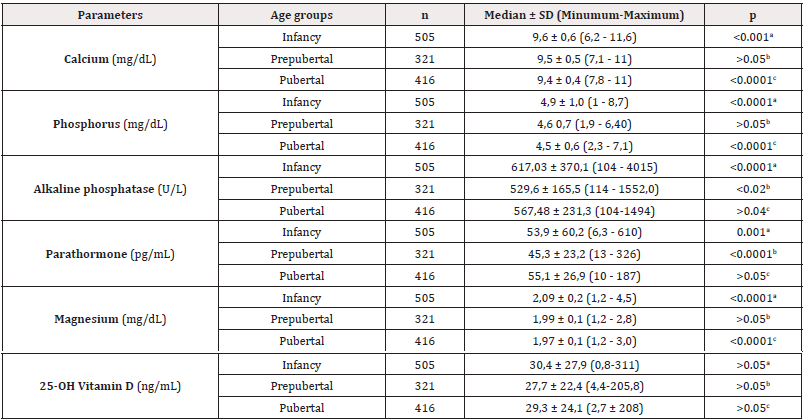
Table 1: Serum calcium, phosphorus, magnesium, alkaline phosphatase, parathormone, and vitamin D levels in infancy, prepubertal and pubertal groups.
Note*: a: Infancy - Prepubertal, b: Prepubertal - Pubertal, c: Pubertal – Infancy
Of 1242 children included in the study, 617 (49.6%) were girls and 625 (50.4%) were boys. The age of our cases was calculated in months. The youngest child was 3 days old, and the oldest was 216 (18 years) months old; the mean age of the study participants was 79,6 ± 63,3 months. Among male children, the youngest child was 4 days old, while the oldest was 216 months old; the mean age of male children was 77,2 ± 64,2 months. 505 (40.6%) of the children were infants, 321 (25.8%) were prepubertal, and 412 (33.4%) were pubertal. The age distribution of these groups was as follows: Infants were 0-36 months old, the youngest was 3 days old, and the oldest was 36 months old; the mean age of infants was 13.8±10.2 months. Among prepubertal children (Tanner stage 1), the youngest child was 37 months old and the oldest was 136 months old; the mean age of prepubertal children was 84.2±26 months. Adolescents (Tanner Stage 2 and above) had a minimum age of 114 months and a maximum age of 216 months; the mean age of adolescents was 154.5±22 months. The serum calcium, phosphorus, magnesium, alkaline phosphatase, parathormone and vitamin D levels were classified according to groups. The minimum, maximum, mean and standard deviation values of these parameters are shown in Table 1.
When our patients were examined by gender, there was no statistical difference between boys and girls in serum calcium, phosphorus and magnesium levels. Serum alkaline phosphatase and parathormone levels were higher in boys than in girls (P <0.05 for alkaline phosphatase; P <0.05 for parathormone). Serum 25-OH vitamin levels did not differ significantly between girls and boys (Table 2).
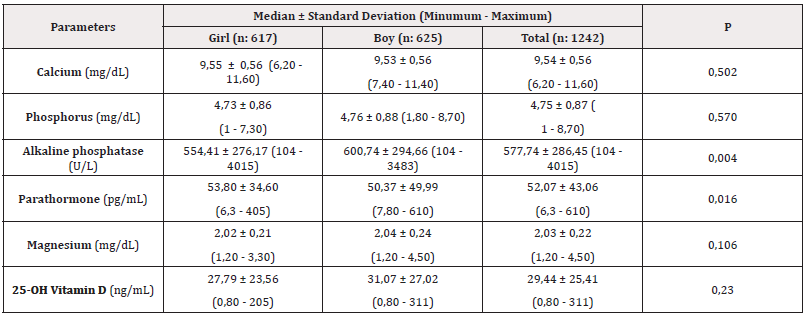
Table 2: Serum calcium, phosphorus, magnesium, alkaline phosphatase, parathormone, and vitamin D levels by gender.
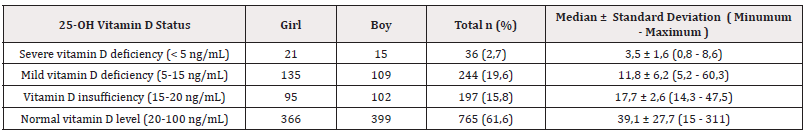
The sufficiency of serum 25-OH vitamin D levels was differentiated according to age groups (infancy, prepubertal, and pubertal) and gender. This situation is shown in Table 3.
When looking at 25-OH vitamin D deficiency by gender, it was noted that the deficiency was more common in girls. Severe deficiency was found in 1.7% of girls (21 cases) and 1.2% of boys (15 cases); mild deficiency was found in 10.9% of girls (135 cases) and 8.8% of boys (109 cases); insufficiency was found in 7.6% (in 95 cases) of girls and 8.2% (in 102 cases) of boys. However, these comparisons did not reveal statistically significant differences (Table 3).
When serum 25-OH vitamin D levels were examined in all study participants (in all groups), severe deficiency was detected in 2.9% (36 children), mild deficiency in 19.6% (244 children), and insufficiency in 15.9% (197 children). Thus, while an overall insufficiency of 38.4% (477) was calculated, 61.6% (765 children) of all participants were found to have sufficient vitamin D levels, i.e., normal, in supply (Table 4).
In our study, vitamin D deficiency (25-OH vitamin D level was less 20 ng/dL) was found in 196 cases (15.7%) in infants, 118 cases (9.5%) in prepubertal children, 163 cases (13.1%) in pubertal children and in a total of 477 cases (38.4%). Vitamin D deficiency was established most frequently in infancy, second most frequently in puberty and least frequently in prepuberty.
A negative correlation was observed between serum 25-OH vitamin D levels and parathormone levels and alkaline phosphatase levels (p <0.05).
Discussion
Vitamin D deficiency has been described as a pandemic and is over 80% in the pediatric age group [7,8].
For this reason, the importance of bone health and vitamin D promotion programs has come to the forefront in recent years. Providing vitamin D to all infants and children has been recognized as an important public health priority in many countries. The European Academy of Pediatrics (EAP) recommends it in doses of 40-1000 IU/day and 600-1000 IU/day in infants (0-1 year) and children (1- 18 years) at risk of vitamin D deficiency for prevention [9]. On the other hand, the American Association of Pediatrics recommends that all infants, children, and adolescents receive a certain amount of daily vitamin D intake to prevent rickets and maintain vitamin D levels at >20 ng/mL [6]. Also in our country, since March 18, 2006, the Ministry of Health has started to distribute free of charge 400 IU of vitamin D supplement per day to all infants aged 0-12 months as soon as a deficiency is detected.
There is no common consensus on what the threshold for vitamin D in children should be. Two opinions prevail in this regard. The first opinion states that vitamin D deficiency, insufficiency and sufficiency are defined as serum levels of 25(OH)D of <15 ng/mL, 15-20 ng/mL and >20 ng/mL, respectively [6]. The second opinion is considered as follows: Vitamin D deficiency, insufficiency and sufficiency are defined as serum levels of 25(OH)D of <20 ng/mL, 20-30 ng/mL and >30 ng/mL, respectively [10] We accepted the limitations of the first opinion [6] in our study.
Different degrees of vitamin D deficiency were reported in different parts of the world. In Italy, vitamin D status was found to be normal in 483 (22.6%) children, mildly deficient in 718 (33.6%) children, moderately deficient in 730 (34.1%) children, and severely deficient in 209 (9.8%) children [11]. In Iran, Vitamin D deficiency was shown in 10.6% of participants, insufficiency in 60.4% and sufficiency in 29% [12]. In a study conducted in China, the overall rate of hypovitaminosis D was 65.60%. Severe deficiency, deficiency and insufficiency were found in 6.57%, 25.51% and 33.52% of the included children, respectively [13].
A review of the studies conducted in our country revealed the following results: Gün, et al. [14] compared vitamin D deficiency between obese and non-obese groups aged 5-17 years and showed that 81.5% of the obese group and 48.3% of the non-obese group had vitamin D deficiency. Moreover, Andiran, et al. [15] found that 40% of 440 children and adolescents aged 0-16 years had vitamin D insufficiency. The only study conducted in our region on vitamin D deficiency in children is the study of Arıca, et al. [16] in 2010. In their study, the researchers found vitamin D deficiency in healthy children aged 0-36 months with a high rate of 49.1% when they set the threshold for deficiency <16.
In our study, depending on serum 25-OH vitamin D levels, severe deficiency was found in 2.9% (36 children), mild deficiency in 19.6% (244 children) and insufficiency in 15.9% (197 children). Thus, overall insufficiency was calculated as 38.4% (477), while it was assessed as sufficient, i.e. normal, in 61.6% (765 children). We believe that this significant improvement could be due to increased awareness in the community and free distribution of Vitamin D initiated by the Ministry of Health. Vitamin D deficiency/insufficiency has been reported in some studies with male predominance [17,18] and others with female predominance [15,19] while there are also studies where no gender difference was found [13,20]. In our study, severe deficiency was found in 1.7% of girls (21 cases) and 1.2% of boys (15 cases), mild deficiency in 10.9% of girls (135 cases) and 8.8% of boys (109 cases) and insufficiency in 7.6% (95 cases) of girls and 8.2% (102 cases) of boys. There was no statistically significant difference with respect to gender in our cases.
Although rickets due to vitamin D deficiency usually occurs in infancy, it can also be observed in school-age children and during puberty, when development proceeds rapidly. For example, in a study conducted in Italy, 35.3% deficiency and 39.1% insufficiency were detected in the age groups below 2.79 years, 53.05% deficiency and 35% insufficiency between 2.79-11.3 years, and 54% deficiency and 35.4% insufficiency between 11.3-18 years [3]. In another study, vitamin D deficiency was most common in Qatari adolescents aged 11-16 years (61.6%), second most common in children aged 5-10 years (% 28.9) and least common in those under 5 years (% 9.5) [21]. In Greece, it was most frequently demonstrated in adolescents aged 13-18 years (75%), followed by children aged 5-12 years (71.2%) and least frequently in infants aged 1-4 years (55.2%) [22].
In our study, when we evaluated the age groups within themselves, vitamin D deficiency was found most frequently in children aged 0-3 years [196 cases (15.7%)], second most frequently in pubertal children [118 cases (9.5%)] and least frequently in prepubertal children [163 cases (13.1%)]. This could be because infants and pubertal children do not benefit much from the sun, while prepubertal children are more exposed to the sun. As can be seen, high rates of vitamin D deficiency are observed in adolescents. It is noteworthy that Hashemipour, et al. [23] revealed the reason for higher prevalence of vitamin D deficiency in adolescent age groups [23]. Vitamin D deficiency with higher prevalence in adolescent age groups results from lifestyle changes, changes in physical activity and dietary habits, increased use of anti-UV sunscreens and spending more time in front of screens. In our study, vitamin D deficiency/ insufficiency was determined to be 13.1% in adolescent children. This rate is quite low compared to other studies conducted on adolescents. One reason for this could be that we set the threshold for deficiency at 20 ng/dl, while other studies have set it at 30 ng/dl.
There is a close relationship between parathormone, one of the most critical hormones in calcium metabolism, and alkaline phosphatase. In a reported study [18], a positive correlation was determined between serum alkaline phosphatase activity and serum parathormone level. Similarly, in our study, a positive correlation was found between parathormone and alkaline phosphatase. It was observed that when serum alkaline phosphatase level increased, serum parathormone level also increased. In accordance with the literature [24,25], a negative correlation was detected between serum 25-OH vitamin D and alkaline phosphatase and parathormones. Serum levels of alkaline phosphatase and parathormones were found to increase, while serum levels of 25-OH vitamin D decreased.
This Study has Some Limitations
Data on residential areas (rural/urban), dietary habits, physical activity, sun exposure, and sunscreen use were not included.
Conclusion
In conclusion, from any perspective, vitamin D deficiency is an important public health problem in terms of its prevalence in different countries. Consequently, we attribute the lower rates of vitamin D deficiency in Van children compared with previous studies to the free distribution of Vitamin D initiated by the Ministry of Health, easy access to healthcare providers, and increased awareness in the community. Therefore, the high rates of vitamin D deficiency point to the need for region-specific, re-adjusted new approaches.
References
- Orbak Z (2014) Rickets in Temel Çocuk Endocrinoloji, F.D. Peyami Cinaz, Ayşehan Akıncı, Behzat Özkan, Bumin N. Dündar, Ayhan Abacı, Teoman Akçay, Editor. Nobel Tıp Kitabevleri: İ p. 595-601.
- Holick MF, Chen (2008) Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition 87(4): 1080s-1086s.
- Irene Rutigliano, Gianpaolo De Filippo, Donatella De Giovanni, Angelo Campanozzi (2021) Is sunlight enough for sufficient vitamin D status in children and adolescents? A survey in a sunny region of southern Italy. Nutrition 84: 111101.
- Wacker M, Holick MF (2013) Vitamin D-Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 5(1): 111-148.
- Thacher TD, Clarke BL (2011) Vitamin D Insufficiency. Mayo Clin Proc 86(1): 50-60.
- Madhusmita Misra, Danièle Pacaud, Anna Petryk, Paulo Ferrez Collett Solberg, Michael Kappy, et al. (2008) Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 122(2): 398-417.
- Jennifer Hilger, Angelika Friedel, Raphael Herr, Tamara Rausch, Franz Roos, et al. (2014) A systematic review of vitamin D status in populations worldwide. Br J Nut 111(1): 23-45.
- Kevin D Cashman, Kirsten G Dowling, Zuzana Škrabáková, Marcela Gonzalez Gross, Jara Valtueña, et al. (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 103(4): 1033-1044.
- Zachi Grossman, Adamos Hadjipanayis, Tom Stiris, Stefano Del Torso, Jean Christophe Mercier, et al. (2017) Vitamin D in European children-statement from the European Academy of Paediatrics (EAP). Eur J Pediatr 176(6): 829-831.
- Christian Braegger, Cristina Campoy, Virginie Colomb, Tamas Decsi, Magnus Domellof, et al. (2013) Vitamin D in the Healthy European Paediatric Population. J Pediatr Gastroenterol Nutr 56(6): 692-701.
- Manuela Ceccarelli, Elena Chiappini, Rosangela Arancio, Mauro Zaffaroni, Simona La Placa, et al. (2020) Vitamin D deficiency in a population of migrant children: an Italian retrospective cross-sectional multicentric study. Eur J Public Health 30(3): 551-556.
- Maryam Bemanalizadeh, Motahar Heidari Beni, Hanieh Sadat Ejtahed, Ramin Heshmat, Fereshteh Baygi, et al. (2020) Association of serum 25-hydroxyvitamin D concentration with anthropometric measures in children and adolescents: the CASPIAN-V study. Eat Weight Disord 26(7): 2219-2226.
- Xuguang Zhang, Yanping Chen, Shanshan Jin, Xinxin Bi, Dongkai Chen, et al. (2020) Association of serum 25-Hydroxyvitamin D with Vitamin D intervention and outdoor activity among children in North China: an observational study. Bmc Pediatr 20(1): 542.
- Emrah Gün, Hakan Uzun, Semih Bolu, İlknur Arslanoğlu, Kenan Kocabay, et al. (2020) Serum 25-hydroxyvitamin D is associated with insulin resistance independently of obesity in children ages 5-17. Prim Care Diabetes 14(6): 741-746.
- Nesibe Andıran, Nurullah Çelik, Halise Akça, Güzide Doğan (2012) Vitamin D Deficiency in Children and Adolescents. J Clin Res Pediatr Endocrinol 4(1): 25-29.
- Vefik Arıca SA, Sebahat Gücük (2010) Tamer Edirne, Level of serum 25-OHD in healthy children aged 0-36 months in Van. Turkish Archives of Pediatrics 45: 286-290.
- Zhiliang Cai, Qiaoxuan Zhang, Ziqiang Xia, Songbai Zheng, Lilan Zeng, et al. (2020) Determination of serum 25-hydroxyvitamin D status among population in southern China by a high accuracy LC-MS/MS method traced to reference measurement procedure. Nutrition & Metabolism 17: 8.
- Kruse K (1995) Pathophysiology of Calcium-Metabolism in Children with Vitamin-D-Deficiency Rickets. J Pediatr 126(5): 736-741.
- Mansbach JM, Ginde AA, Camargo CA (2009) Serum 25-Hydroxyvitamin D Levels Among US Children Aged 1 to 11 Years: Do Children Need More Vitamin D? Pediatrics 124(5): 1404-1410.
- Jong Woo Won, Seong Kwan Jung, In Ah Jung, Yoon Lee (2021) Seasonal Changes in Vitamin D Levels of Healthy Children in Mid-Latitude, Asian Urban Area. Pediatric Gastroenterology Hepatology & Nutrition 24(2): 207-217.
- Bener A, Al Ali M, Hoffmann GF (2009) Vitamin D deficiency in healthy children in a sunny country: associated factors. International Journal of Food Sciences and Nutrition 60: 60-70.
- Gavriela Maria Feketea, Ioana Corina Bocsan, Georgios Tsiros, Panagiota Voila, Luminita Aurelia Stanciu, et al. (2021) Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children Basel 8(2): 111.
- Sima Hashemipour, Bagher Larijani, Hossein Adibi, Ebrahim Javadi, Mojtaba Sedaghat, et al. (2004) Vitamin D deficiency and causative factors in the population of Tehran. Bmc Public Health 4: 38.
- Teodoro Durá Travé, Fidel Gallinas Victoriano, María Malumbres Chacon, Lotfi Ahmed Mohamed, María Jesús Chueca Guindulain, et al. (2021) Are there any seasonal variations in 25-hydroxyvitamin D and parathyroid hormone serum levels in children and adolescents with severe obesity? Eur J Pediatr 180(4): 1203-1210.
- Muhittin A Serdar, Başar Batu Can, Meltem Kilercik, Zeynep A Durer, Fehime Benli Aksungar, et al. (2017) Analysis of Changes in Parathyroid Hormone and 25 (Oh) Vitamin D Levels with Respect to Age, Gender and Season: A Data Mining Study. J Med Biochem 36(1): 73-83.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.