Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Retrospective Analysis of Clinic Pathological Features and Therapeutic Outcomes of Moroccan Patients with Metastatic Sensitive Prostate Cancer
*Corresponding author: Khadija Hinaje, Department of Medical Oncology, Hassan II University Hospital, Faculty of Medicine, Pharmacy and Dental Medicine of Fez, University Sidi Mohamed Ben Abdellah, Fez, Morocco.
Received: April 25, 2024; Published: May 1, 2024
DOI: 10.34297/AJBSR.2024.22.002952
Abstract
Background: Prostate cancer is a global public health issue. It represents the second most common cancer in men after lung cancer and the fifth leading cause of cancer death in men of all ages. 4 935 new cases of prostate cancer are diagnosed each year in Morocco, of which more than 10% are already metastatic.
Aim: The objective of this work is to study the main epidemiological, clinical, histological and therapeutic aspects of metastatic sensitive prostate cancer and to compare our results with those reported in the literature.
Methods: We conducted a retrospective study of 553 cases of metastatic prostate cancer collected at the medical oncology department of CHU Hassan II in Fez, for a period of 10 years from January 2014 to December 2023.
Results: The average age at diagnosis was 72 years. Clinical symptoms were predominantly urinary, with obstructive signs in 38.3% of cases, followed by irritative signs in 21.4% of cases. Other extra-urinary signs related to metastatic spread were described, mainly bone pain (18.2%), as well as neurological signs (7.3%). PSA levels were measured in all patients, ranging from 10 to 2000 ng/ml with an average of 189.5 ng/ml. The predominant Gleason score was 7 (4+3) in 36.4% of cases, followed by a score of 8 (4+4) in 31.8% of cases. All patients had metastatic disease, with 71% being synchronous and 29% metachronous. 54.7% of patients had isolated bone metastases, while 45.3% had associated visceral metastases, of which 26% were hepatic and 13.8% were pulmonary. Regarding the management of metastatic sensitive prostate cancer, all patients underwent castration, with 17.4% undergoing surgical castration and 82.6% undergoing medical castration with LH-RH analogues, 31.9% received castration alone, 9.1% received abiraterone acetate plus prednisone in addition to castration, while 59% received docetaxel-based chemotherapy plus prednisone in addition to castration. The median progression-free survival was 14 months (95% CI: [13.7; 14.2]), 32.6 months (95% CI: [30.5; 41.6]), and 20 months (95% CI: [19.8; 20.18]), respectively.
Conclusion: Metastatic prostate cancer poses a major public health challenge in Morocco. Advances in understanding prognostic factors and therapeutic options offer hope for improving the survival and quality of life of these patients. However, additional efforts are needed to strengthen early detection strategies and access innovative treatments.
Introduction
Metastatic prostate cancer corresponds to a tumor that has spread beyond the tissues surrounding the prostate to reach distant lymph nodes or other parts of the body away from the prostate, such as the lungs, liver, or bones. It is distinguished into two entities: hormone-naive metastatic prostate cancer and castration-resistant metastatic prostate cancer [1]. Hormone-naïve metastatic prostate cancer is a cancer that is sensitive to castration, whether chemical or surgical. It’s a cancer in which tumor cells are stimulated by androgens, mainly testosterone produced by the testicles under the control of the hypothalamic-pituitary axis. Testosterone is the main circulating androgen, with 5 to 10% produced by the adrenal glands. It is then converted in prostatic cells by 5-alpha reductase into dihydrotestosterone [2]. Prostate cancer constitutes a public health problem in the world, it represents the second most common male cancer worldwide, and the fifth leading cause of cancer death in men of all ages [3]. Its diagnosis is suspected upon a suspicious digital rectal examination, and an elevation of prostate-specific antigen (PSA) levels, which can indicate its extension (PSA>100 ng/ml). It’s confirmed through histopathological examination, establishing the type and histological grade. It is a cancer with high metastatic potential locally, locoregionally, and distantly. Its current treatment relies on androgen suppression combined with next-generation hormone therapy or chemotherapy, or the combination of the three +/- local radiotherapy [1]. In developing countries like Morocco, the diagnosis of prostate cancer remains late and is often made at the metastatic stage.
The objective of this work is to study the main epidemiological, clinical, histological and therapeutic aspects of metastatic hormone- sensitive prostate cancer (m HSPC) and to compare our results with those reported in the literature.
Materials and Methods
In order to describe the epidemiological, clinical, histological, and therapeutic profile of patients with mHSPC, we conducted a descriptive retrospective study on 553 cases of metastatic sensitive prostate cancer collected at the medical oncology department of the Hassan II University Hospital in Fez, over a period of 10 years from January 2014 to December 2023. We only included patients treated in our department with histologically proven prostate adenocarcinoma, classified as stage 4. The study excluded patients with incomplete or unusable medical records, non-metastatic prostate cancer (localized or locally advanced), other associated cancer, and histological types other than adenocarcinoma such as sarcoma, lymphoma or others. Statistical analysis was done by SPSS version 23 software, qualitative variables are expressed in frequency and percentage, and quantitative variables are expressed as median and mean. Progression-free survival was calculated using the Kaplan-Meier method.
Results
Epidemiological Data
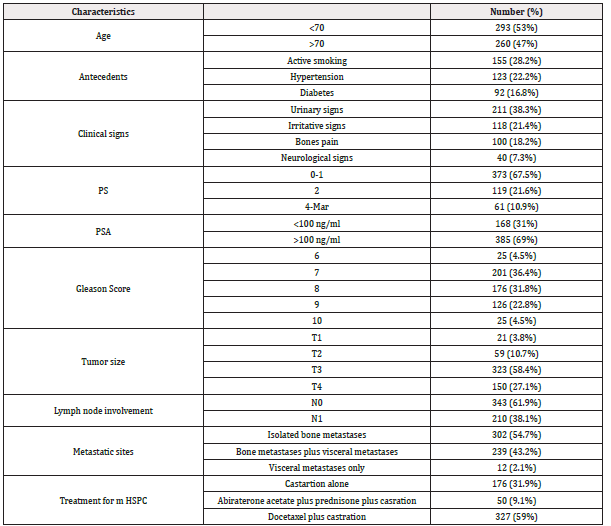
Between January 2014 and December 2023, we included 553 patients with metastatic prostate cancer in the medical oncology department of CHU Hassan II in Fez. The average age of our patients was 72 years with a standard deviation of 8.9 years with age ranges ranging from 46 to 95 years. We observed a clear continual increase in the incidence of new cases in this period. However, a decrease in the number of cases was noted in 2020. The most common antecedents were active smoking (28.2%), followed by hypertension (22.2%), and then diabetes (16.8%).
Clinical Data
The most common symptoms were urinary signs suggestive of obstruction (38.3%), followed by irritative signs (21.4%). Other extra-urinary signs related to metastatic spread were described, mainly bone pain (18.2%) notably in the spine (36%) and pelvis (34%), as well as neurological signs (7.3%). 67.5% of patients were in good general condition (Performance Status (PS) 0 to 1) at the time of diagnosis. 92% of our patients presented with a suspicious prostate on digital rectal examination. Pelvic shielding and induration were the most common abnormalities, with percentages of 43.2% and 34%, respectively. However, 10.4% presented with a nodular prostate.
Biological and Radio- Histological Data
PSA testing was performed on all patients, with levels ranging between 10 and 2000 ng/ml, with an average of 189.5 ng/ml. All patients performed a biological workup including a count blood, an assay of the level of albumin, calcium level, alkaline phosphatase (ALP), testosterone, liver and kidney function. This assessment had demonstrated anemia in 29.8% of patients, hyperleukocytosis in 15.9% of cases, thrombocytosis in 9.8% of cases, hypoalbuminemia in 26% of cases, hypercalcemia in 25% of cases, an increase in the level of ALP in 34.3% of cases, liver function impairment was observed in 11% of cases, predominantly cytolytic, renal insufficiency, primarily obstructive, was found in 20% of cases.
All our patients had histological type adenocarcinoma. The predominant Gleason score was 7 (36.4%), followed by a score of 8 (31.8%).
The radiological staging allowed exploration of loco-regional and distant extension: Thoraco-abdomino-pelvic CT scan was performed in 94.6% of patients, revealing multiple metastatic sites, with bone being the preferential site. All of our patients were metastatic, 71% being de novo (synchronous) and 29% metachronous. 54.7% of patients had isolated bone metastases, while 45.3% had associated visceral metastases, including 26% hepatic and 13.8% pulmonary. Bone scintigraphy was performed in all patients, while magnetic resonance imaging was done in 28% of cases. According to the TNM classification, 27.1% of patients were classified as T4 and lymph node invasion N1 was found in 38.1%.
Therapeutic Data
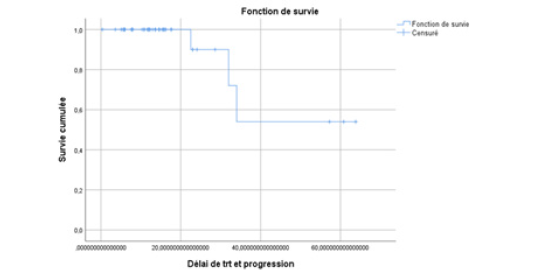
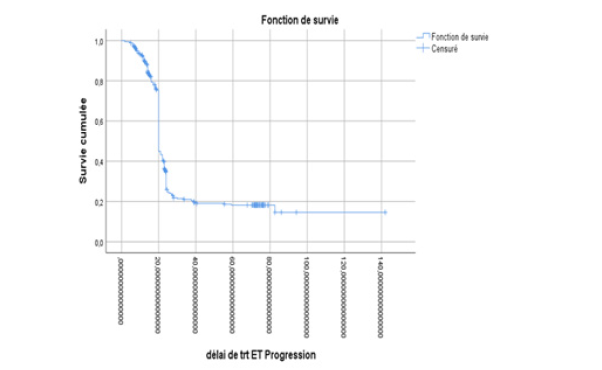
All our patients underwent castration, with 17.4% of cases being surgical and 82.6% being medical using LH-RH analogues. At the hormone-sensitive stage, 31.9% received castration alone, 9.1% received abiraterone acetate plus prednisone in addition to castration, while 59% received docetaxel-based chemotherapy plus prednisone alongside castration. The median progression-free survival (PFS) was 14 months (95% CI: [13.7; 14.2]), 32.6 months (95% CI: [30.5; 41.6]), and 20 months (95% CI: [19.8; 20.18]) respectively (Table 1) (Figures 1-3).
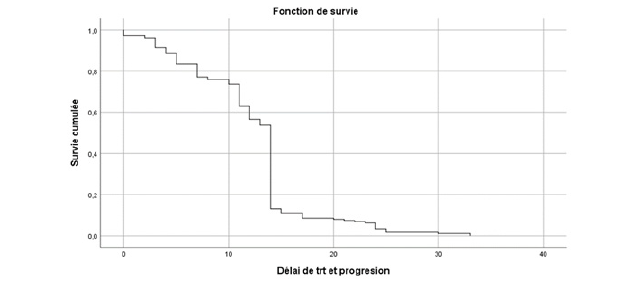
Figure 1: Estimated Kaplan-Meier curve of progression-free survival for patients treated with castration alone in the hormone-naive stage in our series.
Discussion
Prostate cancer has become a leading public health issue over the past fifteen years [4], as evidenced by epidemiological data showing over 1.3 million new cases per year recorded in 2018 [5] and over 1,467,854 new cases in 2022 [6]. In Morocco, prostate cancer is ranked second among cancers, accounting for 16.1% of incident cases of all cancers in men, totaling 4,935 new cases, following lung cancer. This incidence is continuously increasing and currently represents 7.8% of all cancer cases regardless of gender, according to The Global Cancer Observatory Morocco 2022 [7]. In our series, we have observed a significant upward trend and a continuous increase in the incidence of new cases between 2014 and 2023. A sudden drop in the number of cases was noticed in 2020, which could be explained by the COVID-19 pandemic, declared by the WHO in March 2020. The health restriction measures aimed at limiting the spread of the virus reduced access to healthcare and delayed the management of patients, including those with prostate cancer.
The mean age at diagnosis in our case series was 72+/-8.9 years with a range of 46 to 95 years. This figure remains comparable to studies that have found the mean age among metastatic patients to be>70 years: J Rigaud found a mean age of 73.3+/-9 years [8], and the Cooperative Prostate Cancer Study Group (GCECP) noted a mean age of 71 years [9].
Clinical symptoms were predominantly characterized by urinary signs (70%), including obstructive signs in 38.3% of cases, followed by irritative signs in 21.4% of cases. Other extra-urinary signs related to metastatic extension were described, primarily bone pain (18.2%), as well as neurological signs (7.3%). Our study aligns with research conducted by K TENGUE [10] and M BELKHAIMA [11], which showed that the circumstances of discovery were primarily marked by urinary disorders (89% and 93% respectively), associated or not with lower back pain estimated at 67% and 20% of cases respectively. This could be explained by the fact that patients often consult us at a late stage.
In our series, 90% of our patients had a total PSA level ≥100 ng/ml, consistent with those found by K. Tengue 90.5% [10]. We can say that a total PSA ≥100 ng/ml is strongly associated with the presence of metastases.
Adenocarcinoma was the histological type found in our study. This result is similar to those in the literature, where there is a clear predominance of adenocarcinoma in prostate cancer.
Regarding the imaging data, conventional imaging with computed tomography (CT) and skeletal scintigraphy remains the recommended standard for determining the extent of cancer spread. CT scan of the thorax, abdomen, and pelvis was the most commonly performed extension assessment in our patients, accounting for 94.6% of cases, to explore local-regional and distant tumor extension. This is consistent with the study by K TENGUE [10], where 95% of cases underwent a CT scan. This high percentage can be attributed to the inaccessibility and high cost of certain assessments such as MRI and bone scintigraphy. In contrast, in studies conducted by BELKHAIMA [11] and M NDOYE [12], only 16% and 4.3% of cases, respectively, underwent this examination. We need to distinguish between patients with de novo metastatic disease (synchronous) and those who experience recurrence after local treatment (metachronous), with the prognosis of the latter appearing to be better in retrospective studies [13]. The table below summarizes patient stratification according to the timing of metastasis development (synchronous/metachronous) in large phase III randomized trials of hormone-sensitive metastatic prostate cancer (Table 2).
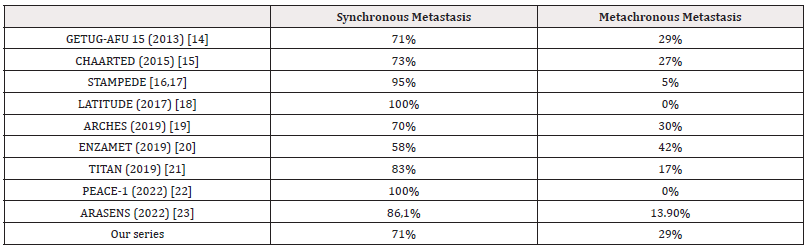
Table 2: Comparison of Different Phase III Trials According to Timing of Metastasis Development (Synchronous/Metachronous).
On the therapeutic level, Androgen deprivation therapy (ADT) was the standard of care in the first-line treatment of mHSPC for many decades. ADT alone achieved disease control over a period of 18 to 24 months in >90% of patients [24]. Today, ADT is the backbone of all systemic therapies of mHSPC and it is continued even in case of a change in therapy. In everyday clinical practice. GnRH agonists are commonly used for ADT, owing to the fact that they are usually easier to administer and better tolerated by patients. A prospective randomized phase III study and meta-analyses, however, point to a lower rate of cardiovascular events with GnRH antagonist treatment, especially in patients with relevant pre-existing conditions [25]. Besides GnRH antagonists, surgical castration is used for androgen deprivation therapy, but only rarely. In our study, all our patients underwent castration, with 17.4% of cases being surgical and 82.6% being medical using LH-RH analogues.
Over the last years, the treatment landscape of mHSPC has changed dramatically with the addition of 4 systemic agents that previously demonstrated benefit in the castrate-resistant setting (docetaxel, abiraterone, enzalutamide, and apalutamide).
Docetaxel was the first substance to trigger a paradigm shift in the management of mHSPC. It was evaluated in two randomized phase III studies: the CHAARTED trial was the first to demonstrate improvement in median overall survival by more than 10 months over a total period of 50 months [15]. It also found that patients with high tumor volume benefited the most. The STAMPEDE trial (arm C and E), published later, confirmed the results of the CHAARTED trial overall, but the benefit was independent of the magnitude of the tumor burden [16]. In our series, 59% received docetaxel-based chemotherapy plus prednisone alongside castration with m PFS at 20 months (95% CI: [19.8; 20.18]).
Various phase III studies have evaluated treatment intensification with novel hormonal agents (NHAs). As the first NHA, Abiraterone was evaluated in combination with prednisone in the LATITUDE trial [18] in patients with synchronous metastatic HSPC and high-risk demonstrating a significant and clinically relevant prolongation of OS through therapy intensification. Similar to what had already been shown for docetaxel, the STAMPEDE trial (arm G) [17] also confirmed the results for intensified hormone therapy. Post-hoc analyses demonstrated an advantage for Abiraterone, irrespective of the subgroup. Nevertheless, the approval of abiraterone for the treatment of mHSPC was limited to patients with synchronous metastatic HSPC with high-risk features according to LATITUDE criteria.
TITAN study [21] that compared an ADT with either apalutamide or placebo. Patients could have received local treatment or docetaxel. After a median follow-up of 22.7 months, the first interim analysis revealed a significant benefit in radiographic progression free survival for the apalutamide group and an overall survival at 24 months of 82.4% for apalutamide versus 73.5% for the placebo. There was no survival benefit in patients previously treated with docetaxel.
Enzalutamide were evaluated in two studies ARCHES [19] and ENZAMET [20] trials. The design of the ARCHES trial is similar to that of the TITAN trial; nearly 18% of patients received pre-treatment with docetaxel and ADT was the treatment standard. Despite the cross-over of approximately 31% of control arm patients, treatment intensification by adding enzalutamide improved overall survival by 34%. In the ENZAMET trial, enzalutamide led to a significant improvement in overall survival in the total patient cohort (HR 0.67; 95% confidence interval (CI]: [0.52; 0.86). In our series, just 9.1% received abiraterone acetate plus prednisone in addition to castration, and this is explained by the still high cost and lack of reimbursement of costs under healthcare. In addition, no patient received enzalutamide or apalutamide due to their lack of accessibility in our context.
Two randomized, multicenter Phase III trials showed the efficacy of Triple combination therapy as a recent treatment approach. The first was the PEACE-1 study [22] on patients with synchronous metastatic HSPC. The combination of ADT, Docetaxel, and Abiraterone significantly increased radiological PFS by 2.5 years. This benefit was observed regardless of tumor volume: HR in high-volume tumors was 0.47 (95% CI: 0.3-0.60), p<0.0001, and 0.58 in low-volume tumors (95% CI: 0.39-0.87), p=0.006. Furthermore, the combination also resulted in improved OS with a 25% reduction in deaths and a gain of 12 to 18 months in patients with high-volume tumors. The toxicity profile remained favorable. In the ARASENS trial [23], hormonal chemotherapy was intensified with darolutamide. Its addition reduces the risk of death by 32.5%. The benefit in terms of OS with the addition of darolutamide was observed in all pre-specified subgroups. Secondary endpoints were also improved: time to castration resistance, time to pain progression, time to first skeletal-related event, and time to first subsequent therapy.
Two randomised trials evaluated the impact of prostate radiotherapy on overall survival in patients with de novo metastatic disease. The HORRAD trial [26] included all patients with de novo metastatic disease, regardless of the clinical features. They all received ADT and were then randomised for prostate irradiation (70Gy in 35 fractions). In 10 years, 446 patients were included; most had more than 5 metastases (65%) and 75% had an ISUP score≥4. With a median follow-up of 47 months, no significant difference in median survival was identified between the two arms (45 months in the RT group and 43 months in the control group). In the subgroup analysis, the hazard ratio was 0.68 in favour of the radiotherapy arm for patients with less than 5 metastases, but it was non-significant.
In the H arm of the STAMPEDE trial [27], 2061 mHSPC patients were randomized. These patients received either systemic therapy (ADT ± docetaxel) alone or in addition hypofractionated prostate radiotherapy. This additional prostate treatment led to a significant improvement in the time to treatment failure by 9% (23% versus 32%; HR 0.76. 95% CI: 0.68; 0.84]; p<0.0001). However, there was no difference in median overall survival. For patients with low metastatic burden, on the other hand, a significant benefit for overall survival and prostate cancer-specific survival was found.
The treatment decision for patients presenting with mHSPC must account for several factors, including patient and disease factors, and importantly the circumstances surrounding drug licensing and cost reimbursements in their healthcare setting.
Conclusion
Metastatic prostate cancer represents a major public health challenge worldwide, including in Morocco. Prostate cancer screening involves both digital rectal examination and PSA testing. This is the only way to ensure early diagnosis and improve the prognosis of our patients and should be offered from the age of 55, especially in the presence of risk factors. In the metastatic prostate cancer stage, management should be multidisciplinary to ensure an appropriate therapeutic strategy. Progress in understanding prognostic factors and therapeutic options offers hope for improving the survival and quality of life of these patients. However, additional efforts are needed to strengthen early screening strategies and access innovative treatments.
References
- G Ploussarda, G Roubaud, E Barret, et al. (2023) French recommendations from the AFU cancer committee, update 2022-2024: prostate cancer. Progress Urology 30(12): 136-251.
- Oudard S, Velev M, Belhaj Y, et al. (2018) Treatment of metastatic prostate cancer, significant advances. Rev Prat 68(7): 707-712.
- (2022) Cancer TODAY | IARC - https://gco.iarc.who.int Data version: Globocan 2022 (version 1.1).
- H A de Santé (2012) Prostate cancer: Identification of risk factors and relevance of screening by prostate-specific antigen (PSA) measurement in a high-risk population of men,” Rapp. Orientation. Saint-Denis La Plaine HAS.
- J F Ferlay (2001) GLOBOCAN 2000. Cancer incidence, mortality and prevalence worldwide, version 1.0. IARC cancerbase.
- (2022) Cancer TODAY | IARC - https://gco.iarc.who.int Data version: Globocan 2022 (version 1.1), Incidence in the world.
- (2022) Cancer TODAY | IARC - https://gco.iarc.who.int Data version: Globocan 2022 (version 1.1), Incidence in MOROCCO.
- Rigaud, LLE Normand, G Karam, P Glemain, J Buzelin, et al. (2002) Prognostic factors for prostate cancer treated with first-line hormonal therapy. pp.232-239.
- Garcia R, Oozeer R, Le Thanh H, Chauvet B, Toy BJ, et al. (1997) Conformal radiotherapy for prostate cancer: contribution of pelvic restraint and new positioning markers. Cancer/Radiotherapy, 1(4): 307-313.
- K Tengue (2016) Epidemiological, diagnostic, therapeutic and progressive profile of prostate cancer in Togo. African J Urol 22(2): 76-82.
- M BELKHAIMA (2007) CLINICAL AND THERAPEUTIC EPIDEMIOLOGICAL PROFILE OF PROSTATE CANCER AT MOHAMED VI CHU. Retrospective study of 159 cases.
- M Nndoye (2010) Epidemiological, diagnostic, therapeutic and progressive profile of prostate cancer in Togo. African Journal of Urology 22(2): 76-82.
- Francini E, Gray KP, Xie W, Shaw GK, Valenca L, et al. (2018) Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 78(12): 889-895.
- Gravis G, Fizazi K, Joly F, et al. (2013) Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol 14:149-158.
- Sweeney CJ, Chen YH, Carducci M, et al. (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737-746.
- James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, et al. (2007) Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 377:338-351.
- James ND, Sydes MR, Clarke NW, et al. (2016) STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomized controlled trial. Lancet 387:1163-1177.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez Antolin A, et al. (2019) Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomized, double-blind, phase 3 trial. Lancet Oncol 20:686-700.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP Holzbeierlein J, Villers A, et al. (2019) ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol.
- Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, et al. (2019) Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 381: 121-131.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, et al. (2018) Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 378: 1408-1418.
- Fizazi K, Foulon S, Carles J (2022) Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomized, phase 3 student with a 2x2 factorial design. Lancet 399(10336): 1695-1707.
- Smith MR, Hussain M, Saad F (2022) Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med 386: 1132-1142.
- Van Poppel H, Klotz L (2012) Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol 19: 594-601.
- Cirne F, Aghel N, Petropoulos JA, Klotz L, et al.: The cardiovascular effects of gonadotropin-releasing hormone antagonists in men with prostate cancer. Eur Heart J Cardiovasc Pharmac.
- Boeve LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, et al. (2019) Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD Trial Eur Urol 75(3): 410-418.
- Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, et al. (2018) Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial Lancet 392(10162): 2353-2366.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.