Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Comparative Analysis of Antimicrobial and in Vitro Cytotoxic Studies in Regenerative and Antioxidant- Containing Products
*Corresponding author: Bahruz Mammadov Samad, Department of Pharmaceutical technology and management, of Azerbaijan Medical University, Baku, Azerbaijan.
Received: May 28, 2024; Published: June 04, 2024
DOI: 10.34297/AJBSR.2024.22.003004
Abstract
Objectives: The aim of the research work is to carry out an ultrasonic extraction process (Stegler DG-360) under the influence of 95% ethanol and ethanol-propylene glycol mixture(in the 95:5 ratio) from Clove buds (Caryophyllos aromaticus L.), hibiscus flowers (Hibiscus sabdariffa L.) and yarrow plants(Achillea millefolium L.), which are rich in biological substances with a high therapeutic effect, in the obtained phytoextracts and in the samples of the ointment prepared on the basis of that phytoextract, antimicrobial activity and in vitro cytotoxicity studies.
Materials and Methods: Phytoextracts containing volatile essential oils (eugenol, acetyleugenol, caryophyllene, etc.) and phenolic and flavonoid compounds (rutin, quercetin, kaempferol, hibicetin, gallic acid, quinic acid, etc.) - antibacterial and antifungal activity was studied by diffusion method. An in vitro cytotoxicity study (MTT method) was carried out with the optimal composition of phytoextract and ointment sample with higher antimicrobial activity.
Results: High antibacterial and antifungal activity was observed in the samples of phytoextract with optimal content and high antioxidant and antibacterial properties, and the ointment prepared based on this phytoextract. Especially the 3rd ointment sample showed the highest antibacterial and antifungal activity compared to other ointment samples and control samples. The in vitro cytotoxicity levels of that ointment sample and phytoextract were studied and it was determined that phytoextract had a cytotoxic effect at a dose of 1 mg/ml, and the ointment sample had a cytotoxic effect at a dose of 500 mg/ml.
Conclusion: Both the studied phytoextract and the 3rd ointment sample had a sufficient antibacterial and antifungal effect in terms of the phytochemical content of the composition. This showed the necessity of preparation and application of various medicinal forms with antioxidants and regenerating effect from those samples.
Keywords: Antioxidant and Antimicrobial activity, Phytocomposition, Cytotoxicity Cell viability, Regenerative effect
Introduction
Cell viability and cytotoxicity assays play an important role in the screening of active ingredients in pharmaceuticals and in the evaluation of the cytotoxic effects of various chemicals [1]. Testing of cell viability and evaluation of quality criteria is the basis for conducting other in vitro studies. Over the past decade, many research studies have developed different guidelines for determining and interpreting cell viability and cytotoxicity based on morphological, biochemical, and functional perspectives [2]. This method is important in the assessment of cell viability and development, as well as in determining the killing power of active substances at the cellular level. This method is also based on cell division or biochemical elements, with particular attention to the mechanical and fundamental aspects of the process. [3,4]. Assays included metabolic activity, enzyme activity, cell membrane permeability and integrity, adenosine 5′-triphosphate content, cell adhesion, reduction equivalents, dye incorporation or exclusion, constitutive protease activity, colony formation and DNA formation, and nuclear fragmentation based on different cellular functions. These assays provide simple, reliable, sensitive, reproducible, cost-effective and high throughput approaches to assess the effect of newly developed chemotherapeutic biomolecules on cell viability during the formulation process. [5,6].
Determining the in vitro cytotoxic effects of the main active ingredient of any medicine, cosmetic products, chemical (such as pesticides, endocrine disruptors, heavy metals, nanoparticles), physical or biological factors of the environment by modern methods has become of great importance in recent years. There are many methods for determining cytotoxicity or cell viability. These methods are classified as staining
i. Colorimetric
ii. Fluorometric
iii. Luminometric
iv. Different methods for determining apoptosis
v. and methods for determining autophagy [7,8].
Which of these methods is more detailed and accurate can be evaluated by researchers using all scientific data. When making a comparison, the physical and chemical properties of the analyzed substance, especially the antimicrobial (antibacterial and antifungal) properties of that substance, the mechanism by which the substance causes cell death, the originality and sensitivity of the method should be taken into account [9]. Currently, MTT method is one of the most widely used cell viability, proliferation and cytotoxicity detection methods. The method developed by Mosmann in 1983 has been called the "gold standard" of cell viability tests [10]. The MTT method is used not only as an in vitro test, but also in tissue culture, and it is also used to measure cell viability in skin irritation and eye irritation tests [11].
Material and Methods
Plant Materials
Samples of phytoextract obtained by ultrasonic extraction from the collection of cloves (Syzygium aromaticus L.), yarrow (A. millefolium L.) - hibiscus (Hibiscus sabdariffa L.) plants and ointments with antibacterial and antifungal activity prepared on the basis of this phytoextract.
Extraction Procedure and Determination of the Yield
For the preparation of the phytoextract, 100 g of dry plant material weighed in the optimal ratio (1 part of yarrow grass, 3 parts of hibiscus flowers and 6 parts of clove buds) and propylene glycol-95% ethanol (5 : 95 ratio) as an extractant were diluted in a ratio of 1:10. The extraction process was carried out by the ultrasonic extraction method at a temperature of 28°C for 7 minutes. The obtained phytoextract was then filtered. The extraction process was carried out in a Stegler DG-460 device [12]. Later, by using the obtained phytoextract, ointment samples containing biopolymer ointment base - chitosan, essential oils of various plants, TVIN-80 emulsifier, silver and gold nanoparticles were prepared.
Determination of Total Phenolic and Flavonoid Content
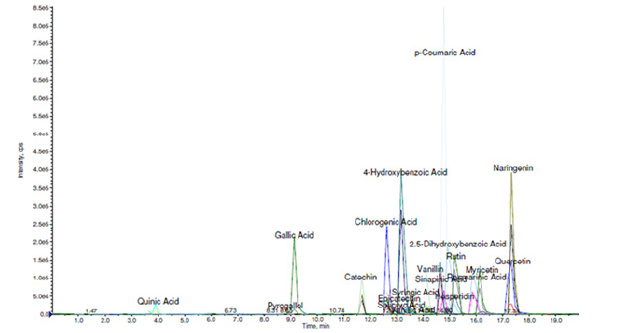
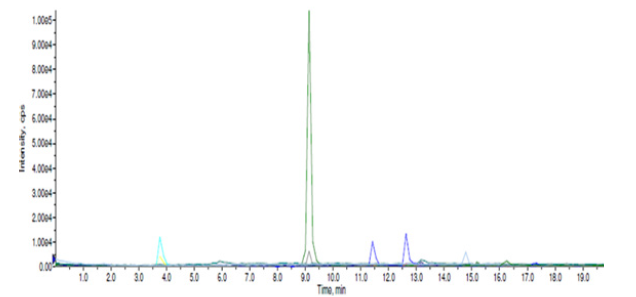
Referring to literature data, as well as the phytochemical studies we carried out on phytoextracts obtained from the collection of clove buds, hibiscus flowers and yarrow plant at the Central Laboratory of Ağrı İbrahim Çeçen University, Republic of Turkey, proved that it contains various volatile essential oils with antibacterial and antifungal activity (eugenol, acetateugenol, caryophyllene, etc.), there are also a variety of flavonoid, flavone and polyphenol derivatives (rutin, quercetin, kaempferol, gallic acid, caffeolchinic acid, hibicetin, etc.) showing rich antioxidant and antibacterial activity [13,14]. The results of the study are detailed in Figures 1,2 and 3.
If we look at the general research works, it is known that the mentioned medicinal plant raw materials are mainly rutin, quercetin, kaempferol, myricetin, catechin, chlorogenic acid, gallic acid, pyrogallol, naringein, etc. contains many derivatives such as these. Also, numerous phytochemical studies conducted on hibiscus flowers (Hibiscus sabdariffa L.) and yarrow (Achillea millefolium L.) have shown that organic acids (hibiscus acid and hydroxycitric acid), phenolic acids (caffeoylquinic acid), fatty acids (linoleic acid)), rich in flavonoids (gossypetin, myricetin, hibicetin and kaempferol) and anthocyanins (hibiscin) [15,16].
Antimicrobial Activity
Disc-Diffusion Method: The antibacterial and antifungal activity of the obtained phytoextracts was investigated and studied in the Scientific Research Laboratory of “The Medical Microbiology and Immunology Department" of AMU. It has been found that when the presented phytocollections are taken in optimal proportions during extraction, the obtained phytoextracts have sufficient antimicrobial activity [17]. Based on this direction, 4 of those phytoextracts were taken as a control, 9 ointment samples with different compositions were prepared and the antibacterial and antifungal effects were analyzed by disk diffusion method in the same laboratory.
Composition of Emulsion Ointments: (Table 1) To study these properties, according to the generally accepted rule, as a test culture, Staphylococcus aureus and MRSA - methicillin-resistant Staphylococcus aureus, as representatives of gram-positive bacteria, Esherichia coli and Pseudomonas aeruginoza from gram-negative bacteria, Candida albicans as representatives of fungi, representative of spore-forming gram-positive rod-shaped bacteria as Bacillus anthracoides and Klebsiella pneumoniae was selected as representative of capsular bacteria. In the disk-diffusion method, a suspension with a turbidity standard of 0.5 Mcfarland is prepared from a daily culture of microorganisms. Then separate microbial suspensions are poured into Petri dishes containing HM peptone B agar (Meat-peptone agar) and Saburo agar. Sterile discs soaked in the studied substance for 3-5 minutes are arranged on the surface of the nutrient medium planted with microbes. After that, seedlings with HM peptone B agar are placed in a thermostat at a temperature of 37°C, and seedlings in Saburo medium at a temperature of 28°C. Results are recorded after 24-48 hours (Table 2).
When we look at the table, we see that the presented samples show sufficient antibacterial and antifungal activity. In particular, the 3rd sample (N3) had a more active effect on all selected test cultures compared to other substances, the zone of inhibition varied from 7 to 34 mm. It should be noted that among the studied substances, the highest zone of inhibition was recorded against Gram-negative bacteria Escherichia coli (34 mm) and Candida albicans cultures from yeast-like fungi as a representative of fungi (24 mm), and this indicator, including controls, was not repeated in a substance. As a continuation of this research work, it was considered appropriate to conduct the In vitro cytotoxicological analysis (MTT method) on that ointment sample.
Reagents and Standarts
As a solution - serum-free cell culture medium (Dulbecco*s MEM High Gluocse, DMEM-HXA, Capricorn Scientific, Germany)
Type and source of cells used - HaCaT, Human keratinocyte cell line (Monolayer)
Seeding number of cells used - 26
Culture test - Dulbecco`s MEM High Glucose, 10% Fetal Bovine Serum (FBS, S181G, Biowest, Fransa), 1% Sodium Piruvat (S8636, Sigma, Germany), 0.1% Penisillin - Streptomisin (A2213, Biochrom, Germany)
Negative Control: A substance known to have no cytotoxic effect on cells, Polyethylene (PE) (0.2 g/ml extract in nutrient medium)
Positive Control: Substance known to have a cytotoxic effect on cells, Dimethyl sulfoxide (DMSO) (20% (v/v) in nutrient medium)
Reactive Control (Standart): Culture prepared with cell culture medium without analyte
Statistical Analysis
It should be noted that all the in vitro cytotoxicity tests were carried out at Ege University, Center for Test and Analysis Laboratory, Application and Research Center (EGE MATAL), Republic of Turkiye. All experiments were repeated 3 times, and the results were statistically verified, and Graphpad Prism-8 was used as a statistical program. The results were analyzed by One-Way ANOVA test, Dunnett was selected as a post-hoc method. When all the results were statistically analyzed, a significant difference was felt in all cell groups treated with plant extract compared to the control. (*** p<0.001, **p<0.01). In the cell groups where the ointment sample was applied, it was confirmed that there was a significant difference compared to the control only at concentrations of 0.5, 1.0 and 500.0 mg/ml (****p<0.0001, **p<0.01).
Results and Discussion
Sample Preparation Procedure
The phytoextract in a concentration of 0.5-1.0-5.0-10.0 mg/ml, and the ointment sample was in a concentration of 0.5-1.0-5.0-10.0-50.0-100.0-500.0 mg/ml was prepared in Dulbecco's MEM High Glucose nutrient medium
Analysis Preparation Procedure
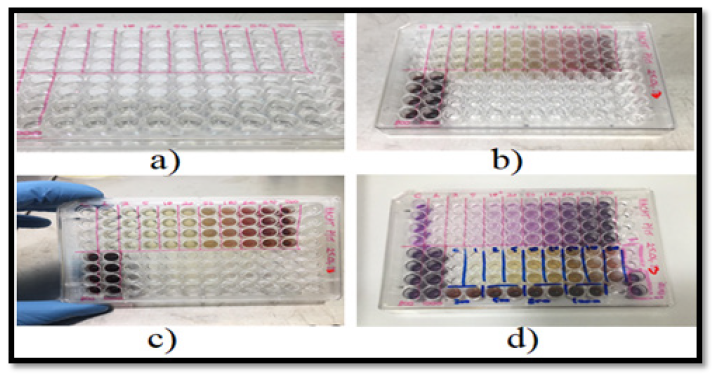
The cytotoxicity test was performed with HaCaT cells (HaCaT is a spontaneously transformed aneuploid immortalized keratinocyte cell line from adult human skin widely used in scientific research). The cytotoxic effect of the samples (at 37°C with 5% CO2 and 95% humidity) applied to the cells at a concentration of 1x105 cells/ml for 24 hours on the cells planted on 96-well microdiscs was evaluated by reading the absorbance frequency in a spectrophotometer (570 nm) with the MTT test (Figure 4).
In Vitro Cytotoxixity Activity
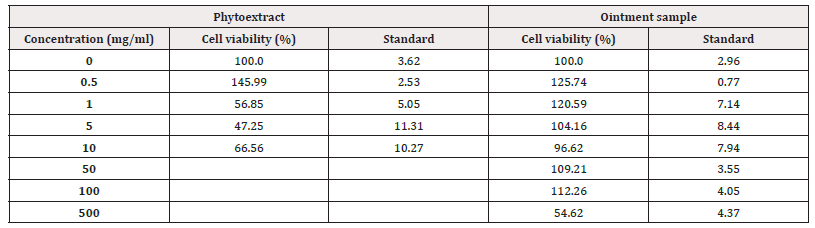
The results of the 4-hour MTT test performed because of the incubation of the cells with the test materials for 24 hours are given below (Table 3).
Negative Control cell viability percentage: 98.1% ± 2.12
Positive Control cell viability percentage: 3.2% ± 1.05
When the MTT results of test materials are evaluated according to ISO 10993-5:2009 Tests for In Vitro Cytotoxicity standard.
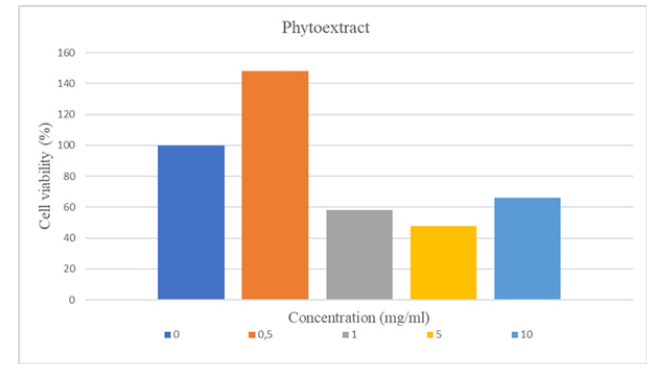
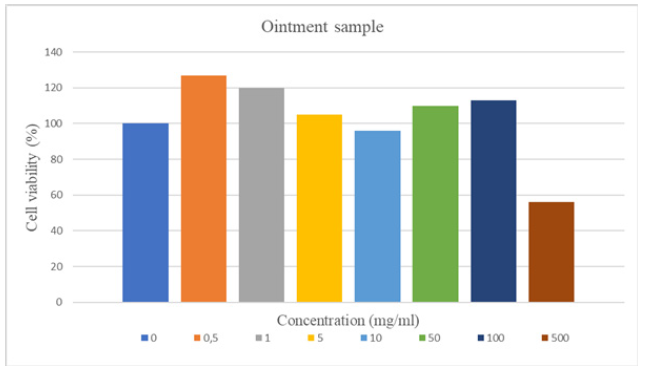
All these samples were confirmed to have a cytotoxic effect, as cell viability was less than 70% in the MTT assay performed with concentrations of 1.0-5.0-10.0 mg/ml of the plant extract. That is, to be more precise, the phytoextract at a concentration of 1mg/ml already had a cytotoxic effect. Similarly, as a result of the MTT test of the cells where the ointment sample was applied at a concentration of 500mg/ml, the cell viability was less than 70%, so it was confirmed that the ointment sample had a cytotoxic effect only from this concentration (Figures 5,6).
Conclusion
This study showed that the extracts obtained from the mentioned phytocomposition are an important source for the regeneration of organs and tissues in the form of preparations with high antioxidant activity as bioactive antioxidants with potential applications in the treatment of a number of diseases and in the development of new medicinal forms. The polyphenol analysis of the mentioned medicinal-plant raw materials made it possible to identify at least twenty bioactive compounds (mainly phenolic acids and flavonoids) that can be applied in the field of medicine. The conducted phytochemical research plays an important role in detecting biologically active compounds - polyphenol derivatives, organic acids - isolated from the medicinal-plant raw materials taken in optimal proportions. All three medicinal-plant raw materials that make up the phytocomposition have been extensively studied pharmacognostically, especially the phytochemical profiles of the polyphenolic compounds of hibiscus flowers and yarrow, with potentially beneficial effects on human health due to the presence of rutin, quercetin, hesperidin and gallic acid.
At the same time, the antibacterial and antifungal activity of both the investigated phytoextract and the ointment prepared on the basis of that phytoextract was quite high. Thus, the ointment sample, which we call the 3rd sample (N3), had a more active effect on all the selected test cultures compared to other substances, the zone of inhibition varied between 7-34 mm. The same sample was recorded as the highest zone of inhibition among the studied substances against Esherichia coli as a representative of gram-negative bacteria (34 mm) and against Candida albicans cultures from yeast-like fungi as a representative of fungi (24 mm), and this indicator, including the control, not repeated in any article. Based on the results of the cytotoxic analysis conducted by the MTT method, it was confirmed that all of these samples had a cytotoxic effect, as the cell viability was less than 70% with the concentrations of 1.0-5.0-10.0 mg/ml of the phytoextract. Based on the results, the phytoextract at a concentration of 1mg/ml already had a cytotoxic effect. Similarly, as a result of the MTT test of the cells where the ointment sample was applied at a concentration of 500mg/ml, the cell viability was less than 70%, so it was confirmed that the ointment sample had a cytotoxic effect only from this concentration. These results, as a continuation of the research, will stimulate the process of obtaining and applying various medicinal forms with regenerating, healing and antioxidant effects.
Acknowledgements
The authors would like to express their gratitude to the staff of the Central Laboratory of Agri İbrahim Cecen University for their unstinting support in conducting preliminary phytochemical studies in the thoughtful development of this manuscript. Finally, we are grateful for the technical assistance provided by the Ege University, Center for Test and Analysis Laboratory, Application and Research Center (EGE MATAL), Republic of Turkiye for conducting in vitro studies.
Conflict of Interest
None.
References
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2): 279‐
- Lefsih Khalef, Radja Lydia, Khettar Filicia, Berkoud Moussa (2024) Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem Funct 42(3): e4007.
- Niles AL, Moravec RA, Riss TL (2008) Update on in vitro cytotoxicity assays for drug development. Expert Opin Drug Discov 3(6): 655‐669.
- Liu X, Rodeheaver DP, White JC, et al. (2018) A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul Toxicol Pharmacol 97: 24‐32.
- Lefsih Khalef, Radja Lydia, Khettar Filicia, Berkoud Moussa (2024) Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem Funct 42(3): 4007-4009
- Majtnerová P, Roušar T (2018) An overview of apoptosis assays detecting DNA fragmentation. Mol Biol Rep 45(5): 1469‐1478.
- Alamoudi WA, Ahmad F, Acharya S, et al. (2018) A simplified colorimetric method for rapid detection of cell viability and toxicity in adherent cell culture systems. J BUON 23(5): 1505‐1513.
- Sali N, Nagy S, Poór M, Köszegi T (2016) Multiparametric luminescent cell viability assay in toxicology models: A critical evaluation. J Pharmacol Toxicol Methods 79: 45-54.
- 2021 Current In Vitro Cytotoxicity Tests Hacettepe University Journal of the Faculty of Pharmacy. 41(1): 45-63.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65 (1-2): 55-63.
- Kumar P, Nagarajan A, Uchil PD (2018) Analysis of Cell Viability by the MTT Assay. Cold Spring Harb Protoc 2018(6).
- Mammadov B S (2021) Selection of the optimal composition of the phytocomposition that stimulates the regeneration process. Health Scie Pract J 2: 175-180.
- Mammadov BS (2022) GC-MS analysis of essential oils in antimicrobial and antifungal effective phytocompositions, scientific-practical magazine named after Aziz Aliyev Med Scie 2(28): 61-66.
- Mammadov B (2023) Identification And Screening Analysis Of Secondary Polyphenol Metabolites In Phytoextracts With Optimal Composition Nature and Science. Inter Scie J pp. 11.
- Ruan J, Yan J, Zheng D, Sun F, Wang J, et al. (2019) Comprehensive Chemical Profiling in the Ethanol Extract of Pluchea indica Aerial Parts by Liquid Chromatography/Mass Spectrometry Analysis of Its Silica Gel Column Chromatography Fractions. Molecules 24(15): 2784.
- Che Y, Wang Z, Zhu Z, Ma Y, Zhang Y, et al. (2016) Simultaneous Qualitation and Quantitation of Chlorogenic Acids in Kuding Tea Using Ultra-High-Performance Liquid Chromatography-Diode Array Detection Coupled with Linear Ion Trap-Orbitrap Mass Spectrometer. Molecules 21(12): 1728.
- Mammadov B, Mehraliyeva S (2020) Acquisition of regenerative phytoextracts and their study of microbiological properties PS-001. Uluslararası Bioteknoloji Kongresi pp. 5-7.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.