Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
An Unusual Case of Secondary Recurrent Idiopathic Hemophagocytic Lymphohistiocytosis (HLH) in a Middle-Aged Male Patient
*Corresponding author: Leonard Ranasinghe, MD, California Northstate University College of Medicine, 9700 W. Taron Dr., Elk Grove, California, 95757, USA.
Received: April 23, 2024; Published: June 11, 2024
DOI: 10.34297/AJBSR.2024.22.003019
Abstract
Hemophagocytic Lymphohistiocytosis (HLH) is a life-threatening, rapidly progressing disease, characterized by a hyperinflammatory cytokine storm. HLH is divided into primary and secondary causes, the former occurring mainly in children due to genetic causes and the latter occurring mainly in adults due to immune dysregulation such as malignancies and viral infections. This study is a rare case of HLH in a healthy, young adult male who had a recurrence after an initial diagnosis of HLH and subsequent death secondary to hepatic encephalopathy. Our goal is to contribute to the limited body of knowledge regarding this rare condition and to improve the efficiency of providing potential HLH patients with an accurate diagnosis. A comprehensive literature review was conducted to find retrospective analyses, other similar case studies, and information on the etiology of HLH that would give insight into the clinical manifestations of this patient. Our patient was initially hospitalized for recurrent fever with unknown etiology and was diagnosed with HLH. The patient had been compliant with treatment. Laboratory workup showed leukocytosis, neutrophilia, thrombocytopenia, acute liver failure, hyponatremia, and hyperferritinemia. Initial treatment included etoposide, anakinra, and dexamethasone with initial improvement; however, the patient exhibited another HLH flare after dexamethasone was tapered down with the goal of completing a lymph node biopsy. This was carried out but provided inconclusive results. He was then empirically started on chemotherapy for a suspected underlying lymphoma. Soon after, the patient was admitted to the ICU due to worsening multiorgan failure and multifactorial encephalopathy as a result of liver failure and uremia and was subsequently intubated and passed away soon after. Although initial treatments improved his symptoms, his condition worsened, ultimately leading to the patient’s death. Our case suggests that, even with the improvement of symptoms with treatment, determining the secondary cause is crucial in the effective treatment of the disease.
Keywords: Hydrocephalus; Cerebral aqueduct stenosis; External ventricular drain; Factor VII deficiency
Abbreviations: AFB: Acid Fast Bacillus Test; ALP: Alkaline Phosphatase; ALT: Alanine Amino Transferase; ANCA: Anti-Neutrophil Cytoplasmic Antibody; AST: Aspartate Amino Transferase; CMV: Cytomegalovirus; CNS: Central Nervous System; CRP: C-Reactive Protein; CRP: C-reactive Protein; CT: Computed Tomography; CXR: Chest X-ray; EBV: Epstein-Barr Virus; Hct: Hematocrit; Hgb: Hemoglobin; HIV: Human Immunodeficiency Virus; IL: Interleukin; LDH: Lactose Dehydrogenase; LFT: Liver Function Test; MRI: Magnetic Resonance Imaging; OSA: Obstructive Sleep Apnea; PMH: Past Medical History; PMH: Past Medical History; Prothrombin Time: PT; RBC: Red Blood Cells; TB: Tuberculosis; WBC: White Blood Cell
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a highly aggressive, life-threatening disease that is associated with abnormal activation of monocytes or macrophages. It is characterized by a hyper-inflammatory cytokine storm of IL-2, IL-6, IL-10, IL-12, IL-18, MCSF, IFN-gamma, and TNF-alpha. If not managed promptly, hypercytokinemia can lead to multi-organ failure and eventually death.
This disease is divided grossly into primary (or familial) and secondary HLH. Primary HLH accounts for about 25% of all HLH cases. [23] It is most often present in children who have genetic mutations that impair the cytotoxic function of natural killer and cytotoxic T cells. These are referred to as familial HLH (fHLH) where patients have genetic mutations in Perforin/PRF1 [1], MUNC 13-4/UNC13D [2], MUNC 18-2/STXBP2 [3], Syntaxin11/STX11 [4]. Of all the familial HLH cases, the FHL-2 subclass accounts for 58% of all familial HLH cases [14]. Secondary HLH is more predominant in adults and is not associated with known genetic mutations that predispose one to HLH and may have a sporadic onset at any age.
The onset of secondary HLH in adults is associated with a multitude of conditions. Usually, HLH in adults is associated with a predisposing condition such as malignancy and autoimmunity that causes immune dysregulation and a trigger typically from viral infections. Malignancies, particularly hematologic ones, such as T-cell lymphomas, B-cell lymphomas, and NK-cell lymphomas, have been strongly associated with adult HLH. The EBV of the Herpesviridae family and the viral agent responsible for infectious mononucleosis, in particular, is found to have a strong association with triggering HLH. Other infectious etiologies associated with secondary HLH include CMV, HIV, Mycobacterium tuberculosis, Rickettsia, Leishmania, and Histoplasma [15].
Clinically, HLH classically presents in patients with a few of the following symptoms: high fever, splenomegaly, cytopenia, enlarged lymph nodes, platelets (< 100 x 109/L), neutropenia (< 1.0 x 109/L), hypertriglyceridemia (≥ 3.0 mmol/L or 265 mg/dL), hypofibrinogenemia (≤ 1.5 g/L), and hyperferritinemia [5]. On bone marrow biopsy, hemophagocytosis may be present; however, this is neither a sensitive nor a specific marker for HLH. Additionally, some symptoms are more specific to adults in HLH such as transaminitis, coagulopathy, elevated LDH, rash, hyponatremia, elevated CRP, and neurologic involvement such as encephalopathy, headache, and seizures [6-10].
Current treatment strategies for secondary HLH in adults are significantly limited as the guidelines that are currently used were established for the treatment of primary HLH in children. Some of the treatment methods are even considered controversial when transferred over to treating adult patients with HLH. Thus, it is no surprise that treatments for adult HLH are highly variable between different facilities [11]. In general, however, initial treatments begin with high-dose corticosteroids such as glucocorticoids or dexamethasone and immunosuppressive agents such as cyclosporine or cyclophosphamide [5,11]. Etoposide has also been recommended in pediatric HLH; however, this has been debated for adult patients [11,12]. Following initial treatments, supportive measures for symptoms caused by HLH such as anemia, thrombocytopenia, or organ failures, and treatment for the underlying cause are fundamental. Thus, it is crucial to find the underlying cause of HLH to better manage the disease and symptoms. According to the HLH-94 guidelines for the treatment of HLH, the recommended protocol consists of an 8-week course of etoposide and tapering doses of dexamethasone [13]. In our case patient, after the initial treatment, once the dexamethasone was tapered off to assist with the lung biopsy for a suspected lymphoma, the patient developed rising ferritin levels, LFTs, and a recurrent fever.
Much of what is known about HLH in adults (secondary) is derived from children (primary), although awareness and research regarding HLH in adults is increasing. Currently, the mechanism underlying the pathologies of HLH remains poorly understood. The median survival rate falls within a few months, varying due to the etiology of secondary HLH [16,17]. Prognostic factors such as malignancy and low serum albumin levels predict worse survival, whereas non-malignant associated HLH favors better survival [17]. In addition, the challenges of diagnosis due to the diverse presentations from varying etiologies further contribute to the poor survival of adult HLH [15]. Oftentimes, the clinical presentation of HLH is mistaken for the more common sepsis, which delays both the diagnosis and treatment of patients who have HLH. Therefore, this study seeks to contribute to a better understanding of HLH to improve outcomes for patients with potential secondary HLH.
Case Report Summary
A 40 y/o male patient with PMH of obesity, gout, and OSA initially presented to the ED with a chief complaint of a 10-day fever (102F), mild headaches, and fatigue. The patient has no history of drug abuse and is a non-smoker. He used alcohol occasionally and also had nephrotic syndrome or glomerulonephritis when he was about fifteen years old which was treated successfully. The patient’s travel history was also unremarkable. Upon physical examination the patient was febrile (36.7 °C), had a respiratory rate of 19 breaths per minute, blood pressure of 116/82 mmHg, and heart rate of 100 beats per minute.
Laboratory evaluation revealed WBC of 2,600/µL (normal range 3,700-11,100), platelet count of 70,000/µL (normal range 150,000-450,000), AST 641 U/ml (normal range 10-40), ALT of 405 U/ml (0-47). The following day, the patient had a Hgb level of 12.5 g/dl (normal range 13.0-17), hematocrit of 36.4% (normal 39.0-51.0), AST 648 U/ml, ALT of 446 U/ml, ALP of 168 U/ml (normal 44-147 U/L), total bilirubin of 1.3mg/dL (normal range: 0.2-1.2), and direct bilirubin of 0.5 (normal <0.3mg/dL). In the following days after, the patient’s liver enzymes (AST, ALT, ALKP, bilirubin) then decreased toward the normal range, the WBC count reached normal levels, and the hemoglobin and hematocrit levels remained below the normal range. Autoimmune antibody screening (anti-Sjogren's Syndrome A/B, anti-ribonucleoprotein, anti-nuclear antibodies, anti-centromere antibodies, anti-chromatin antibodies, anti-Jo-1 antibodies, anti-dsDNA antibodies, rheumatoid factor, and ANCA) were all negative.
A CXR revealed a new opacity in the right middle lung, which was suspicious for pneumonia. The patient was subsequently prescribed amoxicillin and doxycycline, which was later switched to Augmentin and Azithromycin due to lack of improvement with the former treatment. Additional labs such as CBC show leukocytosis, bandemia, and thrombocytopenia. During the next few days, the patient developed new leukopenia, and an enlarged fatty liver (hyperechoic on ultrasound). The patient’s acute leukopenia and thrombocytopenia were suspected of either infectious cause or immune thrombocytopenia purpura (ITP), with malignancy also on the differential.
A bone marrow biopsy revealed histiophagocytosis, suspecting HLH as the new diagnosis. Thus, labs were then sent out to seek the cause of HLH, namely infectious, malignancy, or drug induced. Infectious labs were unremarkable aside from a positive TB quantiferon test with 3 negative AFB results. In consideration of latent TB infection, isoniazid treatment was considered to begin in 6 weeks. Other infectious etiologies considered included EBV, CMV, fungal, parasitic, and bacterial infections. 17 days after the discharge, the patient was re-hospitalized with fever, headache, and palpitations [LR7] since the night before. The patient had been compliant with dexamethasone taper, ruxolitinib, and Bactrim. The temperature was 39.3 °C at the time of admission.
Laboratory workup on the day [LR8] [LR9] of the Emergency Department (ED) visit showed leukocytosis, neutrophilia, thrombocytopenia, acute liver failure, and hyperferritinemia. The white blood cell count was slightly elevated at 12,700/μL. The platelet count was significantly decreased at 76,000 as was the level of fibrinogen at 186 mg/dL (normal range: 209-504). PT was elevated at 16.9 seconds. Hemoglobin and hematocrit values were normal, ruling out anemia. The ferritin level was at 121,674 μg/L (reference value: 10-120 μg/L). Liver enzyme levels were also elevated including AST and ALT at 1228 U/mL and 794 U/mL respectively. The patient also was hyponatremic with a blood sodium level of 127 mEq/L. The lung findings might also be relevant to note here.
The patient was treated with etoposide, anakinra, and dexamethasone with initial improvement, but due to having multiple bland biopsies done, without any conclusive diagnosis for the underlying cause of his HLH, it was decided that his dosage of dexamethasone be tapered down in order to improve the diagnostic power/accuracy of the biopsies. However, the patient began to exhibit test results concerning an HLH flare, which included recurrent fevers, an increase in ferritin levels, and worsening liver function tests (AST and ALT). The patient was then subsequently empirically started on chemotherapy for a suspected underlying lymphoma. He was given a regimen of gemcitabine, cyclophosphamide, and etoposide.
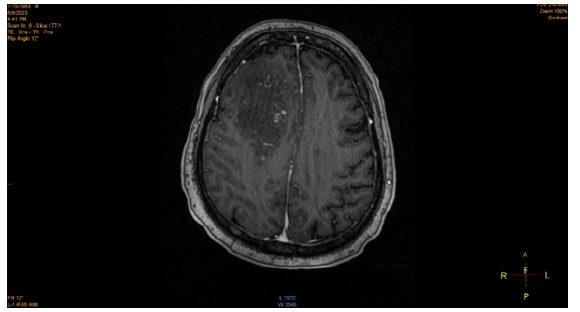
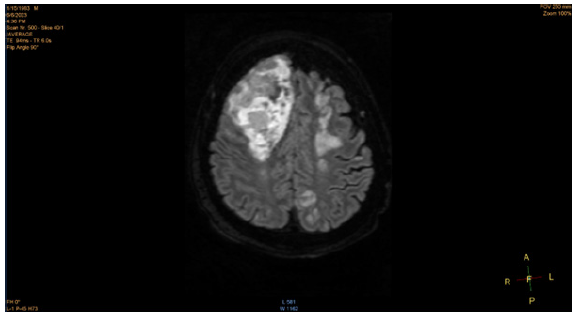
The patient was admitted to the ICU due to worsening multiorgan failure and multifactorial encephalopathy as a result of liver failure and uremia and was subsequently intubated. However, even with minimal sedation, the patient became unresponsive, prompting a CT scan to be ordered. The head CT scan and subsequent MRI scan revealed hypodensities in the upper bifrontal cortex and subcortical white matter, sub-centimeter dense foci in the right subcortical white matter, lobar hemorrhage in the right anterior lobe, and multiple scattered microhemorrhages in the inferior right frontal, left superior frontal, bilateral parietal lobes. In addition to multiple hyperintense signals and humerus cystic spaces seen in the MRI, a poor prognosis of the patient was confirmed. He developed pupil dilation concerning a brain herniation 39 days after hospital admission, which was confirmed by another head CT scan. He was later extubated and passed away after 42 days in the hospital.
Discussion
CNS Involvement in HLH
Due to the manner of this patient’s death, it is crucial to understand the underlying mechanism for CNS involvement in HLH, due to the potentially life-threatening risks that this clinical manifestation can pose to patients.
Central nervous system (CNS) involvement with HLH is a common occurrence and can be characterized by disturbance of consciousness, headaches, dizziness, seizures, and other psychiatric symptoms. Neurological abnormalities often occur in about a third of all HLH patients. [22] In primary HLH, the FHL-3 subclass is often associated with a greater risk of CNS involvement. In one retrospective analysis of 96 HLH patients, 52.1% of the total patients (71.4% of the patients who received brain imaging examinations) exhibited imaging changes. The most common locations of the imaging changes were in the bilateral white matter, basal ganglia, ventricles, sulcus, and meninges [18,19].
While the exact etiology of the CNS involvement in HLH is not fully understood, one of the contributing factors for the neurological symptoms experienced by HLH may be a result of hepatic encephalopathy, resulting from the accumulation of neurotoxins in the brain from acute liver failure. Neurological manifestations, resulting from acute liver failure, can range from disorientation, seizures, intracranial hypertension, and in severe cases, brain herniation, which can result in coma and death. One of the likely neurotoxins that contribute to these neurological manifestations is ammonia (NH4+). Ammonia readily diffuses across the blood-brain barrier (BBB) and accumulates due to urea synthesis impairment in the liver, which would normally metabolize ammonia into readily excreted waste. [20] The brain lacks the ability for urea synthesis, and as a result, ammonia accumulates, prompting astrocytes to clear the excess ammonia via glutamine synthesis. However, the accumulation of glutamine exhibits an osmotic effect, causing swelling of astrocytes, which results in brain edema. In severe cases, this edema can result in an increase in intracranial pressure (ICP) and brain herniation. Additionally, inflammatory cytokines released by microganglia and astrocytes can further contribute to tissue damage. [20] In our patient, his death was ruled as a result of multifactorial encephalopathy from multi-organ failure.
Another risk factor for neurotoxicity may be treatment for HLH itself, especially with the use of cyclosporine. In another retrospective chart review, 5 out of 17 HLH patients from Texas Children’s Hospital developed severe neurotoxicity, with cyclosporine outside of the therapeutic range for 2 of the 5 patients. One of these patients died of intracerebral hemorrhage. In all five patients, all exhibited an increase in systolic blood pressure above the 95th percentile within 24 hours of the onset of any neurological symptoms. According to the HLH-2004 protocol, cyclosporine is prescribed at the time of diagnosis for immunosuppression. Since cyclosporine is metabolized in the liver and mediated by CYP3A4, acute liver failure or injury as a result of HLH, can result in an increase of circulating levels of the calcineurin inhibitor. [18] In one review, the use of cyclosporin A results in neurological side effects in 40% of patients, which include altered mental status, seizures, headaches, peripheral neuropathy, encephalopathy, and white matter changes in the posterior regions of the brain. This is in tandem with other modifiable risk factors, such as hypertension [21].
Conclusion
HLH is a disease that has not been widely studied, mainly due to its rarity and the rapid progression of its clinical manifestations. The speed of progression that symptoms present themselves in patients diagnosed with HLH creates difficulty in initial diagnosis and subsequent treatment. The recurrent nature of this patient’s symptoms and the involvement of CNS in the death of this patient were unique aspects of the case. There is limited documentation of HLH patients having a recurrence of symptoms after undergoing treatment. In our case, this is complicated by the physicians’ attempt to take biopsy samples to confirm a suspected lymphoma underlying the HLH. There is limited to no literature on the effects of tapering dexamethasone on the risk of having a recurrence of secondary HLH.
Furthermore, it is difficult to identify the exact mechanism underlying the CNS involvement in this case. Most likely, there is a multifactorial etiology to the brain edema and herniation experienced by the patient. It is difficult to consider all factors that contributed to the onset of symptoms, secondary recurrence, and death of the patient. This opens the door for greater awareness of this disease, as well as the need for further scrutiny and research into its etiology and progression (Figures 1,2).
Authors offer their sincere thanks to the wife of this patient for providing most of the case details.
Conflict of interest
No conflicts of interest are indicated in this case report.
Acknowledgements
Authors offer their sincere thanks to the deceased patient’s wife for her contributions providing copies of medical records and imaging studies for this case report.
References
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, et al. (1999) Perforin gene defects in familial hemophagocytic lymph histiocytosis. Science 286(5446): 1957-1959.
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, et al. (2003) Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115(4): 461-473.
- zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. (2009) Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 85(4): 482-492.
- zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, et al. (2005) Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 14(6): 827-834.
- Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, et al. (2007) HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48(2):124-131.
- Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, et al. (2014) Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 66(9): 2613-2620.
- Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP (2014) Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc 89(4): 484-492.
- Tsuda H (1997) Hemophagocytic syndrome (HPS) in children and adults. Int J Hematol. 65(3): 215-226.
- Hejblum G, Lambotte O, Galicier L, Coppo P, Marzac C, Aumont C, Fardet L (2014) A web-based delphi study for eliciting helpful criteria in the positive diagnosis of hemophagocytic syndrome in adult patients. PLoS One. 9(4): e94024.
- Gratton SM, Powell TR, Theeler BJ, Hawley JS, Amjad FS, Tornatore C (2015) Neurological involvement and characterization in acquired hemophagocytic lymphohistiocytosis in adulthood. J Neurol Sci 357(1-2): 136-142.
- Hayden A, Park S, Giustini D, Lee AY, Chen LY (2016) Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev 30(6): 411-420.
- Li J, Wang Q, Zheng W, Ma J, Zhang W, Wang W, Tian X (2014) Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 93(2): 100-105.
- B Salunke, S Savarkar, VP Patil (2019) “Hemophagocytic Syndrome-An Approach to the Management,” Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 23(3): S191-S196.
- MR George (2014) “Hemophagocytic lymphohistiocytosis: review of etiologies and management,” J. Blood Med Pp. 5: 69-86.
- Allen CE, McClain KL (2015) Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematology, 2015(1): 177-182.
- Otrock ZK, Eby CS (2015) Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. American Journal of Hematology 90(3): 220-224.
- Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP (2014) Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clinic Proceedings, 89(4): 484-492.
- (2023) Severe Neurologic Side Effects in Patients Being Treated for Hemophagocytic Lymphohistiocytosis - PMC.
- (2023) Central Nervous System Involvement in Hemophagocytic Lymphohistiocytosis in Adults: A Retrospective Analysis of 96 Patients in a Single Center - PMC.
- AC Anand, P Singh (2019) “Neurological Recovery After Recovery From Acute Liver Failure: Is it Complete?,” J. Clin. Exp. Hepatol 9(1): 99-108.
- JM Gijtenbeek, MJ van den Bent, and CJ Vecht (1999) Cyclosporine neurotoxicity: a review,” J. Neurol 246(5): 339-346.
- G Cai, Y Wang, X Liu, Y Han, Z Wang (2017) Central nervous system involvement in adults with haemophagocytic lymphohistiocytosis: a single-center study, Ann. Hematol 96(8): 1279-1285.
- (2023) Hemophagocytic Lymphohistiocytosis: A Diagnostic Conundrum - PMC.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.