Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Study on the Treatment of Rat Temporomandibular Joint Osteoarthritis with Human Umbilical Cord Mesenchymal Stem Cells Exosomes
*Corresponding author: Yingxin Xu, Peking University Shenzhen Hospital Clinical Medical School, Anhui Medical University, 518036, Shenzhen, China, The Fifth Clinical Medical School, Anhui Medical University, 230000, Hefei, China and Peking University Shenzhen Hospital, 518036, Shenzhen, China.
Received: May 01, 2024; Published: May 06, 2024
DOI: 10.34297/AJBSR.2024.22.002963
Abstract
Objective: To investigate the treatment effects of human umbilical cord mesenchymal stem cells exosomes (hUCMSCs-exos) on temporomandibular joint osteoarthritis of rats.
Methods: The hUCMSCs-exos were extracted by ultracentrifugation, and the morphological characteristics and vesicle particle size of hUCMSCs-exos were analyzed by projection electron microscopy and particle size analyzer. The mandibular condylar chondrocytes (MCCs) of rats were cultured in vitro, and the in vitro uptake capacity of hUCMSCs-exos by MCCs was analyzed by immunofluorescence staining. The in vitro temporomandibular joint osteoarthritis (TMJOA) model was constructed, and the rats MCCs were divided into three groups, namely, the blank control group, the TMJOA group, and the Exos group, and the effects of hUCMSCs-exos on the proliferation and migration of rats MCCs under the induction of interleukin-1β (IL-1β) were explored by the Cell Counting Kit-8 (CCK8) assay and the scratch assay. The rat TMJOA model was constructed and divided into blank control group, TMJOA group, and Exos group. The intra-articular inflammatory changes and articular cartilage repair in the joints were evaluated in each group by hematoxylin-eosin (HE) staining. The statistical analysis was conducted using the one-way analysis of variance to compare differences among multiple data groups, adhering to a significance level of P<0.05 for determining statistical significance.
Results: Transmission electron microscopy revealed the teapot-like morphology of HUCMSCs-exos, with a predominant particle size distribution centered around 162nm. The extracted samples were consistent with exosome characterization. HUCMSCs-exos were taken up by rats MCCs, surrounding the nucleus. The scratch assay showed that the wound area of the Exos group decreased significantly compared to the TMJOA group (P<0.05). The CCK8 assays showed that the proliferation capacity of chondrocytes in the Exos group significantly increased compared to the TMJOA group at 48h (P<0.05). The results of in vivo experiments showed that compared with the blank control group, HE staining showed inflammatory manifestations in the condylar cartilage of the TMJOA group, with irregular cartilage surfaces and areas of cellular necrosis. The Exos group showed that the surface of the TMJ condylar cartilage was more continuous, and obvious cellular necrosis was not found.
Conclusion: HUCMSCs-exos improved the migration and proliferation of MCCs under IL-1β HUCMSCs-exos can repair cartilage destruction caused by TMJOA,and keep the continuity of cartilage.
Keywords: Temporomandibular joint, Temporomandibular disorders, Osteoarthritis, Human umbilical cord stem cell, Exosomes
Introduction
The temporomandibular joint (TMJ) is closely related to normal physiological activities and is involved in physiological activities such as chewing, swallowing, breathing, and speak. Temporomandibular joint osteoarthritis (TMJOA) can cause joint pain, joint deformity, and dysfunction, which can negatively affect the physiological function of TMJ and the quality of daily life of patients. According to epidemiological studies, the prevalence of TMJOA in China is 2%~23% [1]. Typical pathological changes of TMJOA are progressive cartilage degradation, loss, and abnormal remodeling of subchondral bone [2]. Currently, the main treatment modalities for TMJOA are symptomatic therapies to control pain and inflammation, including non-surgical conservative treatments, and surgical treatments with condylar resection as the mainstay [3]. Although these treatments can reduce the symptoms and slow the progression of the disease, they cannot completely repair cartilage and subchondral defects. Stem cell therapy has become a novel treatment for TMJOA and other clinical diseases. Human umbilical cord mesenchymal stem cells (hUCMSCs) are more readily available than other stem cells and have no donor site morbidity, are young, abundant in tissues, have high expansion potential and low immunogenicity, and a high paracrine potential to accelerate the tissue repair process [4]. Exosomes (exos) are vesicles with diameters of 50-200nm secreted by cells, which are important regulators of intercellular communication, mediate intercellular communication by delivering biologically active molecules, and participate in a variety of pathological processes [5,6]. Due to their small size, good targeting, stability, ability to cross barriers and deliver drugs into the cytoplasm.
The possibility of exos application for the treatment of osteoarthritis has been demonstrated, but most of the major studies have been conducted on knee osteoarthritis [7,8], and slightly fewer studies have been conducted on TMJOA. Exos come from various sources [9-14], and exos from different cellular sources may possess different functions. In this experiment, we used hUCMSCs-exos for the first time to explore its therapeutic effects on TMJOA in vivo and in vitro. Most of the previous studies have evaluated the effects of exos on the proliferation and migration of rat condylar chondrocytes (MCCs) in normal cellular state. This experiment was the first to investigate the effect of hUCMSCs-exos on the proliferation and migration of rat MCCs induced by interleukin (IL)-1β, which better simulated the repairing effect of hUCMSCs-exos on MCCs under inflammatory environment.
In this study, hUCMSCs were isolated and cultured, and hUCMSCs-exos was extracted. MCCs were isolated and cultured in vitro, and rat MCCs were induced using IL-1β to establish an in vitro TMJOA model, to investigate the effects of hUCMSCs-exos on the proliferation and migration ability of rat MCCs. In this study, we also used complete Freund's adjuvant (CFA) to establish an in vivo TMJOA model and injected hUCMSCs-exos into the cystic cavity of TMJ, and evaluated the in vivo treatment of hUCMSCs-exos for TMJOA by hematoxylin-eosin (HE) staining of the sections. effect of TMJOA. The aim of this study was to preliminarily investigate the reparative effect of hUCMSCs-exos on cartilage regeneration in rat TMJOA, and to explore the repairing ability of hUCMSCs-exos on cartilage regeneration in rat TMJOA, which will provide a basis for future clinical application.
Material & Methods
Materials
4W SD male rats, All the rats were kept in an SPF grade room in one cage of four rats using a timed light device for 12h of light and 12h of dark day and night. Rats had free access to adequate food and water.The experiment followed the 3R principle. MEMα (Bio-Channel, China); FBS (CellMAX, Australia); Penicillin/streptomycin(Gibco, America); APC Mouse Anti-Human CD45, PE Mouse Anti-Human CD73, FITC Mouse anti-Human CD105 (BD Pharmingen, America); CFA (Solaibao, China); IL-1β (Abbkine, China); Toluidine blue staining (sangon biotech, China); CCK8 (Yuanye, China).
Culture of Human Umbilical Cord Mesenchymal Stem Cells
The hUCMSCs were added to complete medium (89% MEMα + 10% FBS + 1% penicillin/streptomycin) and placed in a cell culture incubator at 37°C and 5% CO2.
Extraction and Identification of the Exosomes
The P5 hUCMSCs were taken and cultured in serum-free medium (99% MEMα+1% penicillin/streptomycin) for 48h when the cells proliferated to 70%~80% density, and the supernatant was collected and centrifuged successively at 300g and 2 000g at 4°C for 15 min; after each centrifugation, the supernatant was transferred to a new centrifuge tube and filtered through a filter with a diameter of 0.22μm in order to remove microbubbles. The filtered liquid was then centrifuged by ultracentrifuge at 100000g at 4°C for 1.5h, and the resulting precipitate was hUCMSCs-exos. hUCMSCs-exos was identified by observing the morphology of hUCMSCs-exos using transmission electron microscopy and detecting the range of the particle size of hUCMSCs-exos using NTA.
Extraction and Identification of Rat MCCs
Four 4W SD male rats were taken, euthanized, and then the cartilage of rat condyle was peeled off, rinsed several times with sterile PBS containing 10% penicillin/streptomycin. Then the cartilage tissue was cut into pieces of about 1mm3; the pieces of tissue were spread flatly in T25 cell culture flasks that had been coated with gelatin. The pieces of tissue were also added to complete medium (89% MEMα + 10% FBS + 1% penicillin/streptomycin) and placed in a cell culture incubator at 37°C and 5% CO2. The rat MCCs of P2 were stained with toluidine blue to detect their morphology and structure.
Co-culture of hUCMSCs-exos with Rats MCCs
HUCMSCs-exos were labeled with 200-fold diluted Dil for 15min, and then centrifuge according to method 2.3 to obtain fluorescently labeled hUCMSCs-exos. hUCMSCs-exos labeled at a concentration of 6μg/ml was co-cultivated with rat MCCs at a density of 105/well for 10h in 24-well plates, and the original medium was removed, and the well plates were washed three times using PBS. The well plates were rinsed with PBS for 3 times, fixed with 4% paraformaldehyde for 15min, and rinsed with PBS. The MCCs were stained with 0.1% TritonX-100 permeabilized for 10min, and then the cytoskeleton was stained with ghost pen cyclic peptide for 30min. The nuclei were stained with diamidino-amidino-phenyl-indole (DAPI) for 12min, and the excess staining solution was rinsed with PBS for three times before and after each staining. Antifluorescence quencher was added, and the rat MCCs were observed for red fluorescence expression under fluorescence microscope.
Effect of hUCMSCs-exos on IL-1β Induced Rat MCCs Healing Ability by Scratch Assay
P3 rat MCCs were seeded in 6-well plates at 5×105/well density, and rat MCCs were divided into three groups as blank control group, TMJOA group, and Exos group. After rat MCCs were cultured for 24h, 200μl pipette gun heads were uesed to make the scratch to the bottom of the 6-well plate and all the groups replaced with the FBS-free medium. Besides, the blank control group, 10ng/ml IL-1β was added in the inflammation group, and 10 ng/ml IL-1β and 10μg/ml hUCMSCs-exos were added in the Exos group. Then pictures were taken at 0h and 24h to check the scratch closure of each group.
Effect of hUCMSCs-exos on the Proliferative Capacity of IL-1β Induced Rat MCCs
Rat MCCs were seeded in 96-well plates at 104/well density, grouped at 2.6 and processed accordingly, at 0h, 24h, 48h, all the wells added 10μl CCK-8 solution, and after 2h of incubation, the absorbance values at 450nm were assessed using a spectrophotometer.
The Repairation of hUCMSCs-exos to Cartilage of TMJOA Rats
Nine 4W SD male rats were also randomly divided into blank control group, TMJOA group and Exos group (n=3). The blank control group was injected 50μl PBS in the joint, and the TMJOA group and the Exos group were first injected 50μl CFA for inducing TMJOA in vivo. After 4w injection, the blank control and TMJOA groups were injected 50μl PBS into the rat joint cavity, and the Exos group was injected 50μl PBS containing 500μg hUCMSCs-exos. Rrats were killed after 2w injection intra-articular inflammatory changes and articular cartilage repair in each group were assessed by HE staining.
Statistical Analysis
Analysis of the data was performed using the GraphPad Prism 9.0 software. Measurement data were expressed using ±s and data differences between two groups were compared by t-test, and for multiple groups, one-way ANOVA was used. The difference in data was considered statistically significant if P<0.05.
Results
Characterization of hUCMSCs-exos
The morphology of hUCMSCs-exos was observed by transmission electron microscopy as a typical concave bilayer vesicle (Figure 1A), and the results of the NTA showed that the particle size of hUCMSCs-exos was mainly concentrated at 162nm (Figure 1B).
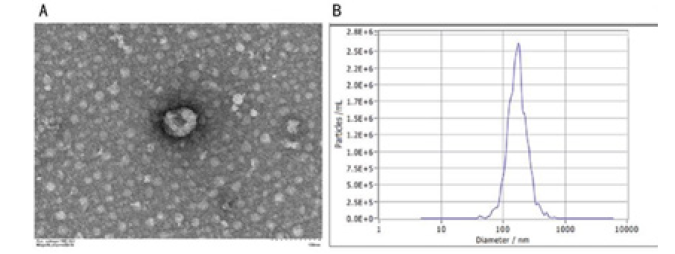
Figure 1: Characterization of hUCMSCs-exos (A)Transmission electron micrograph of hUCMSCs-exos (scale:100μm); (B)The NTA result of hUCMSCs-exos.
Characterization of Rats MCCs
Compared with unstained rats MCCs (Figure 2A) toluidine blue staining showed that rat MCCs were polygonal with bluish-purple nuclei containing a large number of heterostained granules (Figure 2B).
Rat MCCs Uptaken of Exosomes
By co-culturing exosomes with rat MCCs for 10h, it was seen that hUCMSCs-exos labeled with red could be taken up into the cells by rat MCCs and aggregated around the nuclei of the cells (Figure 3).
HUCMSCs-exos Enhances the Migratory Ability of Rat MCCs Induced by IL-1β
As seen by the cell migration assay, the cell scratch area was significantly reduced and more cells migrated toward the middle of the scratch in the exosome-treated group compared with the inflammation group (P<0.001) (Figure 4A). hUCMSCs-exos improved the migration ability of rat MCCs induced by IL-1β (Figure 4B).
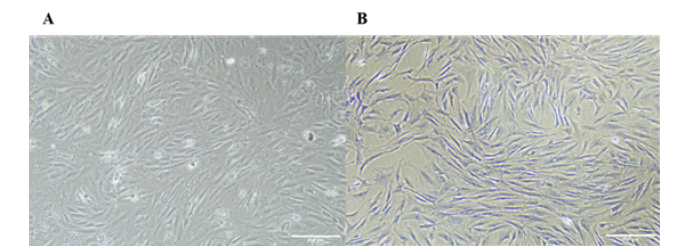
Figure 2: Characterization of rats MCCs (A) Rats MCCs without toluidine blue staining ; (B) Rats MCCs after toluidine blue staining. (Scale: 200μm).
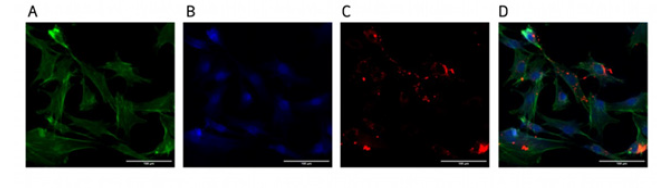
Figure 3: Rat MCCs uptaken of exosomes hUCMSCs-exos were co-cultured with rat MCCs for 10h and observed under fluorescence microscope, (A) Ghost pen cyclic peptide green labeled cytoskeleton; (B) DAPI blue labeled nucleus; (C) Dil red labeled exosome; (D) Exosomes can be uptaken by the cells and aggregated around the nucleus (scale: 100μm).
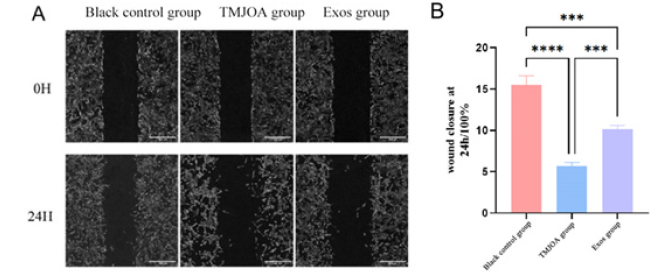
Figure 4: Results of rat MCCs scratch experiment under hUCMSCs-exos intervention (A-B)The scratch healing was observed under an inverted microscope at 0h and 24h, and the scratch healing area was analyzed in each group.
Note*: ***P<0.001 ****P<0.0001(scale: 200μm)
HUCMSCs-Exos Increased the Proliferation Ability of Rat MCCs Induced by IL-1β
The results of CCK8 showed that the cell proliferation capacity of the three groups was unchanged at 0h and 24h. At 48h, compared with the blank control group, the cell proliferation capacity of the TMJOA group was significantly decreased (P<0.05), and the Exos group was significantly higher than TMJOA group (P<0.01), and there was no difference from the blank control group. HUCMSCs-Exos increased the proliferation capacity of rat MCCs induced by IL-1β (Figure 5).
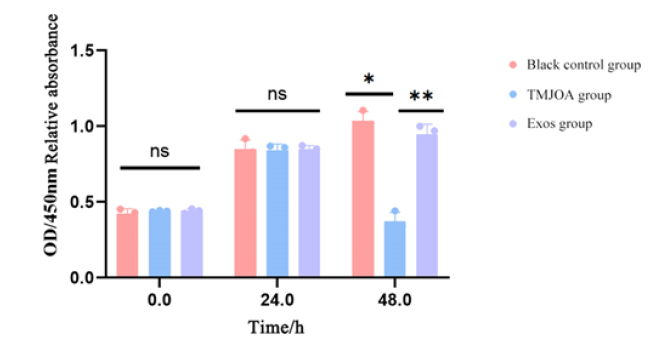
Figure 5: Effect of hUCMSCs-exos on the proliferation capacity of rat MCCs induced by IL-1β Relative absorbance of different experimental groups at different time points at OD 450nm.
Note*: *P<0.05 **P<0.01.
HUCMSCs-exos has A protective Effect on Condylar Cartilage Under TMJOA in Rats
The main pathological changes of TMJOA are progressive cartilage degradation, loss, and abnormal remodeling of subchondral bone. By HE staining, concave defects, roughness, deformation, reduction of chondrocytes, disorganization of hierarchical arrangement, and loss of subchondral bone tissue could be seen on the surface of the condyle in the TMJOA group compared with the blank control group (Figure 6B). In contrast, the condylar surface of the Exos group injected with hUCMSCs-exos in vivo was more continuous, with no obvious defects, more neatly arranged chondrocytes, and less inflammatory changes (Figure 6C).
Discussion
The main pathological manifestation of TMJOA is the destruction of articular cartilage, and the main functional cells of articular cartilage are MCCs [15]. Existing studies have shown that MCCs are one of the main mediators of the pathological changes that occur in TMJOA, and under the action of multiple adverse stimuli, MCCs produce a variety of cytokines and proteases, which result in the degradation of the cells and the matrix [16,17]. Exos, which is secreted by cellular secreted vesicles with diameters ranging from 50~200nm, which are important regulators of intercellular communication, can mediate intercellular communication by delivering bioactive molecules, and are involved in a variety of pathological processes [18]. Due to their small size, good targeting, stability, ability to cross barriers and deliver drugs into the cytoplasm.
Exos uptake cytosis by target cells is the basis of exos regulation of target cells and function [19]. In this study, the uptake of hUCMSCs-exos by rat MCCs was examined, and the results showed that hUCMSCs-exos co-cultured with rat MCCs for 10h was seen to be Dil-stained into rat MCCs and surrounded the nucleus. The uptake assay indicated that hUCMSCs-exos could be cytosolized and taken up by rat MCCs, laying the foundation for its regulation of the biological activities of rat MCCs.
Studies have shown that IL-1β is closely related to the development of TMJOA, which can promote the secretion of a variety of inflammatory factors, increase the level of collagenase, promote the degradation of cartilage matrix, and facilitate the inflammatory response, leading to the death of MCCs, which exacerbates the development of TMJOA [20-22]. Because human condylar cartilage tissue is difficult to obtain clinically, this study used IL-1β to induce rat MCCs and simulate the inflammatory internal environment of MCCs in vitro.
The persistent inflammatory internal environment caused by TMJOA can lead to massive death of MCCs and also inhibit the migration of MCCs, thus the ability of MCCs to migrate to the defect site has an important impact on the treatment of TMJOA. In this study, a scratch assay was used to examine the effect of hUCMSCs-exos on the migration ability of MCCs in rats induced by IL-1β. The experimental results showed that the Exos group had a significantly smaller area in the middle of the scratch compared with the TMJOA group and more MCCs migrated to the inner part of the scratch, which indicated that hUCMSCs-exos could promote the migration of MCCs in the rat under the induction of IL-1β. hUCMSCs-exos has the potential to promote the migration of MCCs to the injury site and may have the ability to promote TMJOA cartilage repair.
The inflammatory state also affects the proliferative capacity of MCCs, leading to a lack of self-renewal to ensure tissue repair. In this study, CCK8 was used to detect the effect of hUCMSCs-exos on the proliferative capacity of rat MCCs induced by IL-1β. The experimental results showed that at 24h after the addition of IL-1β induction, there was no significant difference in cell proliferation ability among the three groups, and at 48h after the addition of IL-1β induction, compared with the control group and the exosome treatment group, there was a significant decrease in cell proliferation ability in the inflammation group, and there was no difference in cell proliferation ability between the exosome treatment group and the control group. It indicates that hUCMSCs-exos can promote the proliferative ability of rat MCCs under IL-1β induction and promote tissue repair.
The main pathological changes of TMJOA are progressive cartilage degradation, loss, and abnormal remodeling of subchondral bone [23-25]. In this study, CFA injection was chosen as a method for modeling TMJOA in vivo. In the in vivo experiments, by HE staining, we found that compared with the blank control group, the condylar surface of the TMJOA group showed typical TMJOA pathological changes such as defects, deformations, chondrocytes becoming fewer and disorganized, and loss of subchondral bone. The condylar surface of the Exos group injected with hUCMSCs-exos was more continuous, with no obvious defects, and the chondrocytes were more neatly arranged, indicating that the in vivo injection of hUCMSCs-exos could reduce the destruction of condylar cartilage in the state of inflammation, maintain a relatively stable state, and repair the damage of the cartilage caused by inflammation.
Conclusions
In summary, in this study, we demonstrated that hUCMSCs-exos can improve the migration ability and cell proliferation ability of rat MCCs induced by IL-1β, and in vivo, it can protect and repair the condylar cartilage damaged by inflammatory state in rats. This study preliminarily investigated the repairing effect of hUCMSCs-exos on the regeneration of condylar cartilage in rat TMJOA, which is expected to provide a basis for the future clinical application of hUCMSCs-exos in TMJOA.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Fundings
This research received no external funding.
Author Contributions
Conceptualization, Hao Zhou; Data curation, Yajie Qi; Formal analysis, Yajie Qi; Investigation, Hao Zhou; Methodology, Hao Zhou and Yajie Qi; Software, Hao Zhou and Yajie Qi; Validation, Hao Zhou ; Visualization, Yajie Qi; Writing-original draft, Hao Zhou and Yajie Qi; Writing-review & editing, Yingxin Xu.
References
- Bansal M (2016) Prevalence and diagnostic features of osteoarthrosis of the temporomandibular joint: A review. International Journal of Research in Orthopaedics 2(1): 1-4.
- Li B, Guan G, Mei L, Kai Jiao, Huang Li (2021) Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med 25(11): 4902-4911.
- Wang D, Qi Y, Wang Z, Anyun Guo, Yingxin Xu, et al. (2023) Recent advances in animal models, diagnosis, and treatment of temporomandibular joint osteoarthritis. Tissue Eng Part B Rev 29(1): 62-77.
- Liang C, Gao S, Gao J, Yanwen Xu, Qilong Li (2023) Comparison of effects of HucMSCs, exosomes, and conditioned medium on NASH. Sci Rep 13(1): 18431.
- Liu H, Huang Y, Huang M, Zhijie Huang, Qin Wang. et al. (2022) Current status, opportunities, and challenges of exosomes in oral cancer diagnosis and treatment. Int J Nanomedicine17: 2679-2705.
- Yuan W, Wu Y, Huang M, Xueman Zhou, Jiaqi Liu, et al. (2022) A new frontier in temporomandibular joint osteoarthritis treatment: Exosome-based therapeutic strategy. Front Bioeng Biotechnol 10:1074536.
- Zhang Z, Zhao S, Sun Z Chuanxing Zhai, Jiang Xia, et al. (2023) Enhancement of the therapeutic efficacy of mesenchymal stem cell-derived exosomes in osteoarthritis. Cell Mol Biol Lett 28(1): 75.
- Cao H, Chen M, Cui X, Yuan Liu, Yuhan Liu, et al. (2023) Cell-Free osteoarthritis treatment with Sustained-Release of Chondrocyte-Targeting exosomes from umbilical Cord-Derived mesenchymal stem cells to rejuvenate aging chondrocytes. ACS Nano 17(14): 13358-13376.
- Ogasawara N, Kano F, Hashimoto N, H Mori, Y Liu, et al. (2020) Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthritis Cartilage 28(6): 831-841.
- Zhang S, Teo K, Chuah S J, Ruenn Chai Lai, Sai Kiang Lim, et al. (2019) MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200: 35-47.
- Liu Q, Wang R, Hou S, Feng He, Yuanjun Ma, et al. (2022) Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res Ther 24(1): 44.
- Mianehsaz E, Mirzaei H R, Mahjoubin Tehran M, Alireza Rezaee, Roxana Sahebnasagh, et al. (2019) Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis. Stem Cell Res Ther 10(1): 340.
- Liu Y, Zhang Z, Wang B, Yunsheng Dong, Congrui Zhao, et al. (2022) Inflammation-Stimulated MSC-Derived small extracellular vesicle miR-27b-3p regulates macrophages by targeting CSF-1 to promote temporomandibular joint condylar regeneration. Small 18(16): e2107354.
- Wang Y, Zhao M, Li W, Yuzhi Yang, Zhenliang Zhang, et al. (2021) BMSC-Derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis via Autotaxin-YAP signaling axis. Front Cell Dev Biol 9: 656153.
- Liu X, Li H, Feng Y, Huilin Guo, Yingjie Li, et al. (2023) Resatorvid alleviates experimental inflammatory TMJOA by restraining chondrocyte pyroptosis and synovial inflammation. Arthritis Res Ther 25(1): 230.
- Wei J M, Tu S Q, Wang Y X, Sai Zhang, Yi Feng, et al. (2023) Clock gene Per1 regulates rat temporomandibular osteoarthritis through NF-kappaB pathway: An in vitro and in vivo study. J Orthop Surg Res 18(1): 817.
- Liu X, Li Y, Zhao J, Zhihui Hu, Wei Fang, et al. (2023) Pyroptosis of chondrocytes activated by synovial inflammation accelerates TMJ osteoarthritis cartilage degeneration via ROS/NLRP3 signaling. Int Immunopharmacol 124(Pt A): 110781.
- Ren S, Lin Y, Liu W, Liqun Yang, Muxin Zhao, et al. (2023) MSC-Exos: Important active factor of bone regeneration. Front Bioeng Biotechnol 11: 1136453.
- Zhu S, Huang H, Liu D, Shimin Wen, Liangliang Shen, et al. (2022) Augmented cellular uptake and homologous targeting of exosome-based drug loaded IOL for posterior capsular opacification prevention and biosafety improvement. Bioact Mater 15: 469-481.
- Shao B, Xu Y, Jia M, Chen-Xi Li, Zhong Cheng Gong, et al. (2023) Association of HMGB1 levels in synovial fluid with the severity of temporomandibular joint osteoarthritis. BMC Musculoskelet Disord 24(1): 183.
- Zhou L, Chen D, Liu P, Lei Chen, Yucheng Su (2022) MiR-132-3p participates in the pathological mechanism of temporomandibular joint osteoarthritis by targeting PTEN. Arch Oral Biol 142: 105511.
- Cheng B, Zhang J, Shen Q, Zheyi Sun, Yingwei Luo, et al. (2024) Liproxstatin-1 alleviates cartilage degradation by inhibiting chondrocyte ferroptosis in the temporomandibular joint. Biol Cell 116(1): e202300042.
- Li Y Y, Feng Y P, Liu L, Xing Long (2022) Inhibition of HMGB1 suppresses inflammation and catabolism in temporomandibular joint osteoarthritis <em>via</em> NF-kappaB signaling pathway. Eur J Histochem 66(3): 3357.
- Feng Y, Hu S, Liu L, Jin Ke, Xing Long, et al. (2021) HMGB1 contributes to osteoarthritis of temporomandibular joint by inducing synovial angiogenesis. J Oral Rehabil 48(5): 551-559.
- He Y, Zhou M, Jian Z, Lingli Fang, Lan Huang, et al. (2022) C-Reactive protein knockout attenuates temporomandibular joint inflammation in rats. J Immunol Res 2022: 8613986.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.